Abstract
Biogas contains significant quantities of undesirable and toxic compounds, such as hydrogen sulfide (H2S), posing severe concerns when used in energy production-related applications. Therefore, biogas needs to be upgraded by removing H2S to increase their bioenergy application attractiveness and lower negative environmental impacts. Commercially available biogas upgradation processes can be expensive for small and medium-scale biogas production plants, such as wastewater treatment facilities via anaerobic digestion process. In addition, an all-inclusive review detailing a comparison of biochar and hydrochar for H2S removal is currently unavailable. Therefore, the current study aimed to critically and systematically review the application of biochar/hydrochar for H2S removal from biogas. To achieve this, the first part of the review critically discussed the production technologies and properties of biochar vs. hydrochar. In addition, exisiting technologies for H2S removal and adsorption mechanisms, namely physical adsorption, reactive adsorption, and chemisorption, responsible for H2S removal with char materials were discussed. Also, the factors, including feedstock type, activation strategies, reaction temperature, moisture content, and other process parameters that could influence the adsorption behaviour are critically summarised. Finally, synergy and trade-offs between char and biogas production sectors and the techno-economic feasibility of using char for the adsorption of H2S are presented. Biochar’s excellent structural properties coupled with alkaline pH and high metal content, facilitate physisorption and chemisorption as pathways for H2S removal. In the case of hydrochar, H2S removal occurs mainly via chemisorption, which can be attributed to well-preserved surface functional groups. Challenges of using biochar/hydrochar as commercial adsorbents for H2S removal from biogas stream were highlighted and perspectives for future research were provided.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since the Industrial Revolution in the early 1760s, extensive use of fossil fuels for industrial activities resulted in several environmental issues, such as the emission of greenhouse gases and other toxic gases into the environment. Given the finite nature of fossil reserves and challenges, such as environmental pollution and climate change, associated with the use of fossil fuels, efforts are made by the scientific community to identify an alternative source of fuel for energy generation that is cheap, readily available, and renewable. Biogas is considered as an alternative energy source that could levy the dependence on fossil resources and, at the same time, reduce the carbon footprint of energy production and consumption (Fonseca-Bermúdez et al. 2023). Hydrogen sulfide (H2S) is one of the traditional acidic gas contaminants in many streams, such as natural gas, refinery gas, landfill gas, fuel gas, and biogas. Hence, removing H2S from the carrier gas stream is crucial due to their acute toxicity and the prevention of corrosion in piping and downstream processing facilities. Biogas, largely a mixture of methane (CH4) and carbon dioxide (CO2), is a product of anaerobic digestion (AD), a process that involves the biological degradation of organic matter in an oxygen-limited environment. Alongside CH4 (53–70%), and CO2 (30–47%), biogas also contains trace concentration of N2 (0–3%), H2O (5–10%), O2 (0–1%), H2S (0–10000 ppm), NH3 (0–100 ppm), hydrocarbons (0–200 mg m−3) and siloxanes (0–41 mg m−3), depending on the type of feedstock and process conditions (Katariya and Patolia 2023).
The concentration of these impurities can be concerning depending on the end-use of the biogas. For example, H2S is a potential biogas contaminant and the presence of H2S in biogas could create several operational and technical issues, such as corrosion of mechanical parts and breakdown of the energy conversion system (Ahmad et al. 2021b). The tolerance limit of H2S in biogas varies depending on the technology considered and applications. For instance, H2S concentrations should not exceed 1 ppm for fuel cell applications (Ghimire et al. 2021). Similarly, when biogas is considered for combustion applications, the recommended levels of H2S are between 200 and 300 ppm (Cattaneo et al. 2023). During combustion, H2S is oxidized into acidic sulfur dioxide (SO2), highly corrosive to metal surfaces. Furthermore, the corrosion process speeds up at higher temperatures and leads to operational failures. In addition to being corrosive, H2S causes catalyst poisoning during steam reforming applications. H2S is highly toxic, and according to the National Institute of Occupational Safety and Health, the recommended exposure limit of H2S is 10 ppm, which is a 10 min ceiling, and 20 ppm is the general industrial ceiling limit. Therefore, it is necessary to remove H2S from biogas before it is considered for any downstream application, particularly for energy generation. H2S-free, biomethane/hydrogen-rich biogas is an important renewable resource in the energy sector for clean thermal energy and power production via direct combustion in gas engines (Bui et al. 2021).
The methods or techniques for capturing H2S from biogas have been classified into ex-situ and in-situ removal techniques. The selection of an appropriate technique depends on various factors, such as biogas composition, degree of removal required, intended application of the treated biogas, and operational conditions (Allegue et al. 2012). Figure 1 summarizes various in-situ and ex-situ H2S removal techniques reported in the literature. In the last decade, significant efforts have been made in the removal of H2S through the use of various techniques, such as wet oxidation (Tian et al. 2022), scrubbing (Liu and Wang 2019), adsorption (Cheng et al. 2024), and biological oxidation (Peluso et al. 2019). However, a common drawback associated with most of the above techniques, particularly wet scrubbing (liquid absorption) is that they are dependent on expensive catalysts or chemicals that may lead to secondary pollution or even equipment corrosion. On the other hand, biological processes are slower and often require strict control of operational conditions. In this regard, adsorption techniques have gained a great deal of attention due to their low cost and energy requirement, ease of operation and excellent removal capacity under low pollutant concentrations (Zhao et al. 2022b).
Several adsorbents, including activated carbon, carbon fibre, graphene, carbon nanotubes, polymers, metal–organic frameworks (MOFs), nanomaterials, and biochar, have been demonstrated for H2S adsorption. Among the various adsorbents, activated carbon is the most widely used, however, the high cost (1100–1700 USD/ton) can limit their large-scale application (Fdez-Sanromán et al. 2020). Cost-effective adsorbents such as biochar and hydrochar are carbon-rich materials obtained from the pyrolysis and hydrothermal processing of biomass, respectively (Vuppaladadiyam et al. 2022), (Mumtaz et al. 2023). Char (in the context of this review refers to both biochar and hydrochar) can be low in cost (90–100 USD/ton) (Fdez-Sanromán et al. 2020). At the same time, char possesses desirable properties, such as high surface area, excellent porosity, and surface functional group, favouring their adsorption performance. Furthermore, char produced from wastes, such as biosolids (stabilised sewage sludge) have high inherent metals/mineral contents (Hakeem et al. 2022) that could further enhance their H2S adsorption capacity (Fdez-Sanromán et al. 2020). Therefore, char is widely used as an adsorbent in various environmental applications such as nutrient recovery (Koulouri et al. 2024) and heavy metal removal from wastewater (Yuan et al. 2024).
In recent times, the application of biochar/hydrochar for gas phase adsorption has gained attention. Several studies have reported the application of char for the removal of various gas components, such as CO2, SO2, H2S, and other gaseous pollutants. However, the removal capacity of char varies with gaseous pollutants and their performance depends on the properties of char and the experimental conditions employed. While some studies elucidated the correlation between biochar’s physiochemical properties and H2S adsorption mechanism, such understanding of hydrochar for H2S adsorption is limited. Specifically, the in-depth understanding of hydrochar efficacy for H2S adsorption and associated mechanisms requires investigations. Therefore, to fill this gap, we present an all-inclusive state-of-art progress on biochar and hydrochar, particularly for the adsorption of H2S from biogas. The production processes, physiochemical properties, mechanisms, removal conditions, and adsorption performances of biochar vs hydrochar are discussed in-depth. In the later part of this review, an attempt was made to emphasize the possibility of creating a sustainable circular economy approach by integrating thermochemical processes for char production from wastewater biosolids and using the produced biochar/hydrochar for biogas upgradation all within wastewater treatment facilities. Additionally, a detailed technical comparison of various H2S removal technologies is conducted based on the understanding gained from this review. Finally, challenges and research perspectives for developing efficient biochar/hydrochar-based adsorbents are highlighted.
2 Overview of H2S removal techniques
2.1 Biological techniques
This technique employs an acidophilic oxidizing group of bacteria naturally occurring in the environment to decompose H2S. The gram-negative bacteria oxidise H2S/sulfides to elemental sulfur and sulfates through two domains (photoautotrophs and chemolithotrophs) via the sulfur oxidising reactions shown in Eqs. 1 and 2 (Rana et al. 2020).
Photoautotrophs bacteria use carbon sources for the photosynthetic reaction where CO2 acts as a terminal electron acceptor and H2S acts as the electron donor. This redox reaction converts H2S to sulfur and sulfate (Nguyen et al. 2022a). H2S oxidation can also be achieved by chemolithotroph bacteria that use O2 (aerobic) or nitrate or nitrate (anaerobic) as an electron acceptor, grow on H2S as an energy source and use CO2 as a carbon source [1]. The oxidation of H2S by biological technique is a well-known process for the abatement of H2S emissions in industrial-scale biogas plants (Cheng et al. 2018; Ho et al. 2013; Lin et al. 2013). The most common approach of microbiological techniques for H2S capture is based on different types of equipment such as biofilter, biotrickling filter, and bioscrubber. For instance, in a biofilter system, the filter bed is packed with organic media that has bacterial supplementing nutrients and a diverse microbial community developing a biofilm (Lestari et al. 2016). The packing media and biofilm formation are responsible for the range of processes (absorption, adsorption, phase transfer) required for the biodegradation of H2S (Ho et al. 2013). A comprehensive review summarising the process mechanisms, operation efficiency, and limitations of H2S removal using biological techniques is provided elsewhere (Barbusiński et al. 2021; Huynh Nhut et al. 2020; Khoshnevisan et al. 2017; Pudi et al. 2022; Syed et al. 2006). Examples of commercial installations of biofilter and bioscrubber for H2S removal from biogas are Sulfothane, DMT Clear Gas Solution Sulfurex®BR and Sulfurex®BF, and THIOPAQ (a reference technology for low-pressure biogas treatment).
2.2 Absorption
This is a traditional industrial technique of scrubbing acidic gases, including H2S, from gas streams such as biogas, hydrocarbons, fuel gas using liquid solvents. Commonly used chemical media for H2S absorption are amine-based solvents, alkalis, ionic liquids, and deep eutectic solvents. In this method, the H2S-containing gas stream is contacted with the chemical solution which selectively removes H2S from the carrier gas and the exiting gas stream is lean in H2S while the exiting solvent is rich in H2S. The mechanism of H2S absorption using chemical solvents can be either a physical or chemical process and may involve the dissolution of trace H2S components by the solvent and a chemical reaction of the dissolved solute with the solvent. The saturated solvent is regenerated by chemical and thermal desorption and reused in the process. Absorption using alkanolamine solvents such as monoethanolamine (MEA) and diethanolamine (DEA) remains one of the most traditional approaches for gas sweetening and is well-advanced in the relevant industry. However, for biogas which typically contains more CO2 than H2S by vol, amine-based absorption might be limited for selective H2S removal, the use of Fe(SO4)3, FeCl2, NaOH, and Fe(OH)3, has been suggested for high H2S removal efficiency from biogas; albeit at the optimum operating conditions (Zulkefli et al. 2016). Nevertheless, the simultaneous removal of CO2 and H2S in biogas using MEA and other aqueous alkaline solvents has been widely demonstrated to improve biogas quality with respect to calorific content and H2S contamination levels (Marín et al. 2020; Tahir et al. 2015; Tippayawong and Thanompongchart 2010). Detailed information about the principle of operations, summaries of lab-scale results and limitations of the H2S absorption method using different solvents can be found in a recent review (Qayyum et al. 2020). Typical commercial installations of absorption technologies for H2S removal from biogas streams include DMT Clean Gas Solutions Sulfurex®CR, ExxonMobil and BASF OASE® sulfex™ and Merichem LO-CAT® sulfur recovery technology.
2.3 Adsorption
Adsorption is a gas–solid separation of H2S from carrier gas stream using solid adsorbent. The H2S-containing gas is passed over a bed of solid adsorbent and by a range of interactions which can be physical or chemical, H2S is selectively removed on the surface or in the bulk of the adsorbent (Chan et al. 2022). A range of adsorbent materials have been demonstrated for removing H2S from biogas – commonly used adsorbents are zeolites, metal oxides, carbon-based (nanomaterials, biochar, activated char), MOFs and porous organic polymers, and composite (Pudi et al. 2022). A review of the most promising adsorbent materials for the removal of H2S from industrial gases has been documented in a recent paper (Georgiadis et al. 2020). The desired features of adsorbent for H2S include a large surface area per unit weight and pores having a larger diameter than the molecular diameter of H2S. The most commonly used commercial adsorbent materials for H2S removal from biogas are activated carbon and metal oxides. Metal oxides catalytically oxidise H2S to elemental sulfur and strongly bind the sulfur in form of metal sulfide. The abundance of porous structure and specific surface area enables activated carbon to capture H2S onto their pores, similar to zeolites. More recently, biochar (and activated char) has been demonstrated as efficient material for removing H2S from biogas with performance comparable to active carbon materials (Choudhury and Lansing 2021; Shang et al. 2016). Biochar adsorbents are considered cheap and sustainable alternatives to expensive conventional materials (such as zeolites, MOFs, and polymers) as they can be sourced from waste biomass, and they do not require tedious synthesis before use for H2S adsorption. Biochar can combine the features of metal oxides and activated carbon with respect to their inorganic compositions and surface properties, therefore, they can offer viable H2S removal capacity. However, biochar use as an efficient commercial adsorbent for H2S removal is still under development and biosolids biochar can be an attractive option for real circular economy solutions for wastewater-generated biogas as detailed in Sect. 5. Adsorption is generally well-suited for treating dilute H2S gas streams. The efficiency of H2S removal by adsorption (gas–solid contact) is highly influenced by the properties of the adsorbent (surface chemistry), as well as the operating parameters (temperature, relative humidity, gas hourly space velocity, and the concentration of H2S, O2 and other contaminants (Sitthikhankaew et al. 2014; Vaziri and Babler 2019). A comprehensive review of H2S adsorption from biogas using waste biomass-derived adsorbent has been reported in the literature (Sitthikhankaew et al. 2014). Commercial H2S adsorption installations based on iron oxide and mixed metal oxide adsorbents are SULFATREAT® and SELECT FAMILY®, respectively.
2.4 Electrochemical
The electrochemical technique for H2S removal uses electrical potential to induce electron flow in an electrochemical cell via a series of oxidation and reduction reactions, which ionises dissolved H2S into 2H+ and S−. H2S removal in electrochemical systems can occur under two mechanisms – oxidation and precipitation (Pudi et al. 2022). The direct oxidation of H2S can produce S0, S2O32−, SO32−, or SO42−, depending on the anode material and applied potential – low applied voltage will result in partial oxidation of sulfide to S0 while at high potential, sulfate and/or thiosulfate are generated (Dutta et al. 2008). However, the formation of S0 is the preferred reaction from an economic viewpoint as it needs less electrical energy, and the solid sulfur deposit on the anode can be easily separated from the system (Pudi et al. 2022). For the electrochemical precipitation process, also referred to as electrocoagulation, a sacrificial anode is made of a metal, such as iron, and releases ferrous/ferric ions into the electrolyte, which react with dissolved H2S to produce insoluble iron sulfide precipitates. The solid residue is then removed from the aqueous solution. The reaction of metal ions with aqueous sulfide to produce metal sulfides at the anode simultaneously cause H2 gas bubbles production at the cathode due to the dissociation of water molecules (Ahmad et al. 2021b; Pikaar et al. 2015). The H2 will aid the electro-floatation of the formed metal sulfide on the surface for easy removal. The interest in electrochemical methods for sulfide removal is due to the simultaneous removal of organic compounds/pollutants from industrial waste streams. While a high electrochemical conversion of gaseous H2S to sulfur can be achieved, a liquid absorber is usually needed to solubilise the sulfide in an aqueous electrolyte before its oxidation in an electrochemical cell (Kang et al. 2019). Elemental sulfur is deposited onto the electrode and deactivates it over time, which is considered a significant limitation of the electrochemical process (Dutta et al. 2009).
2.5 Membrane
Membrane separation is an advanced technology in a variety of industrial processes. Compared to adsorption and absorption, separation of acidic gases by membrane can be accomplished cost-effectively in a decentralised location due to the smaller footprint, high modularity (high membrane area per unit module) and ease of operation (Chan et al. 2022). In membrane separation, physical porous barriers are used for selective gas transport (permeation) driven by transmembrane pressure. The porous membrane materials are diverse and must be permeable, selective, mechanically stable, and chemically inert to separate the gas component. In industrial H2S removal, polymeric membranes, including glassy polymer, rubbery polymer, and hybrid membranes, have been widely used in various configurations (Shi et al. 2020). The development of various polymer-based membranes and membrane processes for H2S separation from natural gas, biogas, and coal gas have been reported in a recent review (Ma et al. 2021b). To improve the simultaneous removal of H2S and CO2 using the membrane process, membrane contactors involving the addition of liquid/solid adsorbent to polymeric membranes have been found to enhance the selective H2S separation from CH4 and CO2-rich gas stream (Ma et al. 2021b). In this type of process using ePTFE-HFM membrane module, H2S removal efficiency ranges from 70 to 85% using water, 90–100% using NaOH, and 94–100% using amine solution contactors (Marzouk et al. 2012). However it should be noted that the use of liquid contactors for enhanced H2S removal using membrane can cause fouling issues and reduce gas flux (Ahmed et al. 2022). Nevertheless, membrane techniques have commercial applications for natural gas sweetening and biogas upgrading for several decades, using materials such as cellulose acetate-based Cynara®/Cameron (SLB 2023) and Separex™/UOP, and polyimide-based MEDAL™/Air Liquide.
3 Biochar vs. hydrochar: production technologies and properties
3.1 Biochar production
Thermochemical processes are commonly used to produce biochar from various biomass feedstocks. While pyrolysis, gasification, and torrefaction are used primarily for producing biochar, hydrothermal processes such as hydrothermal liquefaction and hydrothermal carbonization are used to produce hydrochar. Enormous reviews on char production technologies are published elsewhere (Adeniyi et al. 2023; Fang et al. 2023; Ighalo et al. 2023; Li et al. 2023; Masud et al. 2023; Patel and Panwar 2023; Rathnayake et al. 2023; Roshan et al. 2023; Safarian 2023; Sharma et al. 2023; Supraja et al. 2023; Tawfik et al. 2023; Venkatachalam et al. 2023; Wang et al. 2023; Wu et al. 2023; Yuan et al. 2023; Zakaria et al. 2023). Biochar refers to the solid product obtained from the thermochemical decomposition of biomass resources and generally has stable carbon structures. Pyrolysis is widely acknowledged as one of the primary routes for producing biochar under an inert atmosphere at temperatures typically between 300 and 700 °C. The oxygen-free inert atmosphere prevents carbon combustion in biomass, facilitating a higher degree of carbonisation (Malyan et al. 2021). Pyrolysis is operationally categorized into three types: (i) slow, (ii) fast, and (iii) flash pyrolysis, depending on the process conditions, such as temperature, heating rate, residence time, and feed particle sizes (Sharma et al. 2024). However, other various forms of pyrolysis techniques, such as microwave-assisted pyrolysis, hydro pyrolysis, autothermal pyrolysis, catalytic pyrolysis and co-pyrolysis have emerged with the aim of efficient feed conversion and enhanced product recovery (Vuppaladadiyam et al. 2022). Product distribution during pyrolysis largely depends on the operational conditions and feed materials in all these techniques. For instance, the typical slow heating rate and longer residence time in the slow pyrolysis process favours biochar production, while fast and flash pyrolysis, characterised by a fast heating rate and short residence time favours bio-oil production.
Dry torrefaction, or mild pyrolysis, occurs in the temperature range of 200–300 °C and residence time varies from 30 min to 4 h (Table 1). During dry torrefaction, only 10% of energy contained in biomass is lost in the form of gases and overall, a mass loss of ca. 30% occurs. It is worth noting that torrefaction improves biomass's physical and chemical properties and leads to improved energy density, better ignition, less moisture, better grindability, high C/H and C/O ratios and hydrophobicity (Mamvura and Danha 2020). Dry torrefaction can be done under oxidative (in the presence of air/oxygen) or non-oxidative (inert conditions). Although solids yield is low in oxidative environments, higher reaction rates are expected, resulting in a shorter torrefaction duration (Chen et al. 2021b). Gasification is a high-temperature thermochemical conversion process in a partly oxidizing environment created by adding air, oxygen, steam, carbon dioxide, or other oxidising agents. Gasification is mainly employed to generate syngas as the main product; however, a small fraction of char is produced (Libra et al. 2011; Patel et al. 2020). Table 1 summarises the operational parameters and typical product yields for the various thermochemical techniques for biochar production.
3.2 Hydrochar production
Hydrothermal technologies employ liquid media to convert organic substrate, and in the process, four product streams are generated (hydrochar, biocrude, syngas, and aqueous product). Hydrothermal techniques are promising for processing wet feedstock, as energy-intensive feedstock drying is unnecessary and could be eliminated. Hydrothermal technologies are broadly categorized into hydrothermal carbonization, hydrothermal liquefaction, and supercritical water gasification based on operating conditions (Masoumi et al. 2021). While hydrothermal carbonization is primarily used to produce hydrochar, hydrothermal liquefaction and supercritical water gasification are used to produce biocrude and syngas as primary products, respectively, and hydrochar as a co-product (Marzbali et al. 2021).
Hydrothermal carbonization (HTC) is a low-temperature process, usually carried out below water critical temperatures between 180 and 250 °C under autogenous pressure, and residence time can vary from minutes to several hours (Gupta et al. 2020). Depending on the operational conditions, the product distribution in HTC is 2–5% gas, 40–70% hydrochar and 10–20% biocrude oil (Shyam et al. 2022). HTC comprises three stages, dehydration, decarboxylation, and decarbonylation, and can be used to treat diverse wet organic wastes such as food waste (Periyavaram et al. 2023), sewage sludge (Liu et al. 2021b), fruit waste (Chen et al. 2017a) etc. Product formation, properties, and composition depend on the process parameters and initial feedstock composition (Funke and Ziegler 2010; Libra et al. 2011). Carefully controlling operational parameters and feed may result in highly functionalized carbon materials (Biller and Ross 2016). By decreasing the H/C and O/C molar ratio, the HTC process promotes dehydration and decarboxylation reactions, producing hydrochar with characteristics close to coal (Fang et al. 2018). The mechanism of hydrochar formation from the thermal decomposition of complex organic biopolymers (carbohydrate, proteins, and lipid) into simple intermediate compounds (sugar, amino acid, and fatty acid) followed by series of chemical reactions of the fragements via amidation, hydrolysis, deamination, Millard reaction, dehydration, aromatisation, retro-aldol condensation, among others, during HTC has been summarised elsewhere (Le et al. 2022).
Hydrothermal liquefaction (HTL) is usually carried out in temperatures between 250 and 360 °C, under autogenous water pressures between 10 and 25 MPa to produce solid, liquid, and gaseous product streams. Though HTL produces liquid fuel called biocrude as a major product fraction, hydrochar with high ash content and gases is also produced (Gollakota et al. 2018). Different chemical compounds, including aromatic, aliphatic, phenolics, esters, carboxylic acids, and nitrogenous structures, are available and are distributed in the liquid, solid, and gas phase products. Hydrochar from the HTL process results from recombination reactions in the aqueous phase, and the formation of hydrochar is highly influenced by the partial carbonization reaction of organic molecules released during hydrolysis (Kassim et al. 2022). Supercritical water gasification (SWG) occurs under supercritical conditions at temperatures greater than 375 °C to produce mainly syngas with a higher H2 concentration than conventional gasification (He et al. 2014). SWG is a high-temperature steam reforming process that involves two steps; (i) the hydrolysis of feedstock in supercritical water and (ii) further gasification of aqueous oligomers produced in the previous step. Since the solubility of water under supercritical conditions is high, the intermediate products produced during biomass decomposition are dissolved, resulting in a remarkable reduction in the tar and coke content (He et al. 2014). Unlike conventional thermal gasification, which is carried out at elevated temperatures (> 800 °C), SWG is carried out at relatively low temperatures (< 600 °C). Table 2 summarises the operational parameters and product yields for the various hydrothermal techniques for hydrochar production.
3.3 Properties of biochar vs. hydrochar
3.3.1 Physical properties
Although processes such as gasification, HTL and SWG can produce char, the yields of the processes are not significant, as shown in Tables 1 and 2. Therefore, this section focused on the properties of biochar and hydrochar produced via slow pyrolysis and HTC processes, respectively. The fibre and biochemical compositions of the feedstock directly influence the properties of char produced from the thermal treatment processes. In thermal and hydrothermal processes, with the increase in temperature, the organic components of the feedstock decompose via a set of chemical reactions, leaving a solid residue product with modified surface properties. Feedstock type and operational parameters, such as temperature, feed particle size, heating rate, residence time, and reactor configuration, significantly influence the char properties (Vuppaladadiyam et al. 2023a, b). The higher heating value, carbon content, and specific surface area (SSA) of biochar increase at high temperatures and heating rates at the expense of biochar yield (Ahmad et al. 2021a). Similarly, increased carbon content, fixed carbon content and energy density were reported for hydrochar with increased HTC temperature (Zhang et al. 2019b).
The surface properties of char include SSA, pore volume and pore size. While the SSA is the main indicator of the sorption interphase on the char, pore size and volume could influence effective solid–gas interactions and exchange between the active sites and reactants over the biochar surface (You et al. 2017). In the case of slow pyrolysis, treatment temperature and residence time are the most influential parameters on biochar yield and properties. With the increase in temperature, the volatile content of the biochar decreases and the fixed carbon content increases. The pyrolysis temperature also plays an important role in defining biochar's microstructure and surface properties. Zhao et al. investigated the influence of temperature on apple tree branches-derived biochar properties. The authors noted that the SSA and pore volume of biochar increased with the increase in pyrolysis temperature and had the highest SSA of 108.59 m2 g−1 and pore volume of 0.059 cm3 g−1 at 600 °C (Zhao et al. 2017). Even though an increase in residence time is known to increase the SSA, the effect of residence time on enhancing SSA is less pronounced than the influence of temperature. Results reported in recent studies confirm an increase in the SSA of biochar with an increase in pyrolysis temperature (Jin et al. 2020; Kim et al. 2012; Weber and Quicker 2018). Conversely, hydrochar obtained via HTC generally has a very poor surface area and porosity (Ighalo et al. 2022b). Similar to observations for pyrolysis-derived biochar, the SSA and pore properties of hydrochar also increase with an increase in the reaction temperature (Ighalo et al. 2022a). In addition to reaction temperature, residence time also influences the SSA and pore properties of hydrochar. According to Garlapalli et al., higher residence time would facilitate the devolatilization and polymerization of organic components in the feed and improve the pore properties (Garlapalli et al. 2016). Table 3 presents the structural properties of biochar and hydrochar derived from various biomass sources. It can be noticed from Table 3 that the SSA of biochar and hydrochar vary over a wide range. While for biochar, the SSA varied from 6 to 1495 m2 g−1, for hydrochar, it varied from 3 to 169 m2 g−1, indicating that the SSA is higher in the case of the former than the latter.
The hydrologic properties of char are crucial when it is used as an adsorbent to remove acidic gases, such as H2S. The availability of surface functional groups and a change in porosity during production could define the hydrologic properties of char. While the decrease in functional groups could alter the affinity of biochar to water, an increase in porosity may influence the amount of water that can be adsorbed. Increasing temperature could increase the aromaticity and hydrophobicity of the biochar due to the removal of polar surface functional groups, which is reflected in the low O/C ratio (Kharel et al. 2019). In a study by Pimchuai et al. the authors reported that an increase in the temperature leads to the production of more hydrophobic char. The moisture content of the biochar was significantly lower compared to the raw feedstock. For instance, when sawdust and sawdust-derived biochar were immersed in water for 2 h, the moisture rise with the former was 150.33%, while the moisture rise in the latter was a meagre 2.16% (Pimchuai et al. 2010). However, contradicting results have been reported on the influence of temperature on the hydrophobicity of biochar.
Zornoza et al. produced biochar at 300 °C from cotton crop residue and swine manure, which displayed high hydrophobic characteristics. When the pyrolysis temperature was raised to more than 500 °C, the resulting biochar did not display any hydrophobicity (Zornoza et al. 2016), adhering to results reported in the literature (de Jesus Duarte et al., 2023; Suansa et al. 2021). However, this does not indicate that biochar is hydrophilic when produced at higher temperatures. They can be considered less hydrophobic due to the absence of non-polar functional groups, such as aliphatic, that may be destroyed at temperatures above 400 °C. On the other hand, biochar’s water-holding capacity depends on the porosity and interconnectedness of the pores. Therefore, biochar produced at a higher temperature, though hydrophobic, are expected to hold more moisture in its pore structure. On the other side, hydrochar is slightly less hydrophobic when compared to biochar and the surface hydrophobicity of hydrochar decreases with increasing temperature. The destruction of the surface functional groups with rising temperature could be recognized as a plausible reason for the decreasing hydrophobicity (Cheng et al. 2022; Zhang et al. 2021). However, when compared to biochar, hydrochar retains significant oxygenated functional groups after the hydrothermal process, and the oxygenated functional groups being hydrophilic, hydrochar is relatively less hydrophobic than biochar.
3.3.2 Chemical properties
In addition to the breakdown of the fibrous structure in biomass leading to changes in physical properties, carbonization of biomass could also lead to changes in chemical properties in char. It is worth noting that the physical properties may influence the chemical properties of char. For instance, polycyclic aromatic hydrocarbons (PAHs) can be correlated with the biochar's micropore volume and large surface area. Similarly, minerals' dispersion could influence the biochar morphology (Yip et al. 2010). Biochar and hydrochar are carbon-rich materials, and their main organic contents can typically be assessed using C, H, N, S, and O compositions. Alongside organic elements, biochar contains inorganic elements such as Ca, Mg, P, Si, Al, Fe, and K as well as trace metals (Cu, Ni, Zn, Co, Mn). While the high carbon content defines the quality of char material, the N/C, H/C and O/C are considered a measure of the char's recalcitrancy, aromaticity and biochemical stability, respectively (Rangabhashiyam et al. 2022). Several studies reported the elemental compositions of biochar and hydrochar. Mineral content in the char could influence its pH, surface chemistry and porosity. Biochar produced from high ash-containing feedstocks and at high temperatures generally has a pH above 7 (alkaline). It is believed that the alkaline earth (Ca, Mg) and alkali (Na, K) minerals are mainly responsible for the high pH of such biochar (Bamdad et al. 2018). However, in a recent study, Xu et al. noticed that sludge-derived biochar had a higher mineral content than wheat-straw-derived biochar, but the pH was lower for sludge biochar. The authors attributed the low pH to the abundant availability of Fe in sludge biochar, which may have caused hydrolysis to produce H+ in the solution (Xu et al. 2016). Therefore, it could be understood that the mineral composition rather than the total ash content influences the pH of biochar. In addition to the mineral content, surface functional groups like carboxyl and hydroxyl groups contribute to low biochar pH, particularly when produced at low temperatures. At higher temperatures, acidic functional groups, such as formyl, carboxyl, or hydroxyl groups, get destroyed, promoting the separation of minerals such as NaOH, CaCO3, KOH and MgCO3 from the solid carbon matrix, leading to higher pH values (Xu et al. 2017). Generally, the ash content in the feedstock is directly connected to the percentage of metal species in the char. Demineralization of inorganic composition is common in HTC, as the process is carried out in the presence of a solvent that solubilises the mineral matter into the aqueous phase product. Hydrochar produced via HTC is generally acidic due to the availability of more acidic functional groups than that present in biochar (Fei et al. 2019). In a recent study by Liu et al., the authors compared the oxygenated functional groups of pinewood-derived hydro/biochar. They noticed that the oxygenated functional groups in hydrochar were 340% higher than in biochar (Liu et al. 2020a). In addition, the pH of hydrochar depends on the feedstock. For instance, the pH of hydrochar obtained at 180 °C via HTC of pure cellulose was higher than hydrochar from woody biomass (Saha et al. 2019). Organic content in HTC's effluents may get deposited on the surface of the hydrochar, which could influence its pH when the solids are not properly washed.
The surface functional groups play an essential role in removing gaseous pollutants as they can facilitate the chemical adsorption (in most cases) of gaseous pollutants and stabilize them in biochar. Oxygen and nitrogen-containing functional groups are considered major active sites for the adsorption process (Sajjadi et al. 2019). Oxygenated functional groups include carbonyl, carboxyl, chromene, hydroxyl, ketone, lactone, phenol, and pyrone functional groups (Saha and Kienbaum 2019). The surface oxides may be derived from the feedstock or formed during the conversion process or following an activation process that involves oxidation. While chromenes, ketones and pyrones contribute to the basicity of the char, carbonyl, carboxyl and hydroxyl contribute to the acidity. However, the carboxyl and hydroxyl groups react with metallic cations to form salts, contributing to the alkalinity (Sajjadi et al. 2019). Nitrogen-containing functional groups are usually added to the biochar by treating biochar with agents such as amine, ammonia, nitric acid, and urea. Most common N-containing functional groups include amine-N, pyridinic-N, pyridinic-N-oxide, pyrrolic-N, etc. (Feng et al. 2021). Both biochar and hydrochar are composed of aromatic and aliphatic structures with different surface functional groups.
4 H2S adsorption: mechanisms and processes
4.1 Adsorption mechanism: biochar vs. hydrochar
As highlighted in the previous section, biochar/hydrochar have highly heterogeneous compositions and physicochemical properties largely driven by process conditions and feed type. Therefore, the H2S removal mechanism on char materials is a complex process that can involve the formation of various sulfur products. It is worth noting that adsorptive desulfurization on carbon material undergoes physical and weak chemical adsorption and, therefore, is more suitable at low temperatures. Generally, H2S removal using carbon materials involves two steps; (i) physisorption step: Gaseous H2S is adsorbed on the surface of the carbon material, dissolved in the H2O film, and gets dissociated in the adsorbed state, as shown in Eq. 5. (ii) Oxidation step: In the presence of water and metal impurities, the adsorbed H2S reacts with O2 to form elemental sulfur (S0) and sulfur dioxide and sulfates. Adib et al. (Adib et al. 2000) proposed a mechanism for H2S oxidation (Eqs. 3–6) at low temperatures, which was later extended to alkaline carbons (Eqs. 6–9) by Yan et al. (2002). The basic reactions are as given below.
where \(H_{2} S_{gas}\), \(H_{2} S_{ads}\) and \(H_{2} S_{ads - liq}\) are H2S in gas adsorbed and liquid phases, respectively; KH, Ka, Ks and KR are equilibrium constants for adsorption, gas dissociation, solubility, and surface reaction process, respectively. \(O_{ads}^{*}\) represents dissociative adsorbed O2.
Several researchers expanded the reaction mechanism by studying different biochar under different conditions (Fig. 2). Leuch et al. extended the adsorption mechanism proposed by Adib et al. under dry conditions, which can be found elsewhere (Yan et al. 2002). Similarly, Bagreev and Bandosz suggested a mechanism for the oxidation of H2S on carbon material and according to the authors, H2S is not adsorbed on the carbon surface, instead the carbon surface is expected to play an important role in the oxidation of H2S. According to Chowdary and Lansing, biochar rich in mineral contents can promote H2S adsorption, influence the final sulfur products, and change the products from sulfuric acid to soluble and insoluble metal oxides (Choudhury and Lansing 2021). In addition, these minerals act as catalysts in the catalytic oxidation of H2S to elemental sulfur and sulfur oxides (Bagreev and Bandosz 2005). Hervy et al. (2018a) found that the adsorption of H2S is closely related to the properties of biochar, which includes surface area, basic O-containing functional groups, pore volume, mineral species, and disorganised carbon structure (Hervy et al. 2018a). Under dry and oxygen-deprived conditions, H2S may directly react with the metals available in the ash (Eq. 10) to form metal sulfides. Further oxidation of metal sulfur with organic and inorganic species results in the formation of metal sulphate.
The availability of surface functional groups, particularly O-containing functional groups, could promote H2S adsorption by substitution (Eq. 11) and oxidation reactions (Eq. 12).
where C(O) and (Cfree) are the active and free carbon sites. Further, the defects in carbon structure can provide free carbon sites that could react with H2S according to Eqs. (13) and (14).
The mechanism of adsorption of H2S with hydrochar is presented in Fig. 3. It is worth noting that literature demonstrating the H2S adsorption mechanism in the case of hydrochar is sparsely reported. According to Hu et al. (2024) elemental sulfur is the primary product of the desulfurization process, followed by sulfuric acid and sulfonic acid as by-products, as shown in Fig. 3.
Illustration of H2S removal mechanism of activated hydrochar. Adapted with permissions from (Hu et al. 2024)
Under wet conditions, the authors noted that a water film is formed on the surface of the hydrochar, and the following reactions were reported:
In summary, chemical adsorption occurs on the surface of the char, while physical adsorption mainly occurs in the pore structure of the char matrix. A summary of recent studies that proposed a mechanism for H2S adsorption using biochar and hydrochar is given in Table 4.
Unlike biochar, hydrochar does not have excellent structural properties, such as high surface area and porosity. The dissociation of H2S in the water film is the rate-limiting step in adsorption. It should be noted that an acidic environment hinders the dissociation of H2S, which leads to the availability of H2S ions at low concentrations. In an alkaline environment, the degree of dissociation is high, which favours the formation of elemental sulfur species resistant to further oxidation. While biochar is alkaline in nature, hydrochar is acidic. Therefore, it can be expected that using biochar for adsorption may favour the formation of elemental sulfur, while using hydrochar may favor the formation of sulfur oxides and sulfuric acid (Georgiadis et al. 2020). As biochar has excellent structural properties coupled with alkaline nature and high metal content, both physisorption and chemisorption can be seen as pathways for H2S removal. However, in the case of hydrochar, H2S removal occurs mainly via chemisorption, which can be attributed to well-preserved surface functional groups and high metal content. However, it is worth noting that, H2S adsorption also relies on other influential parameters, such as feedstock properties, type of metals/metal oxides and removal conditions, which are discussed in detail in the following sections.
4.2 Factors influencing H 2 S adsorption
The potential of char in H2S removal is closely related to feedstock type, production conditions, functionalization strategies and removal conditions. Different factors such as feedstock type and preparation factors, activation and functionalization strategies and operational conditions, including reaction temperature, co-existing gases, relative humidity and corresponding adsorption efficiency reported in the literature are presented in Table 5.
4.2.1 Feedstock type and preparation factors
The type of feedstock considered for char production, the conversion process, and production conditions can influence the char properties and, therefore, the performance and removal mechanism of the H2S. Lignocellulosic, aquatic, and sludge biomass are the most commonly used feedstock reported in the literature. Generally, biochar produced from lignocellulosic biomass has a well-developed pore structure compared to biochar produced from sludge (Dissanayake et al. 2020; Zielińska et al. 2015). A study done by Dieguez-Alonso et al. (2018) compared the characteristics of biochar and hydrochar generated from pine wastes. The authors noticed that hydrochar had lower SSA than biochar. However, the pore volume of hydrochar was higher than the biochar produced from both feedstocks. While micropores contribute to the majority of pores in biochar, mesopores and macropores majorly contribute to the pore volume in the case of hydrochar. In the same study, the authors compared the characteristics of hydrochar produced from different feedstocks. They noticed that hydrochar produced at 240 °C with a retention time of 360 min from pine wastes had a significantly higher SSA and pore volume than corn digestate-derived hydrochar (Dieguez-Alonso et al. 2018). In another study done by Juntarchat and Onthong on comparing biochar produced from banana peel and banana empty fruit bunches, the biochar from the latter possessed a higher fixed carbon (ca. 38%), pH (ca. 9) and SSA (3.18 m2 g−1) than the former. In addition, the authors investigated the impact of biochar type and pellet size on the adsorption of H2S using synthetic biochar. They concluded that the smaller the size of the pellet, the higher the removal efficiency. The authors attributed the availability of carboxylic and hydroxide groups to the removal of H2S (Juntarachat and Onthong 2022).
4.2.2 Activation and functionalization strategies
Activation is generally performed to improve the physicochemical properties of char and, thus, the H2S removal efficiency. These methods involve physical activation and chemical activation. The thermal treatment of biomass results in the release of non-carbon elements, mainly hydrogen and oxygen, forming a solid carbon structure with a rudimentary pore structure, incipient pore structure and sparsely distributed functional groups. These attributes of raw bio/hydrochar could limit its potential as an adsorbent (Wen et al. 2023). However, as shown in Fig. 4, activation techniques can be used to enhance char's physical and chemical properties (such as pH, SSA, charge, porosity, and functional groups).
Physical activation techniques include subjecting solid adsorbent material to high temperatures under a controlled flow of reactive activating agents such as CO2, steam, oxygen, air and/or their mixture. Physical activation improves the porosity of the material and introduces a few surface functional groups onto the char. For instance, oxygenated functional groups will be introduced using H2O, CO2, and O2 activation, while N-containing functional groups may be introduced with NH3 activation (Zhao et al. 2022b). In a recent study by Chang et al., the authors reported an increase in the activation temperature and residence time from 800 to 900 °C and 20 to 80 min, respectively, increased the SSA of corn cobs-derived biochar from 405 to 1705 m2 g−1 under CO2 activation. In addition, under similar conditions, when the activation media was changed from CO2 to steam, the SSA increased from 568 to 1063 m2 g−1 (Chang et al. 2000). However, it is worth noting that an increase in temperature may have a negative effect on the structural properties, mainly due to the transformation of micropores into meso- and macropores. On the other hand, as discussed previously, hydrochar generally has a poor porous structure and low SSA when compared to biochar and hence needs activation to enhance structural properties. The physical activation of hydrochar derived from various wastes, including horse manure, grass, sludge and beer wastes, was investigated by Hao et al. The authors utilized CO2 as an activating agent and noticed a rich cluster of ultra micropores post-activation (Hao et al. 2013).
Similar to the physical activation of char, chemical activation is also recognized to enhance the structural properties of char material. Chemical activation with alkalis (KOH, NaOH), acids (HNO3, H3PO4, H2SO4), and salts (ZnCl2, MgCl2, K2CO3) is widely reported (Wang and Wang 2019; Zhao et al. 2022b) and has been used to enhance the formation of micropores in biochar. In addition to enhancing pore structure and SSA, acid or alkali activation increases the acidic and basic groups on the surface of the biochar (Rajapaksha et al. 2016). Dissanayake et al. compared the effectiveness of sole chemical activation and chemical followed by physical activation for biochar produced from pure wood chips, wood chips, and chicken manure. The authors noticed that activation with KOH and KOH + CO2 resulted in a ten-fold increase in the SSA of biochar, from 125.7 to 1281.6 and 1403.9 m2 g−1, respectively. In general, KOH activation results in biochar with a large SSA and high porosity but accompanied with high chemical consumption. While using H3PO4 as an activation agent results in biochar with microporous characteristics (Zhang et al. 2019a) and typical acidic functional groups, such as carboxylic and phenolic (Guo and Lua 2003), HNO3 activation may enhance the carbon and oxygen functional groups via oxidation (Opatokun et al. 2017). Chatir et al. investigated the influence of H3PO4 on the structural properties of argan nut shell-derived hydrochar. The authors noticed that at an impregnation ratio of 3, hydrochar had a remarkable increase in the SSA from 6.45 to 1880 m2 g−1. Also, the micropore, mesopore and total pore volumes of hydrochar increased from 0.002, 0.017 and 0.019 cm3 g−1 to 0.67, 0.69 and 1.36 cm3 g−1, respectively, probably due to the increase in the contact area between H3PO4 and hydrochar (El Hadrami et al. 2022).
Plasma, ultrasonic and microwave radiation treatments were also reported to substantially enhance the pore texture and active sites in bio/hydrochar. Plasma treatment can induce new functional groups under different gas environments, thanks to the oxidative and reductive reactions that occur during the activation process (Xu et al. 2018a). Gupta et al. investigated the impact of low-temperature plasma treatment on the pore structure of pine biochar. The authors reported that plasma treatment improved the porous structure of the biochar and noticed that the SSA, pore volume and pore size increased from 440, 0.455 and 4.8 to 654 m2 g−1, 0.936 cm3 g−1 and 4.9 nm, respectively. The authors compared plasma treatment against chemical activation and mentioned that the plasma-activated biochar contained significantly higher mesopores than its counterpart (Gupta et al. 2015). Shao et al. compared the properties of raw hydrochar and microwave-activated hydrochar produced via HTC of a feedstock comprised of green leaves and wood. The authors noticed that the structural properties, including SSA, pore volume and diameter, increased post-microwave activation. The SSA and pore volume increased by ⁓10 folds from 1.62 m2 g−1 SSA and 0.003 cm3 g−1 pore volume (Shao et al. 2020). However, according to Yan et al., the reaction temperature could play an important role in tuning the properties of hydrochar. The authors noticed that the SSA of the bamboo-derived hydrochar at 220 °C was 5.14 m2 g−1, which increased to 7.81 m2 g−1 at 260 °C. However, with a further increase in operational temperature to 300 °C, the SSA reduced to 3.61 m2 g−1 (Yan et al. 2017).
Alongside modification techniques, a few functionalization strategies are also widely used to activate biochar/hydrochar and make them suitable for engineering applications. Transition metals and noble metals (Co, Ce, Cu, Fe, Mn, Ni and Ti) doping onto char was reported to greatly enhance the adsorption of H2S through catalytic reactions. Biochar serves mainly as a catalyst support or carrier and, in some cases, participates in the removal process as an active catalyst material (Chen et al. 2017b). In a recent study by Wang et al., the authors synthesized Cu-loaded hydrochar to understand the impact of Cu valence on H2S removal capacity. The results of the study indicated that Cu0-activated hydrochar had the highest H2S breakthrough capacity (ca. 348 mg g−1). Catalytic oxidation and reactive adsorption were explained as the major reasons for the high H2S removal performance. In addition, the authors mentioned that the availability of moisture substantially enhanced the catalytic oxidation of H2S to elemental S (Wang et al. 2022). In addition, as mentioned earlier, surface alkaline properties are generally suitable for the removal of acidic gases, such as H2S. Functional modifications to incorporate basic sites onto char to strongly interact with acidic gases could benefit the adsorption of H2S. In this context, ammonia modification to introduce basic nitrogen functionalities appears to be a promising approach. In a recent study by Surra et al., an attempt was made to activate biochar using anaerobic liquid digestate (ALD) and compared the H2S removal efficiency of the ALD activation process pre- and post-pyrolysis of maize cob waste-derived char. The authors considered two cases: (i) ALD as the activation agent for raw maize cob waste, followed by pyrolysis and (ii) pyrolysis of maize cob waste followed by activation of char with ALD. The biochar produced in the former case displayed a higher H2S removal capacity (0.47 mg g−1) than the latter (0.25 mg g−1) (Surra et al. 2019).
4.2.3 Operating conditions during adsorption
In addition to feedstock type, preparation conditions, activation strategies, and functionalization of char, the removal conditions, including reaction temperature, co-existence of other gases such as oxygen, carbon dioxide, and relative humidity, also play a major role in the H2S removal process. It is worth noting that a low reaction temperature favours physical adsorption (Han et al. 2020), whereas a high reaction temperature favours chemical adsorption (Liu et al. 2020a). However, extremely high temperatures may destroy active sites and pore structures in the biochar (Zhao et al. 2019). In addition, the increasing temperature intensifies the thermal movement of gas molecules, lowering their adhesion on the surface of biochar, which in turn lowers the chemical adsorption efficiency and adsorption capacity (Ding and Liu 2020; Xu et al. 2018b). Han et al. investigated the influence of reaction temperature on the H2S removal efficiency of two macroalgae-derived biochar (MC1 and MC2). The authors tested the effect of different reaction temperatures (25, 50, 75 and 200 °C) on H2S removal efficiency and noticed that the breakthrough time was reduced from 10 to 6 min for MC1 and 2 to 1 min for MC2. The authors mentioned that H2S adsorption was favoured at lower temperatures, indicating physical adsorption as a major adsorption mechanism (Han et al. 2020).
For acidic gases, low relative humidity is considered beneficial. For instance, H2S can react with moisture to form sulfuric acid. However, a high relative humidity may facilitate the formation of a water film on the biochar surface and, impede the diffusion of gas molecules, and inhibit H2S removal (Sun et al. 2018). Sitthikhankaew et al. studied the effect of humidity on H2S removal efficiency of activated char. The authors tested activated carbon (AC) at 70% relative humidity and reported that the material displayed excellent H2S removal capacity (0.284 L g−1). In the same study, the authors tested the influence of the existence of co-gases, such as CO2 and O2 with H2S on the removal efficiency of AC. At 70% relative humidity, while the addition of 2% O2 increased the H2S adsorption capacity from 0.01 to 0.059 L g−1, the addition of 40% CO2 increased the adsorption capacity from 0.01 to 0.091 L g−1 (Sitthikhankaew et al. 2014).
The other crucial operational condition that could influence the removal capacity of biochar/hydrochar is the co-existence of H2S with other gases such as O2, CO2, SO2 etc. In a study by Sethupati et al., the authors reported that the H2S removal capacity of biochar in the presence of CO2 was low when compared to H2S alone. They also mentioned that the similar removal mechanism of H2S and CO2 may have resulted in competitive adsorption (Sethupathi et al. 2017). Qin et al. used sludge-derived biochar to remove H2S from MSW landfill-generated biogas, which contained volatile organic compounds (VOCs), and NH3. The authors observed a removal efficiency of 95–100%. Huang et al. (2022) studied the influence of the co-existence of O2, H2O, and CO2 with H2S on H2S removal capacity of food digestate-derived biochar. The authors reported a removal capacity of 1.75 mg g−1 for dry biochar in a pure H2S environment. However, the removal capacity increased to 4.29, 5.29 and 12.50 mg g−1 for dry biochar in H2S + O2, humid biochar in pure H2S and humid biochar in H2S + O2 environments, respectively. The authors reported that the removal of H2S under dry conditions was due to the high mineral content in the biochar. However, with the co-existence of CO2 in the gas stream, the H2S adsorption decreased, possibly due to the physisorption competition with CO2.
5 Potential integration options and circular economy solutions for wastewater treatment plants
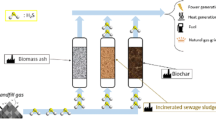
A possible trade-off between biogas and char sectors conceptualized from the literature survey conducted in this study is presented in Fig. 5. The system can be divided into three sectors: wastewater treatment plants (WWTPs), thermal processes (pyrolysis and HTC), and biogas (including anaerobic digestion and other subsidiaries). As discussed in previous sections, adsorption using biochar/hydrochar could be considered as one of the potential routes to remove H2S from biogas. From an economic point of view, the production of biochar/hydrochar from waste feedstock can be cost-effective compared to commercially available adsorbents, such as activated carbon or MOFs. Integration of wastewater treatment/anaerobic digestion with thermal processes, including pyrolysis, gasification and HTC, can offer an efficient approach to cost-effective management of waste, such as sewage sludge, biosolids, and alum sludge (Cho et al. 2023). As shown in Fig. 5, char can be produced from biosolids in three ways. Case (i) demonstrates the production of hydrochar, where sewage sludge or undigested biosolids from WWTP can be directly used as feedstock or direct use of the digestate from the AD process to produce hydrochar. Since HTC requires wet organic feedstocks, case (i) can handle unstabilised and non-dewatered solid streams to convert into hydrochar at 180–250 °C. Case (ii) involves the production of biochar via gasification of digested/stabilised AD residues at 600–900 °C under partly oxidising condition, and case (iii) involves the production of biochar from the pyrolysis of digested/stabilised AD residues at 400–700 °C under oxygen-limited environment. It is worth noting that the production of biochar in case (ii) and case (iii) require an energy intensive drying step before the digestate is feed into process. This is because the solids content and calorific value of the feestock is critical in pyrolysis and gasification, particularly from a thermal energy balance perspective.
Once the char is produced, it could be further activated chemically or physically and/or impregnated with metals to produce activated biochar/hydrochar with enhanced structural properties. Both raw and modified char can be added to an anaerobic digestion unit as additives to boost biogas production (Ahmad et al. 2021a; Masebinu et al. 2019; Zhang et al. 2019b) or can be used as an adsorbent for the continuous removal of pollutants such as CO2, H2S and siloxanes from the biogas stream, termed the biogas upgradation process. The pollutant-free clean biogas is then stored/bottled and used for different mobile and stationary applications. Integrating char production with wastewater treatment process and the onsite application of the produced char as additives in AD systems, adsorbent for wastewater purification, and biogas desulfurisation can offer many environmental and economic benefits, such as:
-
i.
thermal treatment of sewage sludge and final biosolids to produce biochar/hydrochar provides huge potential for contaminant destruction, volume reduction, and odour elimination (Hakeem et al. 2024; Patel et al. 2020).
-
ii.
use of biosolids for char production can allow for maximum recovery of nutrients (N, P, K) and organic carbon, reduce GHG emissions associated with biosolids stockpiling and composting, and reduce risks of diffuse contaminants entry into the environment (air, soil and water) through the traditional land application of biosolids (Nahar et al. 2024; Elgarahy et al. 2024).
-
iii.
use of char materials as additives in AD process can improve the system stability through pH buffering, microbial sheltering, ammonia inhibition, micronutrient supplements, etc., thereby increasing the overall biomethane yield and improving sludge dewaterability (Hassan et al. 2023; Hoang et al. 2022).
-
iv.
adding biochar to AD systems can facilitate in-situ adsorption of H2S and other trace gas pollutants, control odour, and produce contaminants-free and biomethane-rich biogas stream (Choudhury et al. 2020; Liu et al. 2021c).
-
v.
char produced (raw or modified) from wastewater treatment residues can be used as partial replacement for costly activated carbon in wastewater and biogas purification for micropollutant (H2S, siloxanes, CO2, PFAS, heavy metals, dyes, pharmaceuticals, etc.) removal (Patel et al. 2023; Enaime et al. 2020).
6 Comparison of techno-economic feasibility and technology readiness level for selective H2S removal
The technologies for the removal of H2S are industrially advanced, with several commercial installations traditionally used for gas sweetening. Broadly, the method for H2S capture can be classified into physicochemical techniques and biological techniques, which can involve dry or wet desulfurisation methods. Each of these technologies uses a range of materials, has different removal efficiency, and presents different advantages and disadvantages (Pudi et al. 2022), as shown in Table 6. The technological performance of the various H2S removal technologies from biogas is summarised in Table 6. From Table 6, each technique has its strengths and limitations concerning H2S removal efficiency, operating conditions, process selectivity, materials/solvents requirements and commercial application.
For example, adsorption using metal oxide adsorbents is the most established technology on a commercial scale for the fine removal of H2S, while chemical absorption using amine solvent is most industrially matured for bulk removal of CO2 and H2S simultaneously. However, the performance and techno-commercial feasibility of H2S adsorption and absorption can change significantly when using different solvents and materials, biogas compositions, and operating conditions. All technologies have their own specific merits and demerits. No technology offers optimal solutions to every biogas upgrading situation and requirement. The choice of an economically optimal technology will strongly depend on the volume and quality of the raw biogas to be treated, the desired biogas purity and the final utilisation of the gas. The configuration and operation of the anaerobic digestion process can aid the easy integration of a particular H2S removal technology. For instance, biological and membrane techniques might be easy to adopt as in-situ H2S removal technology within the existing wastewater treatment plants as these types of technology are common with wastewater and sludge treatment processes. New WWTP installations might consider adsorption and absorption technology for onsite biogas upgrading. However, the type and management of generated spent substrates/residues and local environmental regulations can guide the appropriate choice of an H2S removal technology. An attempt has been made to provide a relative comparison of the H2S removal technology across various indicators as shown in Table 7.
There are no comprehensive comparative studies on the various technologies for H2S removal under the same/similar conditions. As such, there are discrepancies about the specific capital, operation and maintenance costs, biomethane loss, solvents, and energy requirements for the different technologies at varying treatment scales. In a previous review conducted by Sun et al. (2015), where the energy efficiency and techno-economic analysis of five different biogas upgrading technologies were reported, there was no significant difference in the capital cost of technology investment. The capital expenditures are closely related to the plant capacity, and the larger the plant, the lower the CAPEX for treating a unit volume of biogas. However, energy requirements were observed to vary across the five technologies. For example, the energy consumption in kWh per Nm3 of treated biogas was reported as: 0.45–0.90 for water scrubbing, 0.8–1.54 for cryogenic separation, 0.49–0.67 for physical absorption, 0.12–0.44 for chemical adsorption, 0.3–1.0 for pressure swing adsorption, and 0.25–0.43 for membrane technology. In all these technologies, the energy efficiency ranges from 82 to 98%, the lowest for pressure swing adsorption and the highest for water scrubbing (plus regeneration). However, the operating and maintenance expenditures associated with chemical absorption and cryogenic separation were comparatively higher due to solvent cost and loss on regeneration in the case of chemical adsorption, and low energy efficiency in the case of cryogenic technology. The data presented in Table 7 is for guidance only based on qualitative assessment, detailed comparative studies are required to validate the data. The technology readiness level is based on the best optimum technology using commercially tested materials/solvents, and it can differ if adsorbent/solvents are changed. For instance, while the TRL of adsorption techniques using activated carbon and metal oxides is high, as they have been proven industrially, the TRL of the same adsorption technique using biochar materials will range from low to medium. A similar trend will be observed for the absorption technique using liquid amine against alkali and ionic liquid solvents.
7 Challenges and future research perspectives
7.1 Feedstock availability and quality
Biochar and hydrochar have excellent potential as adsorbents, however, several research gaps still need to be addressed. The first and most significant challenge is the availability of suitable feedstock for char production, which can be later used as an adsorbent for H2S removal. Based on the extensive literature analysis conducted in this study, it is worth mentioning that the feedstock type and quality could significantly influence the properties and effectiveness of char. Ensuring a reliable and stable supply of feedstock is challenging, particularly in areas with limited biomass availability. This presents an opportunity for water utilities and waste treatment facilities to use wastes, such as municipal solid wastes or biosolids, as feedstock for biochar production. In addition, biochar production is an energy-intensive process, which may require significant investment in infrastructure and equipment. This further hinders biochar production in regions with limited resources. Biochar is generally produced from residual biomass such as woodchips, crop residues, biosolids, and municipal solid wastes. Conventional kiln-derived biochar produced from impure feedstock may contain harmful contaminants, such as heavy metals, polycyclic aromatic hydrocarbons, per- and polyfluorinated compounds, and volatile organic compounds. Improper disposal of conventionally produced char post adsorption would negatively impact the ecosystem. Therefore, careful selection of feedstock or use of sophisticated reactors is recommended to avoid unanticipated entry of pollutants into the natural environment.
7.2 Understanding of mechanisms and co-existence of other pollutants
Although H2S adsorption with biochar has been extensively investigated and reported in the literature, there is still a research gap for hydrochar adsorption mechanism. The H2S adsorption mechanism for hydrochar is sparsely available in the literature and research efforts are warranted in this area. For both hydrochar and biochar, the co-existence of other gases or pollutants could increase the complexity of the adsorption process. It is worth noting that the co-existence of multiple organic and inorganic pollutants may compete for the active spots on the biochar and therefore, would enhance/limit the adsorption process. Under instances where H2S is selectively adsorbed, tailoring biochar properties for selective H2S adsorption can be challenging. In addition, most of the literature on using char/activated carbon for H2S adsorption is mainly conducted using feed gas that contains H2S mixed with N2 or air. However, these conditions do not represent the real case scenario. For instance, it is relatively easier to separate H2S from air than from biogas as the former contains O2, which assists in the oxidation reaction (Ahmad et al. 2021b). Even though, biochar is reported to have high adsorption efficiencies, it cannot be concluded in general that similar high efficiencies can be noticed with real biogas stream. Efforts need to be made in this regard to understand the true potential of biochar in real-time applications for biogas desulfurization. In addition, molecular simulation could play an important role in understanding the fundamentals of adsorption on carbon materials. However, much research is needed in developing novel kinetic models to investigate adsorption mechanisms and to model adsorption systems.
7.3 Commercialisation of technology
The major concern during the development of biochar-based adsorption technology is the commercial viability of the technology. Predominantly, the commercial sustainability of using biomass/waste-derived adsorbent for H2S adsorption is still under research. Lifecycle analysis (LCA), together with environmental impact assessment (EIA) and techno-economic analysis (TEA), could provide a detailed understanding of the commercial feasibility of biochar-based H2S adsorption technology. It is worth noting that a decision made after taking into consideration the understandings from LCA, TEA, and EIA would be a strong indicator for investors to transition from commercial adsorbents to biochar/hydrochar. Using biochar for H2S adsorption could support sustainable development by recycling and reusing waste resources. The spent biochar sorbent obtained from the H2S removal process can be used as a soil fertilizer as it is rich in elemental sulfur (Zhang et al. 2017). Cost analysis for using biomass/waste-derived biochar/hydrochar as a replacement for commercially available adsorbents is not reported in the literature. Most of the studies reported in the literature are done at a lab-scale and the char is used without cost justification for modification or activation. It is worth noting that cost is an important parameter for commercialization of any technology and detailed cost-based comparisons between commercial and waste-based adsorbents are currently a research gap that needs to be addressed. Furthermore, the link between biomass/waste-derived char for environmental applications, such as adsorption of H2S, circular economy and sustainable development goals (SDGs) lies in their collective response to promote sustainable practices and contribute to SDGs. Using char for H2S adsorption aligns with several SDGs listed below:
-
SDG 7- Affordable and Clean Energy: Using biomass or waste as a renewable resource to produce biochar could contribute to the production of clean energy.
-
SDG 12-Responsible Consumption and Production: Converting biomass waste to valuable products such as biochar promotes responsible production and encourages sustainable consumption and production.
-
SDG 13-Climate Action: Biochar’s carbon sequestration potential assists in mitigating climate change and attaining carbon reduction targets.
-
SDG 15-Life on Land: Application of biochar to soil could promote biodiversity, combat land degradation, and safeguard ecosystems.
7.4 Regeneration of biochar
Besides the adsorption capacity, the feasibility of using biochar at a large scale also depends on its regenerability (Bamdad et al. 2019). Spent biochar can be recycled several times via various desorption processes before being finally disposed. Several studies have reported the regeneration of biochar; however, most of them are focused on thermal desorption methods. A few studies reported the regeneration of spent sorbents after use in gas phase adsorption. However, only a handful of studies were identified in our literature analysis that reported the regeneration of spent char after the adsorption of H2S (Chen et al. 2021a; Yuan et al. 2021). However, contradicting results were reported in the literature regarding the adsorption capacity of biochar after repetitive regeneration of spent sorbent (Fig. 6). Yuan et al. evaluated the H2S adsorption potential of biochar produced from leftover rice. In their study, the authors used the calcination process to regenerate spent sorbent and subsequently evaluated its regeneration potential. They noticed that the regenerated biochar attained 91.6% of initial breakthrough capacity after five regeneration cycles (Yuan et al. 2021). On the contrary, in a study done by Chen et al. using activated biochar derived from microalgae biomass for H2S adsorption, the authors reported that the performance of biochar declined rapidly after two to three cycles of regeneration (Fig. 6). The authors simply purged air for 12 h at room temperature to regenerate the spent sorbent. The difference in the breakthrough capacity, as seen in Fig. 6, indicates the importance of choosing proper regeneration techniques. 6
In the studies done on using biochar for the adsorption of other acidic gases, such as CO2 and SO2, the authors noticed that thermal regeneration processes conducted between 400 and 500 °C resulted in good regeneration potential (Bamdad et al. 2018, 2019; Shen and Zhang 2019; Xu et al. 2019).
8 Conclusions
H2S from biogas can be removed using commercially available technologies; however, these technologies can be expensive and energy-intensive. Therefore, it is extremely essential to identify low-cost and efficient adsorbents that can be used to upgrade biogas for commercial applications. This review promotes the application of biochar and hydrochar obtained from the pyrolysis and hydrothermal conversion of biomass in upgrading biogas. This study provided a critical analysis of biochar and hydrochar for H2S adsorption from biogas. It was found that the well-developed porous structure, high surface area, and abundant chemical functional groups on char materials make them excellent alternatives to commercially available activated carbons. In addition, biochar has excellent structural properties coupled with an alkaline nature and high mineral matter, which facilitates both physisorption and chemisorption phenomena for H2S removal. However, in the case of hydrochar, H2S removal occurs mainly via chemisorption, which can be attributed to well-preserved surface chemical functional groups and high metal content. Alongside char heterogenous properties, other factors such as production methods, adsorption conditions, and biogas compositions critically affect the H2S adsorption efficiency of biochar/hydrochar. It was also observed that biochar/hydrochar production from wastewater treatment residues (sewage sludge/biosolids) and the subsequent use of the char materials as additives in anaerobic digestion systems to improve biogas production and for further biogas desulfurisation can offer real circular economy opportunities for the water sector. Some of the benefit of integrating char production and onsite utilisation within WWTPs includes increased biomethane yield, in-situ adsorption of H2S in anaerobic digestion unit, and use of clean biogas for combined heat and power generation, albeit, studies are warranted to investigate these potential outcomes.
Despite the promising physicochemical and structural properties of char materials, their industrial application for biogas cleaning are hindered by low adsorption capacity and limited selectivity for H2S as well as regeneration stability. These limitations arose from the diverse feedstock compositions and thermochemical conversion methods for biochar/hydrochar production which strongly affect the yield and properties of the char materials for contaminant adsorption in gas streams. This underscores the need to develop efficient functionalisation strategy to tailor char properties for the desired application as H2S adsorbents. Furthermore, there are still far limited studies reporting the use of biochar/hydrochar for H2S adsorption from simulated or real biogas stream, the observed performance of biochar/hydrochar in the gas phase adsorption of pure H2S might be impacted in multicomponents gas stream typical of biogas. Bench-scale and sub-pilot studies are needed to demonstrate the application beyond lab-scale experiment which are mostly reported. Results from large-scale experiment can provide a more realistic assessment of the overall techno-commercial feasibility of biochar/hydrochar for H2S adsorption.
Abbreviations
- AC:
-
Activated carbon
- ALD:
-
Anaerobic liquid digestate
- DEA:
-
Diethanolamine
- EIA:
-
Environmental impact assessment
- HTC:
-
Hydrothermal carbonization
- HTL:
-
Hydrothermal liquefaction
- LCA:
-
Lifecycle analysis
- MEA:
-
Monoethanolamine
- MOFs:
-
Metal–organic frameworks
- MSW:
-
Municipal solid wastes
- PAHs:
-
Polycyclic aromatic hydrocarbons
- SDG:
-
Sustainability development goals
- SSA:
-
Specific surface area
- SWG:
-
Supercritical water gasification
- TEA:
-
Techno-economic analysis
- VOCs:
-
Volatile organic compounds
- WWTPs:
-
Wastewater treatment plants
References
Adeniyi AG, Iwuozor KO, Muritala KB, Emenike EC, Adeleke JA (2023) Conversion of biomass to biochar using top-lit updraft technology: a review. Biofuels, Bioprod Biorefin 17(5):1411–1424
Adib F, Bagreev A, Bandosz TJ (2000) Analysis of the relationship between H2S removal capacity and surface properties of unimpregnated activated carbons. Environ Sci Technol 34(4):686–692
Ahmad J, Patuzzi F, Rashid U, Shahabz M, Ngamcharussrivichai C, Baratieri M (2021a) Exploring untapped effect of process conditions on biochar characteristics and applications. Environ Technol Innov 21:101310
Ahmad W, Sethupathi S, Kanadasan G, Lau LC, Kanthasamy R (2021b) A review on the removal of hydrogen sulfide from biogas by adsorption using sorbents derived from waste. Rev Chem Eng 37(3):407–431
Ahmed SF, Mehejabin F, Momtahin A, Tasannum N, Faria NT, Mofijur M, Hoang AT, Vo DVN, Mahlia TMI (2022) Strategies to improve membrane performance in wastewater treatment. Chemosphere 306:135527
Allegue LB, Hinge, J, Allé, K (2012) Biogas and bio-syngas upgrading. Danish Technological Institute, pp. 5–97.
Álvarez ML, Gascó G, Palacios T, Paz-Ferreiro J, Méndez A (2020) Fe oxides-biochar composites produced by hydrothermal carbonization and pyrolysis of biomass waste. J Anal Appl Pyrol 151:104893
Ateş A, Mert Y, Timko MT (2023) Evaluation of characteristics of raw tea waste-derived adsorbents for removal of metals from aqueous medium. Biomass Conver Bioref 13(9):7811–7826
Bagreev A, Bandosz TJ (2005) On the mechanism of hydrogen sulfide removal from moist air on catalytic carbonaceous adsorbents. Ind Eng Chem Res 44(3):530–538
Bamdad H, Hawboldt K, MacQuarrie S (2018) A review on common adsorbents for acid gases removal: focus on biochar. Renew Sustain Energy Rev 81:1705–1720
Bamdad H, Hawboldt K, MacQuarrie S, Papari S (2019) Application of biochar for acid gas removal: experimental and statistical analysis using CO2. Environ Sci Pollut Res 26:10902–10915
Barbusiński K, Parzentna-Gabor, A, Kasperczyk D (2021) Removal of Odors (Mainly H2S and NH3) using biological treatment methods, clean technologies. pp. 138–155
Biller P, Ross AB (2016) 17 - Production of biofuels via hydrothermal conversion. In: Luque R, Lin CSK, Wilson K, Clark J (eds) Handbook of biofuels production (Second Edition). Woodhead Publishing, pp 509–547
Bui VG, Tu Bui TM, Hoang AT, Nižetic S, Nguyen Thi TX, Vo AV (2021) Hydrogen-enriched biogas premixed charge combustion and emissions in direct injection and indirect injection diesel dual fueled engines: a comparative study. J Energy Res Technol, Trans ASME 143(12):120907
Cattaneo CR, Muñoz R, Korshin GV, Naddeo V, Belgiorno V, Zarra T (2023) Biological desulfurization of biogas: a comprehensive review on sulfide microbial metabolism and treatment biotechnologies. Sci Total Environ 893:164689
Chan YH, Lock SSM, Wong MK, Yiin CL, Loy ACM, Cheah KW, Chai SYW, Li C, How BS, Chin BLF, Chan ZP, Lam SS (2022) A state-of-the-art review on capture and separation of hazardous hydrogen sulfide (H2S): recent advances, challenges and outlook. Environ Pollut 314:120219
Chang C-F, Chang C-Y, Tsai W-T (2000) Effects of burn-off and activation temperature on preparation of activated carbon from corn cob agrowaste by CO2 and steam. J Colloid Interface Sci 232(1):45–49
Chen X, Lin Q, He R, Zhao X, Li G (2017a) Hydrochar production from watermelon peel by hydrothermal carbonization. Biores Technol 241:236–243
Chen Y, Zhang X, Chen W, Yang H, Chen H (2017b) The structure evolution of biochar from biomass pyrolysis and its correlation with gas pollutant adsorption performance. Biores Technol 246:101–109
Chen G, Yu Y, Li W, Yan B, Zhao K, Dong X, Cheng Z, Lin F, Li L, Zhao H, Fang Y (2020a) Effects of reaction conditions on products and elements distribution via hydrothermal liquefaction of duckweed for wastewater treatment. Biores Technol 317:124033
Chen W, Zhang G, Li D, Ma S, Wang B, Jiang X (2020b) Preparation of nitrogen-doped porous carbon from waste polyurethane foam by hydrothermal carbonization for H2S adsorption. Ind Eng Chem Res 59(16):7447–7456
Chen H, Han X, Liu Y (2021a) Gaseous hydrogen sulfide removal using macroalgae biochars modified synergistically by H2SO4/H2O2. Chem Eng Technol 44(4):698–709
Chen W-H, Lin B-J, Lin Y-Y, Chu Y-S, Ubando AT, Show PL, Ong HC, Chang J-S, Ho S-H, Culaba AB, Pétrissans A, Pétrissans M (2021b) Progress in biomass torrefaction: principles, applications and challenges. Prog Energy Combust Sci 82:100887
Chen L, Jiang X, Chen W, Dai Z, Wu J, Ma S, Jiang W (2022) H2O2-assisted self-template synthesis of N-doped biochar with interconnected mesopore for efficient H2S removal. Sep Purif Technol 297:121410
Cheng Y, Yuan T, Deng Y, Lin C, Zhou J, Lei Z, Shimizu K, Zhang Z (2018) Use of sulfur-oxidizing bacteria enriched from sewage sludge to biologically remove H2S from biogas at an industrial-scale biogas plant. Biores Technol Reports 3:43–50
Cheng S, Hu W, Srinivasakannan C, Xia H, Zhang L, Peng J, Zhou J, Lin G, Zhang Q (2019) Catalytic pyrolysis of the Eupatorium adenophorum to prepare photocatalyst-adsorbent composite for dye removal. J Clean Prod 222:710–721
Cheng C, Guo Q, Ding L, Raheem A, He Q, Shiung Lam S, Yu G (2022) Upgradation of coconut waste shell to value-added hydrochar via hydrothermal carbonization: parametric optimization using response surface methodology. Appl Energy 327:120136
Cheng S, Wang T, Chu L, Li J, Zhang L (2024) Preparation of nitrogen-doped activated carbon used for catalytic oxidation removal of H2S. Sci Total Environ 915:170073
Cho S-H, Lee S, Kim Y, Song H, Lee J, Tsang YF, Chen W-H, Park Y-K, Lee D-J, Jung S (2023) Applications of agricultural residue biochars to removal of toxic gases emitted from chemical plants: a review. Sci Total Environ 868:161655
Choudhury A, Lansing S (2020) Biochar addition with Fe impregnation to reduce H2S production from anaerobic digestion. Biores Technol 306:123121
Choudhury A, Lansing S (2021) Adsorption of hydrogen sulfide in biogas using a novel iron-impregnated biochar scrubbing system. J Environ Chem Eng 9(1):104837
Chowdhury ZZ, Krishnan B, Sagadevan S, Rafique RF, Hamizi NAB, Abdul Wahab Y, Khan AA, Johan RB, Al-Douri Y, Kazi SN (2018) Effect of temperature on the physical, electro-chemical and adsorption properties of carbon micro-spheres using hydrothermal carbonization process. Nanomaterials 8(8):597
Chuang KT, Donini JC, Sanger AR, Slavov SV (2000) A proton-conducting solid state H2S–O2 fuel cell: 2 production of liquid sulfur at 120–145°C. Int J Hydr Energy 25(9):887–894
Cui S, Zhao Y, Liu Y, Pan J (2022) Preparation of copper-based biochar adsorbent with outstanding H2S adsorption capacity and study on H2S removal. J Energy Inst 105:481–490
de Jesus DS, Cerri CEP, Rittl TF, Abbruzzin TF, Blanca Lucia Prado P (2023) Biochar physical and hydrological characterization to improve soil attributes for plant production. J Soil Sci Plant Nutrit 23:3051–3057
Deng J, Liu Y, Liu S, Zeng G, Tan X, Huang B, Tang X, Wang S, Hua Q, Yan Z (2017) Competitive adsorption of Pb (II), Cd (II) and Cu (II) onto chitosan-pyromellitic dianhydride modified biochar. J Colloid Interface Sci 506:355–364
Deng J, Li X, Wei X, Liu Y, Liang J, Tang N, Song B, Chen X, Cheng X (2019) Sulfamic acid modified hydrochar derived from sawdust for removal of benzotriazole and Cu (II) from aqueous solution: adsorption behavior and mechanism. Biores Technol 290:121765
Dieguez-Alonso A, Funke, A, Anca-Couce A, Rombolà AG, Ojeda G, Bachmann J, Behrendt F (2018) Towards biochar and hydrochar engineering—influence of process conditions on surface physical and chemical properties, thermal stability, nutrient availability, toxicity and wettability, energies
Ding S, Liu Y (2020) Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 260:116382
Ding Z, Zhang L, Mo H, Chen Y, Hu X (2021) Microwave-assisted catalytic hydrothermal carbonization of Laminaria japonica for hydrochars catalyzed and activated by potassium compounds. Biores Technol 341:125835
Dissanayake PD, You S, Igalavithana AD, Xia Y, Bhatnagar A, Gupta S, Kua HW, Kim S, Kwon J-H, Tsang DC (2020) Biochar-based adsorbents for carbon dioxide capture: a critical review. Renew Sustain Energy Rev 119:109582
Dutta PK, Rabaey K, Yuan Z, Keller J (2008) Spontaneous electrochemical removal of aqueous sulfide. Water Res 42(20):4965–4975
Dutta PK, Rozendal RA, Yuan Z, Rabaey K, Keller J (2009) Electrochemical regeneration of sulfur loaded electrodes. Electrochem Commun 11(7):1437–1440
El Hadrami A, Ojala S, Brahmi R (2022) Production of activated carbon with tunable porosity and surface chemistry via chemical activation of hydrochar with phosphoric acid under oxidizing atmosphere. Surf Interf 30:101849
Elgarahy AM, Eloffy MG, Priya AK, Yogeshwaran V, Yang Z, Elwakeel KZ, Lopez-Maldonado EA (2024) Biosolids management and utilizations: a review. J Clean Prod 451:141974
Enaime G, Baçaoui A, Yaacoubi A, Lübken M (2020) Biochar for wastewater treatment—conversion technologies and applications. Appl Sci 10(10):3492
Fang J, Zhan L, Ok YS, Gao B (2018) Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J Ind Eng Chem 57:15–21
Fang L, Huang T, Lu H, Wu XL, Chen Z, Yang H, Wang S, Tang Z, Li Z, Hu B, Wang X (2023) Biochar-based materials in environmental pollutant elimination, H2 production and CO2 capture applications. Biochar 5(1):42
Fdez-Sanromán A, Pazos M, Rosales E, Sanromán MA (2020) Unravelling the environmental application of biochar as low-cost biosorbent: a review. Appl Sci 10(21):7810
Fei Y-h, Zhao D, Liu Y, Zhang W, Tang Y-y, Huang X, Wu Q, Wang Y-x, Xiao T, Liu C (2019) Feasibility of sewage sludge derived hydrochars for agricultural application: Nutrients (N, P, K) and potentially toxic elements (Zn, Cu, Pb, Ni, Cd). Chemosphere 236:124841
Feng D, Guo D, Zhang Y, Sun S, Zhao Y, Shang Q, Sun H, Wu J, Tan H (2021) Functionalized construction of biochar with hierarchical pore structures and surface O-/N-containing groups for phenol adsorption. Chem Eng J 410:127707
Fonseca-Bermúdez ÓJ, Giraldo L, Sierra-Ramírez R, Moreno-Piraján JC (2023) Removal of hydrogen sulfide from biogas by adsorption and photocatalysis: a review. Environ Chem Lett 21(2):1059–1073
Funke A, Ziegler F (2010) Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels, Bioprod Biorefin 4(2):160–177
Garlapalli RK, Wirth B, Reza MT (2016) Pyrolysis of hydrochar from digestate: Effect of hydrothermal carbonization and pyrolysis temperatures on pyrochar formation. Biores Technol 220:168–174
Georgiadis AG, Charisiou ND, Goula MA (2020) Removal of hydrogen sulfide from various industrial gases: a review of the most promising adsorbing materials. Catalysts 10(5):521
Ghimire A, Gyawali R, Lens PNL, Lohani SP (2021) Chapter 11 - Technologies for removal of hydrogen sulfide (H2S) from biogas. In: Aryal N, Mørck-Ottosen LD, Wegener-Kofoed MV, Pant D (eds) Emerging technologies and biological systems for biogas upgrading. Academic Press, New York, pp 295–320
Gollakota ARK, Kishore N, Gu S (2018) A review on hydrothermal liquefaction of biomass. Renew Sustain Energy Rev 81:1378–1392
Guo J, Lua AC (2002) Characterization of adsorbent prepared from oil-palm shell by CO2 activation for removal of gaseous pollutants. Mater Lett 55(5):334–339
Guo J, Lua AC (2003) Adsorption of sulphur dioxide onto activated carbon prepared from oil-palm shells with and without pre-impregnation. Sep Purif Technol 30(3):265–273
Guo J, Luo Y, Lua AC, Chi R-a, Chen Y-l, Bao X-t, Xiang S-x (2007) Adsorption of hydrogen sulphide (H2S) by activated carbons derived from oil-palm shell. Carbon 45(2):330–336
Guo S, Gao Y, Wang Y, Liu Z, Wei X, Peng P, Xiao B, Yang Y (2019) Urea/ZnCl2 in situ hydrothermal carbonization of Camellia sinensis waste to prepare N-doped biochar for heavy metal removal. Environ Sci Pollut Res 26:30365–30373
Gupta RK, Dubey M, Kharel P, Gu Z, Fan QH (2015) Biochar activated by oxygen plasma for supercapacitors. J Power Sources 274:1300–1305
Gupta D, Mahajani SM, Garg A (2020) Investigation on hydrochar and macromolecules recovery opportunities from food waste after hydrothermal carbonization. Sci Total Environ 749:142294
Hakeem IG, Halder P, Dike CC, Chiang K, Sharma A, Paz-Ferreiro J, Shah K (2022) Advances in biosolids pyrolysis: roles of pre-treatments, catalysts, and co-feeding on products distribution and high-value chemical production. J Anal Appl Pyrol 166:105608
Hakeem IG, Halder P, Patel S, Selezneva E, Rathnayake N, Marzbali MH, Veluswamy G, Sharma A, Kundu S, Surapaneni A, Megharaj M, Batstone DJ, Shah K (2024) Current understanding on the transformation and fate of per- and polyfluoroalkyl substances before, during, and after thermal treatment of biosolids. Chem Eng J 493:152537
Han M, Jiang K, Jiao P, Ji Y, Zhou J, Zhuang W, Chen Y, Liu D, Zhu C, Chen X (2017) Bio-butanol sorption performance on novel porous-carbon adsorbents from corncob prepared via hydrothermal carbonization and post-pyrolysis method. Sci Rep 7(1):11753
Han X, Chen H, Liu Y, Pan J (2020) Study on removal of gaseous hydrogen sulfide based on macroalgae biochars. J Nat Gas Sci Eng 73:103068
Hao W, Björkman E, Lilliestråle M, Hedin N (2013) Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl Energy 112:526–532
Hassan S, Ngo T, Khudur LS, Krohn C, Dike CC, Hakeem IG, Shah K, Surapaneni A, Ball AS (2023) Biosolids-derived biochar improves biomethane production in the anaerobic digestion of chicken manure. Resources 12(10):123
He C, Chen C-L, Giannis A, Yang Y, Wang J-Y (2014) Hydrothermal gasification of sewage sludge and model compounds for renewable hydrogen production: a review. Renew Sustain Energy Rev 39:1127–1142
He D, Wu J, Yu C, Huang B, Tu X, Li D, Jia X, Dan J, Fang Z, Dai Z, Liu R, Zhou Q (2023) Synthesis of corncob biochar with high surface area by KOH activation for VOC adsorption: effect of KOH addition method. J Chem Technol Biotechnol 98(8):2051–2064
Heo J, Yoon Y, Lee G, Kim Y, Han J, Park CM (2019) Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4–biochar composite. Biores Technol 281:179–187
Hervy M, Minh DP, Gerente C, Weiss-Hortala E, Nzihou A, Villot A, Le Coq L (2018a) H2S removal from syngas using wastes pyrolysis chars. Chem Eng J 334:2179–2189
Hervy M, Pham Minh D, Gérente C, Weiss-Hortala E, Nzihou A, Villot A, Le Coq L (2018b) H2S removal from syngas using wastes pyrolysis chars. Chem Eng J 334:2179–2189
Ho K-L, Lin W-C, Chung Y-C, Chen Y-P, Tseng C-P (2013) Elimination of high concentration hydrogen sulfide and biogas purification by chemical–biological process. Chemosphere 92(10):1396–1401
Hoang AT, Goldfarb JL, Foley AM, Lichtfouse E, Kumar M, Xiao L, Ahmed SF, Said Z, Luque R, Bui VG, Nguyen XP (2022) Production of biochar from crop residues and its application for anaerobic digestion. Biores Technol 363:127970
Hou Y, Huang G, Li J, Yang Q, Huang S, Cai J (2019) Hydrothermal conversion of bamboo shoot shell to biochar: preliminary studies of adsorption equilibrium and kinetics for rhodamine B removal. J Anal Appl Pyrol 143:104694
Hu W, Huang J, Wang J, Xie D, Wang Z, Qiao Y, Xu M (2024) Benign-by-design N-doped activated carbon from wasted aqueous assisted hydrochar of leftover rice for efficient H2S removal. Fuel 358:130233
Huang H, Niu Z, Shi R, Tang J, Lv L, Wang J, Fan Y (2020) Thermal oxidation activation of hydrochar for tetracycline adsorption: the role of oxygen concentration and temperature. Biores Technol 306:123096
Huang D, Wang N, Bai X, Chen Y, Xu Q (2022) The influencing mechanism of O2, H2O, and CO2 on the H2S removal of food waste digestate-derived biochar with abundant minerals. Biochar 4(1):71
Huynh Nhut H, Le Thi Thanh V, Le Tran L (2020) Removal of H2S in biogas using biotrickling filter: recent development. Process Saf Environ Prot 144:297–309
Idrees M, Batool S, Kalsoom T, Yasmeen S, Kalsoom A, Raina S, Zhuang Q, Kong J (2018) Animal manure-derived biochars produced via fast pyrolysis for the removal of divalent copper from aqueous media. J Environ Manage 213:109–118
Ighalo JO, Kurniawan SB, Iwuozor KO, Aniagor CO, Ajala OJ, Oba SN, Iwuchukwu FU, Ahmadi S, Igwegbe CA (2022a) A review of treatment technologies for the mitigation of the toxic environmental effects of acid mine drainage (AMD). Process Saf Environ Prot 157:37–58
Ighalo JO, Rangabhashiyam S, Dulta K, Umeh CT, Iwuozor KO, Aniagor CO, Eshiemogie SO, Iwuchukwu FU, Igwegbe CA (2022b) Recent advances in hydrochar application for the adsorptive removal of wastewater pollutants. Chem Eng Res Des 184:419–456
Ighalo JO, Conradie, J, Ohoro, CR, Amaku, JF, Oyedotun, KO, Maxakato, NW, Akpomie, KG, Okeke, ES, Olisah, C, Malloum, A, Adegoke, KA (2023). Biochar from coconut residues: an overview of production, properties, and applications. Industrial Crops and Products 204.
Jagnade P, Panwar NL (2024) Experiment investigation on the performance of activated bamboo biochar for the adsorption of CO2 and PM 2.5. Emerg Mater 7:1227–1328
Jiang Y-H, Li A-Y, Deng H, Ye C-H, Li Y (2019) Phosphate adsorption from wastewater using ZnAl-LDO-loaded modified banana straw biochar. Environ Sci Pollut Res 26:18343–18353
Jiang Y, Ming C, Sun K, Zhang L, Zhang S, Cui Z, Wang D, Leng C, Hu X (2024) Enhanced abundance of oxygen-containing intermediates from pre-oxidation of poplar sawdust facilitates generation of porous structures in activation with phosphoric acid. Sustain Mater Technol 39:e00815
Jin Q, Wang Z, Feng Y, Kim Y-T, Stewart AC, O’Keefe SF, Neilson AP, He Z, Huang H (2020) Grape pomace and its secondary waste management: biochar production for a broad range of lead (Pb) removal from water. Environ Res 186:109442
Juntarachat N, Onthong U (2022) Removal of hydrogen sulfide from biogas using banana peel and banana empty fruit bunch biochars as alternative adsorbents. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-03430-z
Kambo HS, Dutta A (2015) A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew Sustain Energy Rev 45:359–378
Kang JH, Yoon Y, Song JH (2019) Simultaneous removal of hydrogen sulfide and ammonia using a combined system with absorption and electrochemical oxidation. J Environ Sci Health, Part A 54(14):1430–1440
Kanjanarong J, Giri BS, Jaisi DP, Oliveira FR, Boonsawang P, Chaiprapat S, Singh RS, Balakrishna A, Khanal SK (2017) Removal of hydrogen sulfide generated during anaerobic treatment of sulfate-laden wastewater using biochar: evaluation of efficiency and mechanisms. Biores Technol 234:115–121
Kassim FO, Thomas CLP, Afolabi OOD (2022) Integrated conversion technologies for sustainable agri-food waste valorization: a critical review. Biomass Bioenerg 156:106314
Katariya HG, Patolia HP (2023) Advances in biogas cleaning, enrichment, and utilization technologies: a way forward. Biomass Convers Biorefin 13(11):9565–9581
Kenda GT, Kouteu PAN, Tchuifon DRT, Fotsop CG, Bopda A, Kuete H-IT, Ndifor-Angwafor NG, Anagho SG (2024) Green synthesis of magnetic biochars derived from biobased orange peel materials as sustainable heterogeneous catalytic supports for the Fenton process. Arab J Chem 17(2):105502
Khan N, Chowdhary P, Ahmad A, Shekher Giri B, Chaturvedi P (2020) Hydrothermal liquefaction of rice husk and cow dung in Mixed-Bed-Rotating Pyrolyzer and application of biochar for dye removal. Biores Technol 309:123294
Kharel G, Sacko O, Feng X, Morris JR, Phillips CL, Trippe K, Kumar S, Lee JW (2019) Biochar surface oxygenation by ozonization for super high cation exchange capacity. ACS Sustain Chem Eng 7(19):16410–16418
Khoshnevisan B, Tsapekos P, Alfaro N, Díaz I, Fdz-Polanco M, Rafiee S, Angelidaki I (2017) A review on prospects and challenges of biological H2S removal from biogas with focus on biotrickling filtration and microaerobic desulfurization. Biofuel Res J 4(4):741–750
Khuong DA, Nguyen HN, Tsubota T (2021) Activated carbon produced from bamboo and solid residue by CO2 activation utilized as CO2 adsorbents. Biomass Bioenerg 148:106039
Kim KH, Kim J-Y, Cho T-S, Choi JW (2012) Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Biores Technol 118:158–162
Koottatep T, Fakkaew K, Tajai N, Polprasert C (2017) Isotherm models and kinetics of copper adsorption by using hydrochar produced from hydrothermal carbonization of faecal sludge. J Water, Sanit Hyg Develop 7(1):102–110
Koulouri ME, Templeton MR, Fowler GD (2024) Enhancing the nitrogen and phosphorus content of faecal-derived biochar via adsorption and precipitation from human urine. J Environ Manage 352:119981
Le HS, Chen WH, Forruque Ahmed S, Said Z, Rafa N, Le Tuan A, Ağbulut Ü, Veza I, Phuong Nguyen X, Quang Duong X, Huang Z, Hoang AT (2022) Hydrothermal carbonization of food waste as sustainable energy conversion path. Biores Technol 363:127958
Lee XJ, Ong HC, Gan YY, Chen W-H, Mahlia TMI (2020) State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers Manage 210:112707
Lestari RAS, Sediawan WB, Syamsiah S, Teixeira JA (2016) Hydrogen sulfide removal from biogas using a salak fruit seeds packed bed reactor with sulfur oxidizing bacteria as biofilm. J Environ Chem Eng 4(2):2370–2377
Li X, Li Y (2019) Adsorptive removal of dyes from aqueous solution by KMnO4-modified rice husk and rice straw. J Chem. https://doi.org/10.1155/2019/8359491
Li F, Wang H, Zhang Y, Wang Q (2019a) Preparation of desulfurizing activated carbon from corn stalk and characterization of desulfurizing structure. J Environ Eng Landsc Manag 27(1):33–40
Li Y, Tsend N, Li T, Liu H, Yang R, Gai X, Wang H, Shan S (2019b) Microwave assisted hydrothermal preparation of rice straw hydrochars for adsorption of organics and heavy metals. Biores Technol 273:136–143
Li F, Zimmerman AR, Hu X, Gao B (2020a) Removal of aqueous Cr (VI) by Zn-and Al-modified hydrochar. Chemosphere 260:127610
Li J, Yu G, Pan L, Li C, You F, Wang Y (2020b) Ciprofloxacin adsorption by biochar derived from co-pyrolysis of sewage sludge and bamboo waste. Environ Sci Pollut Res 27:22806–22817
Li B, Zhang Y, Xu J, Mei Y, Fan S, Xu H (2021) Effect of carbonization methods on the properties of tea waste biochars and their application in tetracycline removal from aqueous solutions. Chemosphere 267:129283
Li Y, Shang H, Cao Y, Yang C, Feng Y, Yu Y (2022) High performance removal of sulfamethoxazole using large specific area of biochar derived from corncob xylose residue. Biochar 4(1):11
Li Y, Kumar Awasthi M, Sindhu R, Binod P, Zhang Z, Taherzadeh MJ (2023) Biochar preparation and evaluation of its effect in composting mechanism: A review. Bioresource Technology 384:129329
Libra JA, Ro KS, Kammann C, Funke A, Berge ND, Neubauer Y, Titirici M-M, Fühner C, Bens O, Kern J, Emmerich K-H (2011) Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2(1):71–106
Lin W-C, Chen Y-P, Tseng C-P (2013) Pilot-scale chemical–biological system for efficient H2S removal from biogas. Biores Technol 135:283–291
Liu Y, Wang Y (2019) Removal of gaseous hydrogen sulfide by a photo-Fenton wet oxidation scrubbing system. Energy Fuels 33(11):10812–10819
Liu X, Liao J, Song H, Yang Y, Guan C, Zhang Z (2019) A biochar-based route for environmentally friendly controlled release of nitrogen: urea-loaded biochar and bentonite composite. Sci Rep 9(1):9548
Liu D, Li C, Wu J, Liu Y (2020a) Novel carbon-based sorbents for elemental mercury removal from gas streams: a review. Chem Eng J 391:123514
Liu H, Xu G, Li G (2020b) The characteristics of pharmaceutical sludge-derived biochar and its application for the adsorption of tetracycline. Sci Total Environ 747:141492
Liu D, Tang Y, Li J, Hao Z, Zhu J, Wei J, Liu C, Dong L, Jia B, Chen G (2021a) Eupatorium adenophorum derived adsorbent by hydrothermal-assisted HNO3 modification and application to Pb2+ adsorption. J Environ Chem Eng 9(5):105972
Liu H, Basar IA, Nzihou A, Eskicioglu C (2021b) Hydrochar derived from municipal sludge through hydrothermal processing: a critical review on its formation, characterization, and valorization. Water Res 199:117186
Liu M, Wei Y, Leng X (2021c) Improving biogas production using additives in anaerobic digestion: a review. J Clean Prod 297:126666
Liu X, Li G, Chen C, Zhang X, Zhou K, Long X (2022) Banana stem and leaf biochar as an effective adsorbent for cadmium and lead in aqueous solution. Sci Rep 12(1):1584
Lu J, Zhang C, Wu J, Luo Y (2017) Adsorptive removal of bisphenol A using N-doped biochar made of Ulva prolifera. Water Air Soil Pollut 228:1–9
Lu X, Liu X, Zhang W, Wang X, Wang S, Xia T (2020) The residue from the acidic concentrated lithium bromide treated crop residue as biochar to remove Cr (VI). Biores Technol 296:122348
Ma Q, Chen W, Jin Z, Chen L, Zhou Q, Jiang X (2021a) One-step synthesis of microporous nitrogen-doped biochar for efficient removal of CO2 and H2S. Fuel 289:119932
Ma Y, Guo H, Selyanchyn R, Wang B, Deng L, Dai Z, Jiang X (2021b) Hydrogen sulfide removal from natural gas using membrane technology: a review. J Mater Chem A 9(36):20211–20240
Malyan SK, Kumar SS, Fagodiya RK, Ghosh P, Kumar A, Singh R, Singh L (2021) Biochar for environmental sustainability in the energy-water-agroecosystem nexus. Renew Sustain Energy Rev 149:111379
Mamvura TA, Danha G (2020) Biomass torrefaction as an emerging technology to aid in energy production. Heliyon 6(3):e503531
Marcilla A, Catalá L, García-Quesada JC, Valdés FJ, Hernández MR (2013) A review of thermochemical conversion of microalgae. Renew Sustain Energy Rev 27:11–19
Marín D, Vega M, Lebrero R, Muñoz R (2020) Optimization of a chemical scrubbing process based on a Fe-EDTA-carbonate based solvent for the simultaneous removal of CO2 and H2S from biogas. J Water Process Eng 37:101476
Marzbali MH, Kundu S, Halder P, Patel S, Hakeem IG, Paz-Ferreiro J, Madapusi S, Surapaneni A, Shah K (2021) Wet organic waste treatment via hydrothermal processing: a critical review. Chemosphere 279:130557
Marzouk SAM, Al-Marzouqi MH, Teramoto M, Abdullatif N, Ismail ZM (2012) Simultaneous removal of CO2 and H2S from pressurized CO2–H2S–CH4 gas mixture using hollow fiber membrane contactors. Sep Purif Technol 86:88–97
Masebinu S, Akinlabi E, Muzenda E, Aboyade A (2019) A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renew Sustain Energy Rev 103:291–307
Masoumi S, Borugadda, VB, Nanda, S, Dalai AK (2021) Hydrochar: a review on its production technologies and applications, catalysts
Masud MAA, Shin WS, Sarker A, Septian A, Das K, Deepo DM, Iqbal MA, Islam ARMT, Malafaia G (2023) A critical review of sustainable application of biochar for green remediation: Research uncertainty and future directions. Sci Total Environ 904:166813
McGeogh M, Annath H, Mangwandi C (2024) Turning teawaste particles into magnetic bio-sorbents particles for arsenic removal from wastewater: isotherm and kinetic studies. Particuology 87:179–193
Mumtaz H, Sobek S, Werle S, Sajdak M, Muzyka R (2023) Hydrothermal treatment of plastic waste within a circular economy perspective. Sustain Chem Pharm 32:100991
Muradov N, Fidalgo B, Gujar AC, Garceau N, T-Raissi A, (2012) Production and characterization of Lemna minor bio-char and its catalytic application for biogas reforming. Biomass Bioenerg 42:123–131
Nahar K, Thulasiraman AV, Vuppaladadiyam AK, Hakeem IG, Shah K (2024) Current understanding on the fate of contaminants during hydrothermal treatment of sewage sludge. Curr Opin Green Sustain Chem 49:100960
Nguyen DH, Tran HN, Chao H-P, Lin C-C (2019) Effect of nitric acid oxidation on the surface of hydrochars to sorb methylene blue: An adsorption mechanism comparison. Adsorpt Sci Technol 37(7–8):607–622
Nguyen PM, Do PT, Pham YB, Doan TO, Nguyen XC, Lee WK, Nguyen DD, Vadiveloo A, Um M-J, Ngo HH (2022a) Roles, mechanism of action, and potential applications of sulfur-oxidizing bacteria for environmental bioremediation. Sci Total Environ 852:158203
Nguyen T-B, Truong Q-M, Chen C-W, Doong R-a, Chen W-H, Dong C-D (2022b) Mesoporous and adsorption behavior of algal biochar prepared via sequential hydrothermal carbonization and ZnCl2 activation. Biores Technol 346:126351
Nicolae SA, Szilágyi PÁ, Titirici MM (2020) Soft templating production of porous carbon adsorbents for CO2 and H2S capture. Carbon 169:193–204
Nicolae SA, Louis-Therese J, Gaspard S, Szilágyi PÁ, Titirici MM (2022) Biomass derived carbon materials: Synthesis and application towards CO2 and H2S adsorption. Nano Select 3(1):165–177
Opatokun SA, Prabhu A, Al Shoaibi A, Srinivasakannan C, Strezov V (2017) Food wastes derived adsorbents for carbon dioxide and benzene gas sorption. Chemosphere 168:326–332
Patel MR, Panwar NL (2023) Biochar from agricultural crop residues: environmental, production, and life cycle assessment overview. Resour, Conserv Recycl Adv 19:200173
Patel S, Kundu S, Halder P, Ratnnayake N, Marzbali MH, Aktar S, Selezneva E, Paz-Ferreiro J, Surapaneni A, de Figueiredo CC, Sharma A, Megharaj M, Shah K (2020) A critical literature review on biosolids to biochar: an alternative biosolids management option. Rev Environ Sci Bio/technol 19(4):807–841
Patel S, Hedayati Marzbali M, Hakeem IG, Veluswamy G, Rathnayake N, Nahar K, Agnihotri S, Bergmann D, Surapaneni A, Gupta R, Sharma A, Shah K (2023) Production of H2 and CNM from biogas decomposition using biosolids-derived biochar and the application of the CNM-coated biochar for PFAS adsorption. Waste Manage 159:146–153
Peluso A, Gargiulo N, Aprea P, Pepe F, Caputo D (2019) Nanoporous materials as H2S adsorbents for biogas purification: a review. Sep Purif Rev 48(1):78–89
Periyavaram SR, Uppala L, Sivaprakash S, Reddy PHP (2023) Thermal behaviour of hydrochar derived from hydrothermal carbonization of food waste using leachate as moisture source: kinetic and thermodynamic analysis. Biores Technol 373:128734
Pikaar I, Likosova EM, Freguia S, Keller J, Rabaey K, Yuan Z (2015) Electrochemical abatement of hydrogen sulfide from waste streams. Crit Rev Environ Sci Technol 45(14):1555–1578
Pimchuai A, Dutta A, Basu P (2010) Torrefaction of agriculture residue to enhance combustible properties. Energy Fuels 24(9):4638–4645
Pioquinto García S, Garza Rodríguez LÁ, Bustos Martínez D, Cerino Córdova FdJ, Soto Regalado E, Giraudet S, Dávila Guzmán NE (2021) Siloxane removal for biogas purification by low cost mineral adsorbent. J Clean Prod 286:124940
Pudi A, Rezaei M, Signorini V, Andersson MP, Baschetti MG, Mansouri SS (2022) Hydrogen sulfide capture and removal technologies: a comprehensive review of recent developments and emerging trends. Sep Purif Technol 298:121448
Qayyum A, Ali U, Ramzan N (2020) Acid gas removal techniques for syngas, natural gas, and biogas clean up – a review. Energy Sour Part A: Recov, Utilizat Environ Effects,. https://doi.org/10.1080/15567036.2020.1800866
Raheem A, Prinsen P, Vuppaladadiyam AK, Zhao M, Luque R (2018) A review on sustainable microalgae based biofuel and bioenergy production: recent developments. J Clean Prod 181:42–59
Rajapaksha AU, Chen SS, Tsang DCW, Zhang M, Vithanage M, Mandal S, Gao B, Bolan NS, Ok YS (2016) Engineered/designer biochar for contaminant removal/immobilization from soil and water: potential and implication of biochar modification. Chemosphere 148:276–291
Rana K, Rana N, Singh B (2020) Chapter 10 - Applications of sulfur oxidizing bacteria. In: Salwan R, Sharma V (eds) Physiological and biotechnological aspects of extremophiles. Academic Press, New York, pp 131–136
Rangabhashiyam S, dos Santos Lins PV, de Magalhães Oliveira LM, Sepulveda P, Ighalo JO, Rajapaksha AU, Meili L (2022) Sewage sludge-derived biochar for the adsorptive removal of wastewater pollutants: a critical review. Environ Pollut 293:118581
Rathnayake D, Schmidt HP, Leifeld J, Mayer J, Epper CA, Bucheli TD, Hagemann N (2023) Biochar from animal manure: a critical assessment on technical feasibility, economic viability, and ecological impact. GCB Bioenergy 15(9):1078–1104
Roshan A, Ghosh D, Maiti SK (2023) How temperature affects biochar properties for application in coal mine spoils? A meta-analysis. Carbon Res 2(1):3
Safarian S (2023) Performance analysis of sustainable technologies for biochar production: a comprehensive review. Energy Rep 9:4574–4593
Saha D, Kienbaum MJ (2019) Role of oxygen, nitrogen and sulfur functionalities on the surface of nanoporous carbons in CO2 adsorption: a critical review. Micropor Mesopor Mater 287:29–55
Saha N, Saba A, Reza MT (2019) Effect of hydrothermal carbonization temperature on pH, dissociation constants, and acidic functional groups on hydrochar from cellulose and wood. J Anal Appl Pyrol 137:138–145
Sahoo SS, Vijay VK, Chandra R, Kumar H (2021) Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Cleaner Eng Technol 3:100101
Sajjadi B, Chen W-Y, Egiebor NO (2019) A comprehensive review on physical activation of biochar for energy and environmental applications. Rev Chem Eng 35(6):735–776
Sethupathi S, Zhang M, Rajapaksha AU, Lee SR, Mohamad Nor N, Mohamed AR, Al-Wabel M, Lee SS, Ok YS (2017) Biochars as potential adsorbers of CH4, CO2 and H2S. Sustainability 9(1):121
Shang G, Li Q, Liu L, Chen P, Huang X (2016) Adsorption of hydrogen sulfide by biochars derived from pyrolysis of different agricultural/forestry wastes. J Air Waste Manag Assoc 66(1):8–16
Shao Y, Tan H, Shen D, Zhou Y, Jin Z, Zhou D, Lu W, Long Y (2020) Synthesis of improved hydrochar by microwave hydrothermal carbonization of green waste. Fuel 266:117146
Sharma AK, Ghodke PK, Goyal N, Bobde P, Kwon EE, Lin KYA, Chen WH (2023) A critical review on biochar production from pine wastes, upgradation techniques, environmental sustainability, and challenges. Biores Technol 387:129632
Sharma T, Hakeem IG, Gupta AB, Joshi J, Shah K, Vuppaladadiyam AK, Sharma A (2024) Parametric influence of process conditions on thermochemical techniques for biochar production: a state-of-the-art review. J Energy Instit 113:101559
Shen Y, Zhang N (2019) Facile synthesis of porous carbons from silica-rich rice husk char for volatile organic compounds (VOCs) sorption. Biores Technol 282:294–300
Shi Y, Liang B, Lin RB, Zhang C, Chen B (2020) Gas separation via hybrid metal-organic framework/polymer membranes. Trends Chem 2(3):254–269
Shyam S, Arun J, Gopinath KP, Ribhu G, Ashish M, Ajay S (2022) Biomass as source for hydrochar and biochar production to recover phosphates from wastewater: a review on challenges, commercialization, and future perspectives. Chemosphere 286:131490
Sitthikhankaew R, Chadwick D, Assabumrungrat S, Laosiripojana N (2014) Effects of humidity, O2, and CO2 on H2S adsorption onto upgraded and KOH impregnated activated carbons. Fuel Process Technol 124:249–257
SLB (2023) Cynara H2S and CO2 separation membranes: https://www.slb.com/products-and-services/innovating-in-oil-and-gas/well-production/processing-and-separation/gas-treatment/cynara-h2s-and-co2-separation-membranes. Accessed on 10 April 2024
Suansa NI, Al-Mefarrej HA, Alshahrani TS (2021) Biochar hydrological characteristics in related to branch wood properties. BioResources 16(2):2824–2837
Sun Q, Li H, Yan J, Liu L, Yu Z, Yu X (2015) Selection of appropriate biogas upgrading technology-a review of biogas cleaning, upgrading and utilisation. Renew Sustain Energy Rev 51:521–532
Sun Y, Zhang JP, Wen C, Zhang L (2016) An enhanced approach for biochar preparation using fluidized bed and its application for H2S removal. Chem Eng Process 104:1–12
Sun X, Ruan H, Song X, Sun L, Li K, Ning P, Wang C (2018) Research into the reaction process and the effect of reaction conditions on the simultaneous removal of H2S, COS and CS2 at low temperature. RSC Adv 8(13):6996–7004
Sun N, Wang T, Qi B, Yu S, Yao Z, Zhu G, Fu Q, Li C (2024) Inhibiting release of phenanthrene from rice-crab coculture sediments to overlying water with rice stalk biochar: performance and mechanisms. Sci Total Environ 908:168385
Supraja KV, Kachroo H, Viswanathan G, Verma VK, Behera B, Doddapaneni TRKC, Kaushal P, Ahammad SZ, Singh V, Awasthi MK, Jain R (2023) Biochar production and its environmental applications: recent developments and machine learning insights. Bioresour Technol 387:129634
Surra E, Costa Nogueira M, Bernardo M, Lapa N, Esteves I, Fonseca I (2019) New adsorbents from maize cob wastes and anaerobic digestate for H2S removal from biogas. Waste Manage 94:136–145
Syed M, Soreanu G, Falletta P, Béland M (2006) Removal of hydrogen sulfide from gas streams using biological processes-A review. Can Biosyst Eng 48:1–14
Tahir MS, Shahid Z, Shahzad K, Sagir M, Rehan M, Nizami AS (2015) Producing methane enriched biogas using solvent absorption method. Chem Eng Trans 45:1309–1314
Tawfik A, Eraky M, Osman AI, Ai P, Zhou Z, Meng F, Rooney DW (2023) Bioenergy production from chicken manure: a review. Environ Chem Lett 21(5):2707–2727
Tian X, Chen Y, Chen Y, Chen D, Wang Q, Li X (2022) Removal of gaseous hydrogen sulfide by a FeOCl/H2O2 wet oxidation system. ACS Omega 7(9):8163–8173
Tippayawong N, Thanompongchart P (2010) Biogas quality upgrade by simultaneous removal of CO2 and H2S in a packed column reactor. Energy 35(12):4531–4535
Truong Q-M, Nguyen T-B, Chen C-W, Chen W-H, Bui X-T, Dong C-D (2024) KHCO3-activated high surface area biochar derived from brown algae: a case study for efficient adsorption of Cr(VI) in aqueous solution. Environ Res 247:118227
Tsai W-T, Lin Y-Q, Huang H-J (2021) Valorization of rice husk for the production of porous biochar materials. Fermentation 7(2):70
Tsvetkov M, Zaichenko A, Podlesniy D, Repina M, Glukhov A (2024) Pyrolysis of Laminaria japonica with biochar production and its characteristics, E3S web of conferences. EDP Sci 474:01012
Vamvuka D, Loupasis E, Chamilaki E, Sdoukou E (2024) Adsorption of ammonium from wastewaters by an almond kernel derived biochar modified by potassium hydroxide or dolomite and activated by steam. Environ Adv 15:100465
Vaziri RS, Babler MU (2019) Removal of hydrogen sulfide with metal oxides in packed bed reactors—a review from a modeling perspective with practical implications. Appl Sci 9:5316
Venkatachalam CD, Sekar S, Sengottian M, Ravichandran SR, Bhuvaneshwaran P (2023) A critical review of the production, activation, and morphological characteristic study on functionalized biochar. J Energy Storage 67:10525
Vuppaladadiyam AK, Vuppaladadiyam SSV, Awasthi A, Sahoo A, Rehman S, Pant KK, Murugavelh S, Huang Q, Anthony E, Fennel P, Bhattacharya S (2022) Biomass pyrolysis: a review on recent advancements and green hydrogen production. Bioresour Technol 364:128087
Vuppaladadiyam AK, Vuppaladadiyam SSV, Sikarwar VS, Ahmad E, Pant KK, Murugavelh S, Pandey A, Bhattacharya S, Sarmah A, Leu SY (2023) A critical review on biomass pyrolysis: reaction mechanisms, process modeling and potential challenges. J Energy Instit 108:101236
Vuppaladadiyam AK, Vuppaladadiyam SSV, Sahoo A, Murugavelh S, Anthony E, Bhashkar T, Zheng Y, Zhao M, Duan H, Zhao Y, Antunes E, Sarmah AK, Leu SY (2023) Bio-oil and biochar from the pyrolytic conversion of biomass: a current and future perspective on the trade-off between economic, environmental, and technical indicators. Sci Total Environ 857:159155
Wang J, Wang S (2019) Preparation, modification and environmental application of biochar: a review. J Clean Prod 227:1002–1022
Wang S, Nam H, Nam H (2020) Preparation of activated carbon from peanut shell with KOH activation and its application for H2S adsorption in confined space. J Environ Chem Eng 8(2):103683
Wang Z, Huang J, Zhong Y, Hu W, Xie D, Zhao C, Qiao Y (2022) Copper supported on activated carbon from hydrochar of pomelo peel for efficient H2S removal at room temperature: role of copper valance, humidity and oxygen. Fuel 319:123774
Wang L, Deng J, Yang X, Hou R, Hou D (2023) Role of biochar toward carbon neutrality. Carbon Res 2(1):2
Weber K, Quicker P (2018) Properties of biochar. Fuel 217:240–261
Wei Y, Wang H, Zhang X, Liu C (2021) Ammonia-assisted hydrothermal carbon material with schiff base structures synthesized from factory waste hemicelluloses for Cr (VI) adsorption. J Environ Chem Eng 9(5):106187
Wen C, Liu T, Wang D, Wang Y, Chen H, Luo G, Zhou Z, Li C, Xu M (2023) Biochar as the effective adsorbent to combustion gaseous pollutants: preparation, activation, functionalization and the adsorption mechanisms. Prog Energy Combust Sci 99:101098
Wu P, Singh BP, Wang H, Jia Z, Wang Y, Chen W (2023) Bibliometric analysis of biochar research in 2021: a critical review for development, hotspots and trend directions. Biochar 5(1):6
Wu W, Wu C, Liu J, Yan H, Zhang G, Li G, Zhao Y, Wang Y (2024) Nitrogen-doped porous carbon through K2CO3-activated bamboo shoot shell for an efficient CO2 adsorption. Fuel 363:130937
Xu X, Cao X, Zhao L, Sun T (2014) Comparison of sewage sludge- and pig manure-derived biochars for hydrogen sulfide removal. Chemosphere 111:296–303
Xu X, Kan Y, Zhao L, Cao X (2016) Chemical transformation of CO2 during its capture by waste biomass derived biochars. Environ Pollut 213:533–540
Xu X, Zhao Y, Sima J, Zhao L, Mašek O, Cao X (2017) Indispensable role of biochar-inherent mineral constituents in its environmental applications: a review. Biores Technol 241:887–899
Xu W, Hussain A, Liu Y (2018a) A review on modification methods of adsorbents for elemental mercury from flue gas. Chem Eng J 346:692–711
Xu Y, Deng F, Pang Q, He S, Xu Y, Luo G, Yao H (2018b) Development of waste-derived sorbents from biomass and brominated flame retarded plastic for elemental mercury removal from coal-fired flue gas. Chem Eng J 350:911–919
Xu Y, Luo G, He S, Deng F, Pang Q, Xu Y, Yao H (2019) Efficient removal of elemental mercury by magnetic chlorinated biochars derived from co-pyrolysis of Fe (NO3)3-laden wood and polyvinyl chloride waste. Fuel 239:982–990
Yan R, Liang DT, Tsen L, Tay JH (2002) Kinetics and mechanisms of H2S adsorption by alkaline activated carbon. Environ Sci Technol 36(20):4460–4466
Yan W, Perez S, Sheng K (2017) Upgrading fuel quality of moso bamboo via low temperature thermochemical treatments: dry torrefaction and hydrothermal carbonization. Fuel 196:473–480
Yip K, Tian F, Hayashi J-i, Wu H (2010) Effect of alkali and alkaline earth metallic species on biochar reactivity and syngas compositions during steam gasification. Energy Fuels 24(1):173–181
You S, Ok YS, Chen SS, Tsang DCW, Kwon EE, Lee J, Wang C-H (2017) A critical review on sustainable biochar system through gasification: energy and environmental applications. Biores Technol 246:242–253
Yuan Y, Zhang N, Hu X (2020) Effects of wet and dry ball milling on the physicochemical properties of sawdust derived-biochar. Instrum Sci Technol 48(3):287–300
Yuan Y, Huang L, Zhang TC, Ouyang L, Yuan S (2021) One-step synthesis of ZnFe2O4-loaded biochar derived from leftover rice for high-performance H2S removal. Sep Purif Technol 279:119686
Yuan X, Cao Y, Li J, Patel AK, Dong CD, Jin X, Gu C, Yip ACK, Tsang DCW, Ok YS (2023) Recent advancements and challenges in emerging applications of biochar-based catalysts. Biotechnol Adv 67:108181
Yuan J, Wang C, Tang Z, Chu T, Zheng C, Han Q, Chen H, Tan Y (2024) Biochar derived from traditional Chinese medicine residues: an efficient adsorbent for heavy metal Pb (II). Arab J Chem 17(3):105606
Zakaria MR, Ahmad Farid MA, Andou Y, Ramli I, Hassan MA (2023) Production of biochar and activated carbon from oil palm biomass: current status, prospects, and challenges. Indust Crops Prod 199:116767
Zhang H, Voroney RP, Price GW, White AJ (2017) Sulfur-enriched biochar as a potential soil amendment and fertiliser. Soil Res 55(1):93–99
Zhang X, Gao B, Fang J, Zou W, Dong L, Cao C, Zhang J, Li Y, Wang H (2019a) Chemically activated hydrochar as an effective adsorbent for volatile organic compounds (VOCs). Chemosphere 218:680–686
Zhang Z, Zhu Z, Shen B, Liu L (2019b) Insights into biochar and hydrochar production and applications: a review. Energy 171:581–598
Zhang Y, Qin J, Yi Y (2021) Biochar and hydrochar derived from freshwater sludge: characterization and possible applications. Sci Total Environ 763:144550
Zhao S-X, Ta N, Wang X-D (2017) Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 10(9):1293
Zhao R, Jia L, Yao Y-x, Huo R-p, Qiao X-l, Fan B-g (2019) Study of the effect of adsorption temperature on elemental mercury removal performance of iron-based modified biochar. Energy Fuels 33(11):11408–11419
Zhao M, Ma X, Liao X, Cheng S, Liu Q, Wang H, Zheng H, Li X, Luo X, Zhao J, Li F, Xing B (2022a) Characteristics of algae-derived biochars and their sorption and remediation performance for sulfamethoxazole in marine environment. Chem Eng J 430:133092
Zhao Z, Wang B, Theng BKG, Lee X, Zhang X, Chen M, Xu P (2022b) Removal performance, mechanisms, and influencing factors of biochar for air pollutants: a critical review. Biochar 4(1):30
Zielińska A, Oleszczuk P, Charmas B, Skubiszewska-Zięba J, Pasieczna-Patkowska S (2015) Effect of sewage sludge properties on the biochar characteristic. J Anal Appl Pyrol 112:201–213
Zornoza R, Moreno-Barriga F, Acosta JA, Muñoz MA, Faz A (2016) Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 144:122–130
Zulkefli NN, Masdar S, Jahim J, Majlan EH (2016) Overview of H2S removal technologies from biogas production. Int J Appl Eng Res 11(20):10060–10066
Acknowledgements
This work is supported by the ARC Training Centre for the Transformation of Australia’s Biosolids Resource, RMIT University, Australia under Grant No. IC190100033.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vuppaladadiyam, A.K., Jena, M.K., Hakeem, I.G. et al. A critical review of biochar versus hydrochar and their application for H2S removal from biogas. Rev Environ Sci Biotechnol 23, 699–737 (2024). https://doi.org/10.1007/s11157-024-09700-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-024-09700-8