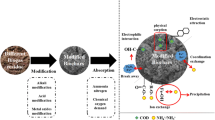
Abstract
Hydrogen sulfide (H2S) removal has been a significant concern in various industries. In this study, food waste digestate-derived biochar (DFW-BC), a by-product of food waste treatment with abundant minerals, was assessed for removing H2S from different simulated biogas containing oxygen (O2) and carbon dioxide (CO2) and under different moisture (H2O) contents (0% and 20%) of biochar. The influencing mechanisms of the gas conditions combined with the moisture contents were also investigated. The results showed an H2S removal of 1.75 mg g−1 for dry biochar under pure H2S, 4.29 mg g−1 for dry biochar under H2S + O2, 5.29 mg g−1 for humid biochar under H2S, and 12.50 mg g−1 for humid biochar under H2S + O2. For dry DFW-BC, the high Fe content was responsible for the O2 enhancement. In contrast, O2 + H2O activated the catalytic H2S oxidation of the less reactive minerals (mainly Ca). The inhibition of CO2 on H2S adsorption was not obvious for dry DFW-BC; the specific pore structure may have provided a buffer against the physisorption competition of CO2. However, when H2O was present on DFW-BC, the changes in critical biochar properties and sulfur speciation as opposed to that without H2O implied an evident occurrence of CO2 chemisorption. This CO2 chemisorption partially hindered O2 + H2O enhancement, decreasing the H2S removal capacity from 12.50 to 8.88 mg g−1. The negative effect was ascribed to mineral carbonation of CO2, neutralizing the alkaline surface and immobilizing metal oxides, which thus reduced the acceleration in H2S dissociation and activation in catalytic H2S oxidation by O2 + H2O.
Graphical Abstract

Highlights
-
Ash-dominated digestate-derived biochar (DFW-BC) was evaluated for H2S removal.
-
O2 enhanced the H2S removal on dry DFW-BC and H2O + O2 achieved further improved performance.
-
H2O-induced CO2 chemisorption reduced H2O + O2 promotion due to mineral carbonation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hydrogen sulfide (H2S) is an odorous and toxic gas emitted from various industries. It is corrosive to pipeline equipment during biogas utilization (Li et al. 2020) and can cause acute and chronic health problems if directly emitted into the environment (Ro et al. 2021). Besides, the odor threshold of H2S is within a very low range of 0.008–0.13 ppm (NRC 2010), making the H2S removal very desirable even when it is at low concentrations. Among various biogas purification or deodorization strategies, H2S adsorption onto solid media is one of the most commonly used methods (Velasco et al. 2019). Hydrogen sulfide adsorption onto activated carbons has been widely reported, as well as its excellent adsorption capacity (Elsayed et al. 2009; Bazan-Wozniak et al. 2017). However, activated carbon production requires an activation process, which increases the cost and may have adverse effects (Shang et al. 2013). Metal oxide and carbon nanomaterials (e.g., carbon nanotubes, graphene, and graphene oxide) also present significant effects in capturing H2S (Kailasa et al. 2020; Revabhai et al. 2022; Patel and Kailasa 2022), but they need special fabrication similar to activated carbon production. As a precursor of activated carbons, biochar is produced through a one-step biomass waste pyrolysis at relatively low temperatures with less sophisticated equipment. In this perspective, biochar is more eco-friendly and cost-effective than activated carbons or nanomaterials (Sahota et al. 2018; Sun et al. 2014; Creamer et al. 2014). Therefore, using biochar absorbents for H2S treatment has gained increasing attention in recent years (Bamdad et al. 2018).
The reaction mechanism during the H2S removal on carbons can be very complex with a variety of sulfur products (Elsay et al. 2009). Generally, the H2S removal on carbons involves two steps (Adib et al. 2000; Choudhury and Lansing 2021: (1) physical adsorption. Gaseous H2S is adsorbed on carbon surface (Eq. 1), dissolved in water (H2O) film (Eq. 2), and dissociated in an adsorbed state (Eq. 3); (2) oxidation step. The adsorbed H2S reacts with oxygen (O2) to form ending products of elemental sulfur (S0; Eq. 4), sulfur dioxide (SO2; Eq. 5), and sulfate (\({\text{SO}}_{4}^{2 - }\); Eq. 6) in the presence of water and metal impurities. The basic reactions are as follows:
where H2Sgas, H2Sads-liq, and H2Sads are H2S in gas, liquid, and adsorbed phases, respectively; KH, KS, Ka, and KR are equilibrium constants for processes of adsorption, gas solubility, dissociation, and surface reaction constant; O*ads is dissociatively adsorbed O2. Other researchers also expanded the reaction mechanism by studying various biochars. Particularly, rich minerals on biochars were emphasized as they could significantly promote the H2S removal capacity and affect the final sulfur products. Metal minerals can react with dissolved H2S in the water film to form metal sulfides (Choudhury and Lansing 2021), change the sulfate product from sulfuric acid to soluble or insoluble metal sulfates that would cause less acidification on biochar surface (Xu et al. 2014), and carry out catalytic H2S oxidation with the products of S0 and metal sulfides (Bagreev and Bandosz 2005). Under dry and O2-absent conditions, gaseous H2S could also directly react with reactive metals in the ash (Eq. 8), or is removed by substitution reaction (Eq. 9) and oxidation reaction (Eq. 10) with O-containing groups for food waste and sludge-derived chars (Hervy et al. 2018):
where C(O) is an active site; C(S) is a sulfur site; Cfree is a free carbon site. The performance of various types of biochars in H2S removal has been reported, and the effects of pH, surface chemistry, pore structure, and metal minerals were all examined to understand the adsorption mechanism. Most previous related studies highlight the importance of the alkaline biochar surface in H2S removal. As the rate-determining step is regarded as the dissociation of H2S into HS− as expressed by Eq. (3) (Sun et al. 2017), a highly alkaline nature of the adsorbent can facilitate H2S dissociation for further oxidation reactions (Xu et al. 2014). A neutral or acidic pH could significantly inhibit the dissociation of H2S and consequently suppress the following oxidative reactions (Sun et al. 2016). A water film would help dissolve H2S (Eq. 2), promoting the further dissociation (Xu et al. 2014). Caustic impregnants (sodium, potassium, etc.) on carbon materials also play a significant role in the dissociation of H2S into HS− as they are often strongly basic (Zhang et al. 2016a). Elsayed et al. (2009) reported that the amount of adsorbed H2S depended on the number of basic groups on the char surface. Additionally, a power function relationship between surface area and biochar desulfurization efficiency was proposed (Su et al. 2021). Su et al. (2021) reported that H2S removal performance was also closely related to the pore size distribution. While researchers usually emphasize the micropores as critical places for H2S oxidation (Choudhury and Lansing 2021; Ma et al. 2021; Surra et al. 2019), the mesopores have also been reported as the active site for H2S adsorption (Zhu et al. 2020). Reactive oxides in biochar minerals, such as iron (Fe) oxides and copper oxide, can directly react with gaseous H2S because of their high affinity for sulfur (Ciahotný et al. 2019).
The function of these inherent biochar properties was also influenced by extrinsic factors, including H2O and O2. For example, in dry conditions, H2S physisorption in super- and ultra-micropores is the most likely mechanism of H2S retention. In contrast, in the presence of H2O, the combination of local pH and pore size distribution is predominant (Scheufele et al. 2021). A significant presence of O2 may increase active sites in the form of oxygenated groups (Scheufele et al. 2021). The reactivity of non-reactive metal oxides, including calcium oxide and magnesium oxide to H2S, is very low in dry conditions, but their catalytic role is activated in humid aerobic conditions (Bagreev and Bandosz 2005). Choudhury and Lansing (2021) suggested that the dissociation of H2S can be facilitated by a pH higher than its pKa (7.2), but further catalytic oxidation of H2S and increased removal efficiency are likely driven by reactive oxygen and metal oxides.
Additionally, there is limited information available regarding the influence of carbon dioxide (CO2), which is acidic and can co-exist in biogas, on the adsorption capacity and mechanism of H2S, although individual CO2 adsorption to chars-based adsorbents has been widely reported (Goel et al. 2021). In a few related studies, inconsistent CO2 impact has been found, including (1) competitive adsorption for perilla, soybean stover, Korean oak, and Japanese oak biochars with 20% humidity in purifying a simulated biogas containing H2S (0.3%), CO2 (40%), and CH4 (59.7%) (Sethupathi et al. 2017), (2) cooperative effect in the 8.9 Å pores of commercial activated carbons when CO2 concentration is greater than 5% in adsorbing 200 ppm of H2S (Gonçalves et al. 2018), and (3) no evident effect for neither wood pallets nor food waste and sludge-derived biochars using a simulated syngas consisting of 30% H2, 39.98% CO, 15% CH4, 15% CO2, and 200 ppm of H2S (Hervy et al. 2018). More research is required to gain a full understanding of the CO2 impact. Regarding carbon element-dominated biochars, the role of CO2 physisorption was especially highlighted in elucidating its impact on H2S removal (Sethupathi et al. 2017). In addition to physisorption, the chemisorption of CO2 on chars has also been reported by their functional groups (Creamer et al. 2014) or the introduced metal oxides (Goel et al. 2021). Xu et al. (2020) discovered that physisorption was the dominant CO2 adsorption mechanism for the biochar composite with low iron content, but as the Fe content increased, chemical reactions became dominant. Moreover, the influence of CO2 on H2S removal of biochars could also be affected by the presence of O2 and H2O, related to the activated complex reactions with metal minerals (Huang et al. 2022a). Nevertheless, the influence of CO2 chemisorption on H2S removal of biochars, particularly mineral-rich biochars, has rarely been explored. The information could be essential for predicting or optimizing H2S removal of biochars in scenarios with considerable CO2.
Compared to wood or crop waste-derived chars, biochars or activated chars made from anaerobically digested fiber have been much less reported (Pelaez-Samaniego et al. 2018). In our previous study, we found that the pyrolytic solid digestate from food waste (DFW) was characterized by a highly alkaline char surface, considerable pore volume, large surface area, and especially high ash content (> 70%) consisting of abundant minerals (Wang et al. 2022a), which were all favorable for H2S adsorption and oxidation. Thus, DFW-derived biochar (DFW-BC) has a great potential to be an excellent H2S adsorbent; however, it could have CO2 chemisorption effects owing to its high ash content. To the best of our knowledge, no research has been carried out to examine the H2S adsorption performance of the DFW-BC and the influencing mechanisms of extrinsic factors, such as O2, H2O, and CO2. The utilization of DFW-BC for gas pollutant removal would also expand the resource utilization options of DFW since its treatment is still a challenge (Wang et al. 2021).
Given the research needs, we employed DFW-BC to conduct H2S adsorption tests simulating different adsorption scenarios. The two major objectives of the experiment were as follows: (1) to reveal the performance and mechanism of DFW-BC in H2S removal under O2 and H2O influence; and (2) to examine a potential interaction between CO2 chemisorption and H2S removal to mineral-dominated biochars using DFW-BC as an example.
2 Material and methods
2.1 Preparation and characterization of DFW-BC
The feedstock (DFW) for producing DFW-BC was sourced from a two-stage food waste anaerobic digestion plant in Shenzhen, China. The detailed characteristics of the feedstock were reported by Wang et al. (2021). The moisture content of raw DFW was about 60%. Before pyrolysis, the DFW was freeze-dried for 48 h using a freeze dryer (FreeZone 2.5, Labconco, USA) and was adjusted to a moisture content of 5%. The pyrolysis procedure followed the previous study (Wang et al. 2022a, b). First, a vertical chamber furnace (SLGL-1200L, Siomm, China) was heated to reach a center temperature of 600℃ using a K-type thermocouple. Then, the furnace was purged with nitrogen (N2) (purity > 99.999%) for 20 min at a flow rate of 1 L min−1 to acquire an O2-free condition. Next, the pre-treated DFW was placed into the center of furnace and went through fast pyrolysis that lasted for 20 min. This rapid pyrolysis process favors the DFW treatment as it is time-saving. The pyrolysis experiment was in triplicate, from which the average yield of biochar was 31.86%.
The following properties were measured for the raw DFW-BC. The water-extractable fraction was acquired from a 1:50 (w/v) mixture of DFW-BC and water. Then the fraction was directly measured for the pH value (FiveEasy Plus, Mettler Toledo, Switzerland). For the concentrations of soluble \({\text{SO}}_{4}^{2 - }\), an ion chromatography (Dionex Aquion, Thermo Fisher, USA) was applied to analyze the filtrate’s composition (by a 0.45 μm filter membrane). The microstructure of DFW-BC was observed by scanning electron microscopy (SEM) (ZEISS SUPRA® 55, Carl Zeiss, German). Nitrogen adsorption and the Brunauer–Emmett–Teller (BET) method were used to estimate the specific surface area while the Barret-Joyner-Halenda (BJH) desorption method was adopted to obtain the pore distribution and average pore volume. The surface functional groups were determined using Fourier transform infrared (FTIR) spectroscopy (IRTrancer-100, Shimadzu, Japan). An elemental analyzer (Perkin Elmer 2400 II, USA) was applied to measure the organic element (C, H, S & N) composition and it automatically output the averages from triplicate measurements. The ash content was obtained via a specific standard method in China (GB/T 212-2008) for proximate analysis of coal, i.e., weighing the residue of biochar after it was combusted to a constant mass in a muffle furnace at a high temperature (around 800℃). The content of O2 was then calculated by a simple formula, i.e., O = (1 – C – H – N – S – Ash) × 100% (Wang et al. 2022a). For measuring the metal concentrations, samples were prepared following the procedures of Wang et al. (2022a). First, biochar samples of 100 mg were digested with 10 mL of acid solution consisting of HCl, HNO3, and HF (3:1:1). The liquid from the last step was filtered by a 0.22 μm membrane filter. And then the filtered liquid was analyzed for metal concentrations by inductively coupled plasma mass spectrometry (iCAP RQ, ThermoFisher, USA). Additionally, an analysis of X-ray photoelectron spectroscopy (XPS) was performed using a PHI 5500 multi-technique system (Physical Electronics) to identify the chemical states of sulfur on DFW-BC.
2.2 Design of H2S adsorption tests
The H2S adsorption tests were all conducted at lab temperature. For each test, a fixed amount of 1 g DFW-BC was packed in a vertical plexiglass column that was 26 cm tall with an interior diameter of 15 mm. The gas inlet and outlet were at the bottom and top of the column, respectively. H2S concentration in biogas can be as low as hundreds of ppb in landfill gas (Ding et al. 2012) and as high as 3.5% for syngas produced from sulfur-rich coal gasification (Hervy et al. 2018). The adsorption kinetics and mass transfer rate can also be significantly different between low and high H2S concentrations (e.g., 10 vs. 3000 ppm) (Elsay et al. 2009). In this study, we chose a low level of H2S concentration (i.e., 150 ppm) to evaluate the H2S removal performance of DFW-BC. The inlet flow rate was controlled at 100 mL min−1 using a pre-calibrated mass flow controller. To continuously monitor the outlet H2S concentrations, an infrared biogas analyzer (Gasboard-3200plus, Hubei Cubic-Ruiyi Instrument Co., Ltd, China) was employed. Zero gas (99.999% N2) and standard gas (150 ppm H2S) calibrations were performed on the analyzer before each adsorption test. A schematic diagram of the experimental setup and the demonstration of its reliability and repeatability can be found in our previous study (Huang et al. 2022a).
To investigate the individual impact of O2, H2O, and CO2 as well as their combined impact, eight adsorption scenarios were created using two DFW-BC moisture contents (MCs) (0% and 20%), two O2 contents (0% and 21% simulating the air), and two CO2 contents (0% and 40%) in the syngas. The raw biochars were oven-dried to obtain dry DFW-BC. Thereafter, a calculated mass of water was added to the dried DFW-BC for the DFW-BC with 20% MC. Note that special efforts were taken during this procedure, i.e., instilling the water as slowly and evenly as we can for the most MC homogeneity of the samples.
2.3 Calculations
The H2S removal capacity q (mg g−1) was calculated using Eq. (11):
where FR is the inlet H2S flow rate (mL min−1); \({\rho }_{\mathrm{H}_{2} \mathrm{S}}\) is the H2S density (mg mL−1); t is the time (min); Ci is the inlet H2S concentration (ppm); Ct is the outlet concentration (ppm); m is the biochar mass (g).
2.4 Adsorption kinetics
Pseudo-first-order equation and Pseudo-second-order equation were derived following Eqs. (12) and (13):
where qt and qe (mg g−1), respectively, are adsorbed H2S mass at time t (min) and at equilibrium; k1 (min−1) and k2 (g mg−1 min−1), respectively, are the rate constants for the pseudo-first-order and pseudo-second-order adsorption kinetics.
3 Results
3.1 Properties of DFW-BC
The biochar had a strong alkaline surface proved by its high pH value (11.47), which is beneficial for H2S dissociation (Xu et al. 2014). High BET surface area and large micropore volume have been reported to promote the reaction rate of H2S oxidation (Ma et al. 2021). The DFW-BC is superior to most biochars made from other biomass wastes in terms of pore volume and surface area according to Additional file 1: Table S1 listing the critical properties of biochars from different feedstocks.
Micropores have been widely identified as a critical area where H2S oxidation takes place on carbons (Choudhury and Lansing 2021; Surra et al. 2019). Hervy et al. (2018) concluded that mesopores lower than 7 nm were also important adsorption sites for H2S oxidation on food waste and sludge-derived biochar in dry gas conditions. Additionally, a relatively larger pore size distribution (i.e., averaged 2.34 nm) could provide support for the adsorbates to reach the adsorption site on municipal sludge-derived biochar easily (Lin et al. 2021). The biochar exhibited an average pore diameter of 4.05 nm, with the majority of the pore size ranging from 2 to 6 nm (as shown in Fig. 1), suggesting a possible favorable pore structure for H2S adsorption. Compared to wood or crop waste-derived biochars with regular porous carbon skeletons, the DFW-BC presented a more heterogeneous surface and agglomerated morphology (Fig. 2), which is attributed to the high mineral content in the feedstock (Xu et al. 2020) (Table 1).
The ash content of the DFW-BC was particularly high (70.07%) than that of the wood and straw waste-derived biochars (Weber and Quicker 2018; Gwenzi et al. 2021). This high ash content of biochar is attributed to the high ash content (24.30%) of the parent biomass (DFW). High ash content implied abundant mineral constituents in the biochar, which were anticipated to improve the sorption of acidic gases (Xu et al. 2017). The deposition of the metal minerals on the surface of DFW-BC was evidenced by SEM–EDS mapping (Fig. 2). Ca, Fe, and Al were the most abundant metal elements, followed by Na, Mg, and K. The high amount of Ca in the DFW-BC originated from food waste and the added CaO for dewatering the DFW (Yu et al. 2020). XRD analysis results showed that CaCO3 was the major metal mineral in the DFW (Wang et al. 2022a) and the thermal decomposition of CaCO3 commences at 550–600 ℃, with a loss of CO2 and a product of CaO on the biochar (Rodriguez-Navarro et al. 2009; Singh et al. 2010). Furthermore, our previous study revealed that the main portion of Fe was reducible, while over 65% of Ca, Na, K, and Mg were acid extractable and exchangeable on the DFW-BC (Wang et al. 2022a). The existence and abundance of the above metal minerals supported the proposed reactions in the discussion section.
3.2 Removal capacities
The adsorption curves are presented in Fig. 3. Under the dry condition, the H2S adsorption capacity was only 1.75 mg g−1. In contrast, the adsorption capacity of the biochar was significantly improved to 5.29 mg g−1 in the presence of 20% MC. The influence of O2 was positive for the DFW-BC even under dry conditions, with a removal capacity of 4.29 mg g−1. When the DFW-BC was humid, the combination of O2 and H2O enhanced the H2S removal up to 12.50 mg g−1, indicating the need for both O2 and H2O to reach an optimal performance of the DFW-BC in removing H2S.
Adsorption curves of H2S on the DFW-BC under different conditions. a is under pure H2S; b is under H2S+CO2; c is under O2; and d is under H2S+CO2+O2. The legends in the top left indicate the syngas composition, in which H2S is 150 ppm, CO2 is 40%, and O2 is 21%; “BC” and “BC-20MC” mean dry DFW-BC and DFW-BC with 20% moisture content, respectively
The role of CO2 was also examined. However, no evident impact on the H2S removal for DFW-BC under dry conditions was observed. In contrast, the promotion of H2O in the H2S removal capacity was partially disrupted when CO2 was present. This conclusion was drawn from the fact that the H2S removal capacity was only 2.97 mg g−1 under 20% CO2 + 20% MC as opposed to 5.29 mg g−1 under 20% MC. Similarly, the presence of CO2 in the syngas only slightly inhibited the stimulation of O2 in H2S removal on dry DFW-BC; the removal capacity was 3.37 mg g−1 under H2S + CO2 + O2 and 4.29 mg g−1 under H2S + O2. However, this CO2 inhibition was induced to a more significant degree in the presence of H2O, reducing the removal capacity from 12.50 mg g−1 under H2S + O2 + 20% MC to 8.88 mg g−1 under H2S + O2 + CO2 + 20% MC. In conclusion, the critical roles of O2 and H2O in activating more efficient H2S removal on DFW-BC while an adverse influence of CO2 on their promotion was demonstrated (Table 2).
Studies on H2S adsorption of digestate-derived biochars are very limited (Table 3). Comparing those materials to commercial activated chars, they showed competitive H2S adsorption capacities (Pelaez-Samaniego et al. 2018; Ayiania et al. 2019). The DFW-BC in this study had a relatively lower H2S adsorption capacity; however, it should be noted that the above adsorbents were all with activation treatment either through CO2 activation or Na2CO3 impregnation while only raw DFW-BC was evaluated. The much higher H2S adsorption in the study of Kanjanarong et al. (2017) was probably explained by the high biochar mass and moisture content (80–85%) but low inlet flow rate that were used in their adsorption system. Appropriate height of fixed-bed char and gas velocity are both necessary to avoid significant pressure drop and for a sufficient residence time for the gas molecules in the fixed-bed (Hervy et al. 2018). For example, Han et al. (2020) indicated that a higher gas velocity led to a reduced contact time that decreased the H2S removal of biochar derived from rice husk. In contrast, increasing the adsorption bed height would benefit the H2S removal due to the prolonged contact time (Meri et al. 2018; Papurello et al. 2020; Sawalha et al. 2020). However, the resistance to the flow also would become higher (i.e., higher head loss or pressure drop) because of the strong mechanical energy dissipation (Scheufele et al. 2021). The concentration level is another factor influencing the H2S adsorption kinetics and mass transfer rate (Elsay et al. 2009). Han et al. (2020) reported that the increase in H2S concentrations shortened the breakthrough time by decreasing the number ratio of adsorption sites to H2S molecules, but eventually increased the H2S removal capacity probably due to the increased partial pressure in the biochar bed.
3.3 Adsorption kinetics
Without regard to the presence of H2O, the adsorption process more closely matched the Pseudo-first-order equation (Table 4), indicating a dominant role of physisorption in controlling the adsorption process (Gwenzi et al. 2021). In contrast, the adsorption process was more aligned with the Pseudo-second-order equation when other gas components existed in the syngas, implying that chemisorption mainly determined the adsorption rate (Goel et al. 2021). It was interesting to notice that the major rate controlling process was once again physisorption with the introduction of H2O on the DFW-BC as suggested by the higher R2 values for the Pseudo-first-order equations.
4 Discussion
4.1 Influence of feedstock type on biochar properties
The factors affecting the biochar properties can be categorized into feedstock and pyrolysis conditions, including temperature, heating rate, residence time, etc. Temperature is claimed to be the most significant factor while residence time and heating rate are less important (Weber and Quicker 2018; Guo et al. 2021). To elaborate how the feedstock combining temperature would affect critical biochar properties that determine H2S removal capacity, Additional file 1: Table S1 summarizes the results from previous studies. For most of the feedstocks, an increased temperature leads to increased carbon content, pH value, ash content, surface area, and pore volume. However, a higher temperature would cause decreased mass yield and functional groups, and extremely high pyrolysis temperatures (e.g., 800–1000 ℃) may result in a decrease in surface area and pore volume likely due to the shrinking solid matrix (Weber and Quicker 2018).
Additionally, feedstock containing abundant inorganic nutrients would produce biochars with higher ash but lower carbon content than plant-based biomass mainly consisting of cellulose, hemicelluloses, lignin, etc. As listed in Additional file 1: Table S1, woody waste-derived biochars exhibit carbon content of 53.99–83.60% and even ≥ 95% at high pyrolysis temperatures (Weber and Quicker 2018), while manure- and sewage sludge-derived biochars own a relatively lower carbon content but high ash content of > 60% and > 80%, respectively. The higher the ash content, the higher the concentration of the mineral components on the biochar (Ayiania et al. 2019). Mineral components (e.g., carbonates) contributed to the alkalinity of biochar in an aqueous medium (Masebinu et al. 2019). This explains the relatively higher pH values for ash-dominated biochars than carbon-dominated biochars. For example, under a pyrolysis temperature of 600℃, the ash content and pH value were 3.59–4.38% and 9.60–9.82 for straw-derived biochars (Huang et al. 2022a) but were 70.07% and 11.47 for DFW-BC in this study. Feedstock type is also one crucial factor determining the functional group compositions besides the pyrolytic temperature. Higher fixed carbon and lignin in the feedstock led to a high amount of surface functional groups (Kumar et al. 2021). However, manure and sewage sludge-derived biochars present more N- and S-functional groups than woody biomass-based materials (Masebinu et al. 2019).
In brief, understanding the influence of feedstock type and pyrolysis conditions (mainly temperature) on biochar properties can help select or produce a promising H2S adsorbent.
4.2 Promotion by H2O in H2S removal on DFW-BC
H2S adsorption on carbons can result from several interactions, including physisorption by van der Waals forces or a more intense bond with O-containing groups and chemisorption through which H2S is oxidized by the O-containing groups (generating elemental sulfur S0) or minerals (to metal sulfides, sulfites, or sulfides) (Han et al. 2020; Elsayed et al. 2009; Guo et al. 2006; Moreno-Castilla 2004). In humid conditions, a water film would be created on the biochar surface (Sahota et al. 2018). According to the aforementioned gas–liquid–solid reaction mechanism on carbon-based adsorbents (Li et al. 2020), this water film should have enhanced H2S dissolution and ionization, improving H2S adsorption on biochars (Xu et al. 2014; Sitthikhankaew et al. 2014). The product S0 is highly active atom and can self-assembly to small S clusters, which could diffuse along the carbon surface, covering or blocking the pores (Sun et al. 2022). Water can then promote sulfur deposition at different carbon sites and mechanically remove sulfur from active sites, decelerating the biochar deactivation process (Zhang et al. 2016b). Additionally, the reactivity of metal minerals in oxidizing H2S is inert under dry biochar conditions but stimulated under humid conditions (Bagreev and Bandosz 2005). These reasons were responsible for the improved H2S removal when DFW-BC became humid.
The results in Fig. 4a suggest that greater consumption of those basic O-containing groups occurred (including C=O, −COO, and −OH) after H2S adsorption on humid DFW-BC as opposed to dry DFW-BC. Given the improved H2S removal capacity in the presence of H2O (from 1.75 to 5.29 mg g−1), enhanced participation of these O-containing functional groups was expected for removing H2S. However, no such decreasing stretching vibration in those O-containing groups was observed for humid DFW-BC after H2S removal when O2 or CO2 was present, indicating their negligible roles in the further O2-induced promotion or CO2-induced inhibition.
The FTIR spectra of the DFW-BC before and after H2S adsorption. a is under pure H2S; b is under H2S+O2; c is under H2S+CO2; and d is under H2S+CO2+O2. "BC" and "BC-20MC" mean dry DFW-BC and DFW-BC with 20% moisture content, respectively. 466–468 cm−1: Si–O–Si; 559 cm−1: Fe–O, P=O; 873 cm−1: C–H; 1030–1050: C–O; 1415 cm−1: –COO; 1586–1602 cm−1: C=O; 3425 cm−1: O–H
Sulfur speciation and proportions on DFW-BC after H2S removal can be found in Fig. 5, which is supplemented by Additional file 1: Fig. S1 to show a more visible variation of sulfur composition. The XPS results in Fig. 5a, b show that H2O mainly stimulated the conversion of H2S to an end product of S0 to improve the H2S removal on the DFW-BC. This explains why its pH value was not any lower than that of the dry DFW-BC. Additionally, the content of sulfate increases from 16.65% to 23.53% in Fig. 5a, b, among which soluble \({\text{SO}}_{4}^{2 - }\) content displays an increase from 0.6 to 1.81 mg g−1. This soluble \({\text{SO}}_{4}^{2 - }\) content is attributed to the reactions with metal minerals including Ca, Na, K, and Mg, which are the major metal constituents in the ash and are mainly present in an extractable form as mentioned above. Similarly, in the study of Xu et al. (2014) on pig manure and sewage sludge-derived biochars, \({\text{SO}}_{4}^{2 - }\) was the dominant sulfur form mainly present as soluble (K,Na)2SO4 and CaSO4 precipitate, respectively, evidencing the significance of abundant minerals in removing H2S. Hervy et al. (2018) ascribed the high H2S removal capacity of food waste/sludge-based chars to their high amount of ash and active mineral species, and Ca-containing particles was proved to contribute to its high H2S with the formation of CaSO4. In brief, H2O improved the H2S removal on DFW-BC with no generation of sulfides, but it increased S0 and sulfates with no further decrease in pH.
The XPS spectra of S2p for the DFW-BC after H2S adsorption. a is under pure H2S for dry DFW-BC; b is under pure H2S for DFW-BC with 20% moisture content; c is under H2S+O2 for dry DFW-BC; d is under H2S+O2 for DFW-BC with 20% moisture content; e is under H2S+CO2 for dry DFW-BC; f is under H2S+CO2 for DFW-BC with 20% moisture content; g is under H2S+O2+CO2 for dry DFW-BC; and h is under H2S+O2+CO2 for DFW-BC with 20% moisture content
4.3 Promotion by O2 in H2S removal on DFW-BC
In dry biochar conditions, the presence of O2 significantly improved the H2S removal on the DFW-BC from 1.75 to 4.29 mg g−1. Along with the improved H2S removal capacity for DFW-BC, a less reduced pH (9.39) than that without O2 (pH = 8.65) was observed. The result contradicted the finding of Hervy et al. (2018), which revealed that O2 in the dry syngas decreased the H2S adsorption capacity of wood pallets and food waste-derived chars owning to the formation of acidic sulfur species. In our previous study, more surface acidification and inhibited H2S removal were also observed for dry straw biochars when O2 was present (Huang et al. 2022a).
The reasons for the enhancement of H2S removal by O2 in this study include the following: (1) direct oxidation of H2S by O2 (He et al. 2011; Wu et al. 2018), (2) formation of more active sites (Sitthikhankaew et al. 2014); and (3) catalytic oxidation of H2S by metal oxides in a basic carbon surface environment (Bagreev and Bandosz 2005). Considering the more reactive nature of Fe and its high content, the major reactions explaining the O2 improvement in H2S removal on dry DFW-BC should be dominated by iron oxides:
The XPS analysis confirmed the newly generated sulfur and sulfides in the presence of O2 (Fig. 5a vs. c). The results explained why H2S removal was improved while surface acidification on dry DFW-BC was reduced since the formation of sulfur and metal sulfides was expected to cause less influence than sulfuric acids (Bagreev and Bandosz 2005).
When O2 was further mixed with 20% MC, a remarkable promotion in H2S removal capacity was observed for the DFW-BC; the removal capacity increased to the highest value among all adsorption scenarios. From Table 2 and Fig. 5c vs. d, there is no much further decrease in pH; however, sulfates, sulfur, and sulfides contents for the DFW-BC are observed to be higher under O2 + H2O than under O2. The added H2O helped generate a water film that accelerated the dissociation of H2S, as mentioned above. As a result, the significantly increased content of soluble sulfate (from 1.06 to 6.39 mg g−1) could be ascribed to the following reactions (17)–(18) (Xu et al. 2014):
As discussed in Sect. 4.2, the generation of sulfides from direct reactions between metal minerals and H2S was not observed under either dry or humid DFW-BC conditions. Therefore, the more generated sulfides should be attributed to the enhanced catalytic H2S oxidation by O2 and metal minerals in the presence of H2O (Choudhury and Lansing 2021). In contrast to the dry biochar conditions under O2, where the catalytic oxidation of Fe was emphasized, H2O should have activated the participation of Ca, Na, K, and Mg in oxidizing H2S. Consequently, an H2O-induced O2 enhancement in the H2S adsorption capacities was rationalized. Calcium was the most abundant on DFW-BC with a content of 146.72 ± 13.71 mg g−1 dw, and was therefore expected to have the most critical role. The catalytic effects of Ca in the presence of O2 + H2O were expressed in reactions (19) − (21) (Bagreev and Bandosz 2005):
4.4 Effect and mechanism of CO2 in interfering with the O2 and H2O promotion
Under dry DFW-BC conditions, CO2 did not significantly inhibit H2S adsorption (via competition for adsorption sites) (Sethupathi et al. 2017). Additionally, no evident difference in the speciation of sulfur products between Fig. 5a, e was observed. When H2O was present, the presence of CO2 in the syngas had an inhibitory effect on H2S removal, which decreased by 44% from that without CO2. When comparing the sulfur speciation between Fig. 5b, f, CO2 induced 3.58% of sulfides that were newly generated while reduced the proportion of S0 from 12.37% to 5.58%. These results indicated that the co-existing CO2 modified the mechanism of H2S removal on humid DFW-BC but not dry DFW-BC.
Generally, CO2 removal on biochar is believed to be physisorption, owing to its textural property (surface area and micropore volume) (Chen et al. 2017; Bamdad et al. 2018; Sethupathi et al. 2017). This process involves gas diffusion in the macropore, mass transfer of gas–solid interface reaction in the mesopore, and CO2 deposition in the micropore. As for increased CO2 physisorption, previous studies demonstrated the benefits of large surface area and micropore volume but smaller pore size via producing biochar at higher pyrolysis temperatures (Creamer et al. 2014), or biochar modification through air activation (Plaza et al. 2014), CO2-ammonia treatment (Zhang et al. 2014), impregnation with sodium lignosulfonate (Zhang et al. 2022), pre-carbonization + KOH impregnation (Song et al. 2014), and so on. The insignificant effect of CO2 presence on H2S adsorption to dry DFW-BC suggested that its pore structure (mainly small mesopores) had advantages over other wood-derived or crop waste-derived biochars in buffering the CO2 competition for adsorption sites (Sethupathi et al. 2017; Huang et al. 2022a). However, surface chemistry, which includes chemisorption through functional groups (Zhang et al. 2014) and mineral carbonation of CO2 (Xu et al. 2020), could play an important role in capturing more CO2. According to Xu et al. (2020), the governing CO2 sorption mechanism was switched from physical adsorption to chemical reaction when the metal content of biochars increased. Since the DFW-BC has a strong alkaline surface with abundant minerals, an alkaline water film could have formed on the carbon surface, leading to a chemisorption competition between H2S and CO2. Mineral carbonation of CO2 occurs in the presence of H2O, with Ca and K as examples through the following reactions (Sitthikhankaew et al. 2014):
The reactions were supported by the decreased sulfur generation from the catalytic effects of metal minerals, but newly produced sulfides, as shown in Fig. 5b vs. f. When comparing the decreased H2S removal under O2 + CO2 + H2O to that under O2 + H2O, the notably increased S0 and sulfite contents were associated with decreased sulfide and sulfate fractions in Fig. 5 (among which soluble sulfate reduced from 6.39 to 1.88 mg g−1). As elaborated in Sect. 4.3, the three mechanisms for O2 enhancing the H2S removal comprised direct oxidation of H2S, generation of more active sites, and catalytic oxidation by Fe oxides. Based on O2 enhancement, the further H2O improvement was attributed to the promoted dissociation of H2S in the alkaline water film and catalytic oxidation by Ca, Na, K, and Mg oxides. However, in the presence of CO2 + H2O, owing to the consumption of metal oxides to form carbonates and bicarbonate by the aforementioned mineral carbonation of CO2, the alkaline surface (OH−) for dissociating H2S was neutralized and the catalytic oxidation of H2S by metal oxides was reduced. Consequently, the further H2O improvement based on the O2 enhancement was partially hindered by the presence of CO2 in the syngas.
4.5 Biochar and sulfur recovery
As for the recovery of active carbons, chemical treatment, solvent washing, microwave, and thermal treatment have all been adopted as regeneration methods (Song et al. 2014). However, given the relatively low cost, wide sources of feedstock, and the environment-friendly nature of biochar, reuse of the spent biochar may be more appealing than its regeneration. Firstly, the spent biochar can be directly applied to land as a source of micro-nutrient in sulfur deficient soils. Zhang et al. (2017) demonstrated that biochar after H2S adsorption significantly increased corn plant biomass by 31–49% and soybean biomass by 4–14% through a 90-day greenhouse study. Additionally, several studies have reported the multiple merits of biochar as a soil amendment for enhanced microbial activities, including methanotrophic activities in landfill cover soil that can mitigate fugitive methane emission from landfills (Reddy et al. 2014). In our previous study, H2S-saturated biochar could still maintain its capacity for improving the methane oxidation efficiency of landfill cover soil (Huang et al. 2022b). The spent biochar can also be secondly utilized for the removal of other pollutants, such as mercury from condensate oil. It is well known that sulfur owns a high affinity for mercury. Thus, H2S sulfurized biochar was investigated for removing ionic mercury from gasoline by Wang et al. (2020). They found that the removal efficiency of H2S-treated tobacco biochar was increased by 87% based on that without H2S treatment, and the inorganic sulfides played a major role through ion exchange with metals. Apparently, these are only a few examples depicting the recovery potential of the spent biochar and its sulfur, and more diverse utilization approaches can be expected.
5 Practical applications and future research prospects
Exploring DFW-BC as an H2S adsorbent is a win–win strategy as it expands the resource utilization options of DFW with less environmental impact and offers an alternative low-cost adsorbent for H2S removal. This study demonstrates DFW-BC as a promising H2S adsorbent. In the future, modification of DFW-BC can be conducted for assessing the improvement in the H2S removal efficacy. For example, physical activation (e.g., through the introduction of steam or CO2) during the pyrolysis process or chemical activation (e.g., through Na2CO3 impregnation of DFW-BC) is worth investigating investigated (Hervy et al. 2018; Pelaez-Samaniego et al. 2018). In scaling up the H2S adsorption system for practical industrial applications, a deep understanding of the operational parameters (H2S concentration, temperature, gas velocity, fixed-bed height, etc.) would also be required for optimized design.
6 Conclusions
In this study, the removal capacities of H2S on DFW-BC with high ash content (70.07%) and abundant metal minerals were investigated. The influence of O2 and H2O on DFW-BC performance and particularly the impact of CO2 chemisorption were explored. By integrating the evolution of biochar properties and speciation of sulfur products, the promotion by O2, H2O, and O2 + H2O in adsorbing and oxidizing H2S on DFW-BC was rationalized. Under dry conditions, the high Fe content of the DFW-BC was beneficial for ensuring O2 enhancement rather than O2 inhibition and surface acidification. In contrast, O2 + H2O induced the catalytic oxidation by those less reactive minerals (mainly Ca) in oxidizing H2S. Unlike wood or crop waste-derived chars, dry DFW-BC displayed no evident CO2 physisorption disturbance; this buffering capacity was attributed to its specific pore structure. For humid DFW-BC, the presence of CO2 partially neutralized the H2O and O2 + H2O enhancement in the H2S removal. This negative effect was due to the CO2 chemisorption through the mineral carbonation on the humid DFW-BC, which consequently hindered the acceleration of H2S dissociation and reduced the activated catalytic oxidation by metal minerals. This study provides new insights into potential CO2 interaction with H2S removal on biochars with high ash content and abundant minerals as opposed to the previously emphasized physisorption competition. The information would also assist in optimizing the desulfuration or deodorization performance of digestate-derived biochars in different scenarios, especially when considerable amounts of CO2 are present in the biogas.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adib F, Bagreev A, Bandosz TJ (2000) Analysis of the relationship between H2S removal capacity and surface properties of unimpregnated activated carbons. Environ Sci Technol 34:686–692. https://doi.org/10.1021/es990341g
Ayiania M, Carbajal-Gamarra FM, Garcia-Perez T, Frear C, Suliman W, Garcia-Perez M (2019) Production and characterization of H2S and PO43− carbonaceous adsorbents from anaerobic digested fibers. Biomass Bioenerg 120:339–349. https://doi.org/10.1016/j.biombioe.2018.11.028
Bagreev A, Bandosz TJ (2005) On the mechanism of hydrogen sulfide removal from moist air on catalytic carbonaceous adsorbents. Ind Eng Chem Res 44:530–538. https://doi.org/10.1021/ie049277o
Bamdad H, Hawboldt K, MacQuarrie S (2018) A review on common adsorbents for acid gases removal: focus on biochar. Renew Sust Energ Rev 81:1705–1720. https://doi.org/10.1016/j.rser.2017.05.261
Bazan-Wozniak A, Nowicki P, Pietrzak R (2017) The influence of activation procedure on the physicochemical and sorption properties of activated carbons prepared from pistachio nutshells for removal of NO2/H2S gases and dyes. J Clean Prod 152:211–222. https://doi.org/10.1016/j.jclepro.2017.03.114
Chen Y, Zhang X, Chen W, Yang H, Chen H (2017) The structure evolution of biochar from biomass pyrolysis and its correlation with gas pollutant adsorption performance. Bioresour Technol 246:101–109. https://doi.org/10.1016/j.biortech.2017.08.138
Choudhury A, Lansing S (2021) Adsorption of hydrogen sulfide in biogas using a novel iron-impregnated biochar scrubbing system. J Environ Chem Eng 9:104837. https://doi.org/10.1016/j.jece.2020.104837
Ciahotný K, Kyselová V (2019) Hydrogen sulfide removal from biogas using carbon impregnated with oxidants. Energy Fuels 33:5316–5321. https://doi.org/10.1021/acs.energyfuels.9b00624
Creamer AE, Gao B, Zhang M (2014) Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem Eng J 249:174–179. https://doi.org/10.1016/j.cej.2014.03.105
Ding Y, Cai C, Hu B, Yu Y, Zheng X, Chen Y, Wu W (2012) Characterization and control of odorous gases at a landfill site: a case study in Hangzhou, China. Waste Manage 32:317–326. https://doi.org/10.1016/j.wasman.2011.07.016
Elsayed Y, Seredych M, Dallas A, Bandosz TJ (2009) Desulfurization of air at high and low H2S concentrations. Chem Eng J 155:594–602. https://doi.org/10.1016/j.cej.2009.08.010
Goel C, Mohan S, Dinesha P (2021) CO2 capture by adsorption on biomass-derived activated char: a review. Sci Total Environ 798:149296. https://doi.org/10.1016/j.scitotenv.2021.149296
Gonçalves DV, Paiva MAG, Oliveira JCA, Bastos-Neto M, Lucena SMP (2018) Prediction of the monocomponent adsorption of H2S and mixtures with CO2 and CH4 on activated carbons. Colloid Surface A 559:342–350. https://doi.org/10.1016/j.colsurfa.2018.09.082
Guo J, Luo Y, Lua AC, Chi R, Chen Y, Bao X, Xiang S (2006) Adsorption of hydrogen sulphide (H2S) by activated carbons derived from oil-palm shell. Carbon 45:330–336. https://doi.org/10.1016/j.carbon.2006.09.016
Guo J, Zheng L, Li Z, Zhou X, Cheng S, Zhang L (2021) Effects of various pyrolysis conditions and feedstock compositions on the physicochemical characteristics of cow manure-derived biochar. J Clean Prod 311:127458. https://doi.org/10.1016/j.jclepro.2021.127458
Gwenzi W, Chaukura N, Wenga T, Mtisi M (2021) Biochars as media for air pollution control systems: contaminant removal, applications and future research directions. Sci Total Environ 753:142249. https://doi.org/10.1016/j.scitotenv.2020.142249
Han X, Chen H, Liu Y, Pan J (2020) Study on removal of gaseous hydrogen sulfide based on macroalgae biochars. J Nat Gas Sci Eng 73:103068. https://doi.org/10.1016/j.jngse.2019.103068
He R, Xia F, Wang J, Pan C, Fang C (2011) Characterization of adsorption removal of hydrogen sulfide by waste biocover soil, an alternative landfill cover. J Hazard Mater 186:773–778. https://doi.org/10.1016/j.jhazmat.2010.11.062
Hervy M, Minh DP, Gérente C, Weiss-Hortala E, Nzihou A, Villot A, Coq LL (2018) H2S removal from syngas using wastes pyrolysis chars. Chem Eng J 334:2179–2189. https://doi.org/10.1016/j.cej.2017.11.162
Huang D, Liang M, Wang N, Xu Q (2022a) The efficiency and mechanism of humidity in alleviating CO2 inhibition on H2S adsorption to straw biochars. Environ Res 210:113008. https://doi.org/10.1016/j.envres.2022.113008
Huang D, Xu W, Wang Q, Xu Q (2022b) Impact of hydrogen sulfide on biochar in stimulating the methane oxidation capacity and microbial communities of landfill cover soil. Chemosphere 286:131650. https://doi.org/10.1016/j.chemosphere.2021.131650
Kailasa SK, Koduru JR, Vikrant K, Tsang YF, Singhal RK, Hussain CM, Kim KH (2020) Recent progress on solution and materials chemistry for the removal of hydrogen sulfide from various gas plants. J Mol Liq 297:111886. https://doi.org/10.1016/j.molliq.2019.111886
Kanjanarong J, Giri BS, Jaisi DP, Oliveira FR, Boonsawang P, Chaiprapat S, Singh RS, Balakrishna A, Khanal SK (2017) Removal of hydrogen sulfide generated during anaerobic treatment of sulfate-laden wastewater using biochar: evaluation of efficiency and mechanisms. Bioresour Technol 234:115–121. https://doi.org/10.1016/j.biortech.2017.03.009
Kumar M, Dutta S, You S, Luo G, Zhang S, Show PL, Sawarkar AD, Singh L, Tsang DCW (2021) A critical review on biochar for enhancing biogas production from anaerobic digestion of food waste and sludge. J Clean Prod 305:127143. https://doi.org/10.1016/j.jclepro.2021.127143
Li D, Chen W, Wu J, Jia CQ, Jiang X (2020) The preparation of waste biomass-derived N-doped carbons and their application in acid gas. J Mater Chem A 8:24977. https://doi.org/10.1039/D0TA07977D
Lin Q, Zhang J, Yin L, Liu H, Zuo W, Tian Y (2021) Relationship between heavy metal consolidation and H2S removal by biochar from microwave pyrolysis of municipal sludge: effect and mechanism. Environ Sci Pollut R 28:27694–27702. https://doi.org/10.1007/s11356-021-12631-4
Ma Q, Chen W, Jin Z, Chen L, Zhou Q, Jiang X (2021) One-step synthesis of microporous nitrogen-doped biochar for efficient removal of CO2 and H2S. Fuel 289:119932. https://doi.org/10.1016/j.fuel.2020.119932
Masebinu SO, Akinlabi ET, Muzenda E, Aboyade AO (2019) A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renew Sust Energ Rev 103:291–307. https://doi.org/10.1016/j.rser.2018.12.048
Meri NH, Alias AB, Talib N, Rashid ZA, Ghani WAWAK (2018) Comparison of H2S adsorption by two hydrogel composite (HBC) derived by empty fruit bunch (EFB) biochar and coal fly ash (CFA). Conf Ser Mater Sci Eng 334:012038. https://doi.org/10.1088/1757-899X/334/1/012038
Moreno-Castilla C (2004) Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 42:83–94. https://doi.org/10.1016/j.carbon.2003.09.022
National Research Council (NRC) (2010) Acute exposure guideline levels for selected airborne chemical. http://www.nap.edu/catalog/12978.html.
Papurello D, Lanzini A, Bressan M, Santarelli M (2020) H2S removal with sorbent obtained from sewage sludges. Processes 8:130. https://doi.org/10.3390/pr8020130
Patel MR, Kailasa SK (2022) Carbon nitride nanomaterials: properties, synthetic approaches and new insights in fluorescence spectrometry for assaying of metal ions, organic and biomolecules. ChemistrySelect 7:e202201849. https://doi.org/10.1002/slct.202201849
Pelaez-Samaniego MR, Smith MW, Zhao Q, Garcia-Perez T, Frear C, Garcia-Perez M (2018) Charcoal from anaerobically digested dairy fiber for removal of hydrogen sulfide within biogas. Waste Manage 76:374–382. https://doi.org/10.1016/j.wasman.2018.03.011
Plaza MG, González AS, Pis JJ, Rubiera F, Pevida C (2014) Production of microporous biochars by single-step oxidation: effect of activation conditions on CO2 capture. Appl Energ 114:551–562. https://doi.org/10.1016/j.apenergy.2013.09.058
Reddy KR, Yargicoglu EN, Yue D, Yaghoubi P (2014) Enhanced microbial methane oxidation in landfill cover soil amended with biochar. J Geotech Geoenviron 140:04014047. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001148
Revabhai PM, Singhal RK, Basu H, Kailasa SK (2022) Progress on boron nitride nanostructure materials: properties, synthesis and applications in hydrogen storage and analytical chemistry. J Nanostruct Chem. https://doi.org/10.1007/s40097-022-00490-5
Ro KS, Woodbury B, Spiehs M, Szogi AA, Silva PJ, Hwang O, Cho S (2021) Pilot-scale H2S and swine odor removal system using commercially available biochar. Agron 11:1611. https://doi.org/10.3390/agronomy11081611
Rodriguez-Navarro C, Ruiz-Agudo E, Luque A, Rodriguez-Navarro AB, Ortega-Huertas MO (2009) Thermal decomposition of calcite: mechanisms of formation and textural evolution of CaO nanocrystals. Am Mineral 94:578–593. https://doi.org/10.2138/am.2009.3021
Sahota S, Vijay VK, Subbarao PMV, Chandra R, Ghosh P, Shan G, Kapoor R, Vijay V, Koutu V, Thakur IS (2018) Characterization of leaf waste based biochar for cost effective hydrogen sulphide removal from biogas. Bioresource Technol 250:635–641. https://doi.org/10.1016/j.biortech.2017.11.093
Sawalha H, Maghalseh M, Qutaina J, Junaidi K, Rene ER (2020) Removal of hydrogen sulfide from biogas using activated carbon synthesized from different locally available biomass wastes-a case study from Palestine. Bioengineered 11:607–618. https://doi.org/10.1080/21655979.2020.1768736
Scheufele FB, da Silva ES, Cazula BB, Marins DS, Sequinel R, Borba CE, Patuzzo GS, Lopez TFM, Alves HJ (2021) Mathematical modeling of low-pressure H2S adsorption by babassu biochar in fixed bed column. J Environ Chem Eng 9:105042. https://doi.org/10.1016/j.jece.2021.105042
Sethupathi S, Zhang M, Rajapaksha AU, Lee SR, Nor NM, Mohamed AR, Al-Wabel M, Lee SS, Ok YS (2017) Biochars as potential adsorbers of CH4, CO2 and H2S. Sustainability-Basel 9:121. https://doi.org/10.3390/su9010121
Shang G, Shen G, Liu L, Chen Q, Xu Z (2013) Kinetics and mechanisms of hydrogen sulfide adsorption by biochars. Bioresource Technol 133:495–499. https://doi.org/10.1016/j.biortech.2013.01.114
Singh B, Singh BP, Cowie AL (2010) Characterisation and evaluation of biochars for their applications a soil amendment. Aust J Soil Res 48:516–525. https://doi.org/10.1071/SR10058
Sitthikhankaew R, Chadwick D, Assabumrungrat S, Laosiripojana N (2014) Effects of humidity, O2, and CO2 on H2S adsorption onto upgraded and KOH impregnated activated carbons. Fuel Process Technol 124:249–257. https://doi.org/10.1016/j.fuproc.2014.03.010
Song J, Shen W, Wang J, Fan W (2014) Superior carbon-based CO2 adsorbents prepared from poplar anthers. Carbon 69:255–263. https://doi.org/10.1016/j.carbon.2013.12.024
Su L, Chen M, Zhuo G, Ji R, Wang S, Zhang L, Zhang M, Li H (2021) Comparison of biochar materials derived from coconut husks and various types of livestock manure, and their potential for use in removal of H2S from biogas. Sustainability-Basel 13:6262. https://doi.org/10.3390/su13116262
Sun Y, Gao B, Yao Y, Fang J, Zhang M, Zhou Y, Chen H, Yang L (2014) Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem Eng J 240:574–578. https://doi.org/10.1016/j.cej.2013.10.081
Sun Y, Zhang J, Wen C, Zhang L (2016) An enhanced approach for biochar preparation using fluidized bed and its application for H2S removal. Chem Eng Process 104:1–12. https://doi.org/10.1016/j.cep.2016.02.006
Sun Y, Yang G, Zhang L, Sun Z (2017) Preparation of high performance H2S removal biochar by direct fluidized bed carbonization using potato peel waste. Process Saf Environ 107:281–288. https://doi.org/10.1016/j.psep.2017.02.018
Sun L, Gong P, Sun Y, Qin Q, Song K, Ye J, Zhang H, Zhou B, Xue Y (2022) Modified chicken manure biochar enhanced the adsorption for Cd2+ in aqueous and immobilization of Cd in contaminated agricultural soil. Sci Total Environ 851:158252. https://doi.org/10.1016/j.scitotenv.2022.158252
Surra E, Nogueira MC, Bernardo M, Lapa N, Esteves I, Fonseca I (2019) New adsorbents from maize cob wastes and anaerobic digestate for H2S removal from biogas. Waste Manage 94:136–145. https://doi.org/10.1016/j.wasman.2019.05.048
Velasco A, Morgan-Sagastume JM, González-Sánchez A (2019) Evaluation of a hybrid physicochemical/biological technology to remove toxic H2S from air with elemental sulfur recovery. Chemosphere 222:732–741. https://doi.org/10.1016/j.chemosphere.2019.02.005
Wang J, Wang J, Zhang Y, Wang T, Pan W (2020) Ionic mercury captured by H2S sulfurized biochar in liquid hydrocarbons: mechanism and stability evaluation. Fuel 278:118413. https://doi.org/10.1016/j.fuel.2020.118413
Wang N, Huang D, Zhang C, Shao M, Chen Q, Liu J, Deng Z, Xu Q (2021) Long-term characterization and resource potential evaluation of the digestate from food waste anaerobic digestion plants. Sci Total Environ 794:148785. https://doi.org/10.1016/j.scitotenv.2021.148785
Wang N, Chen Q, Zhang C, Dong Z, Xu Q (2022a) Improvement in the physicochemical characteristics of biochar derived from solid digestate of food waste with different moisture contents. Sci Total Environ 819:153100. https://doi.org/10.1016/j.scitotenv.2022.153100
Wang N, Huang D, Shao M, Sun R, Xu Q (2022b) Use of activated carbon to reduce ammonia emissions and accelerate humification in composting digestate from food waste. Bioresource Technol 347:126701. https://doi.org/10.1016/j.biortech.2022.126701
Weber K, Quicker P (2018) Properties of biochar. Fuel 217:240–261. https://doi.org/10.1016/j.fuel.2017.12.054
Wu H, Zhu Y, Bian S, Ko JH, Li SFY, Xu Q (2018) H S adsorption by municipal solid waste incineration (MSWI) fly ash with heavy metals immobilization. Chemosphere 195:40–47. https://doi.org/10.1016/j.chemosphere.2017.12.068
Xu X, Cao X, Zhao L, Sun T (2014) Comparison of sewage sludge and pig manure derived biochars for hydrogen sulfide removal. Chemosphere 111:296–303. https://doi.org/10.1016/j.chemosphere.2014.04.014
Xu X, Zhao Y, Sima J, Zhao L, Mašek O, Cao X (2017) Indispensable role of biochar-inherent mineral constituents in its environmental applications: a review. Bioresource Technol 241:887–899. https://doi.org/10.1016/j.biortech.2017.06.023
Xu X, Xu Z, Gao B, Zhao L, Zheng Y, Huang J, Tsang DCW, Ok YS, Cao X (2020) New insights into CO2 sorption on biochar/Fe oxyhydroxide composites: kinetics, mechanisms, and in situ characterization. Chem Eng J 384:123289. https://doi.org/10.1016/j.cej.2019.123289
Yu Q, Li H, Deng Z, Liao X, Liu S, Liu J (2020) Comparative assessment on two full-scale food waste treatment plants with different anaerobic digestion processes. J Clean Prod 263:121625. https://doi.org/10.1016/j.jclepro.2020.121625
Zhang X, Zhang S, Yang H, Feng Y, Chen Y, Wang X, Chen H (2014) Nitrogen enriched biochar modified by high temperature CO2-ammonia treatment: characterization and adsorption of CO2. Chem Eng J 257:20–27. https://doi.org/10.1016/j.cej.2014.07.024
Zhang J, Sun Y, Woo M, Zhang L, Xu K (2016a) Preparation of steam activated carbon from black liquor by flue gas precipitation and its performance in hydrogen sulfide removal: experimental and simulation works. J Taiwan Inst Chem E 59:395–404. https://doi.org/10.1016/j.jtice.2015.09.005
Zhang Z, Wang J, Li W, Wang M, Qiao W, Long D, Ling L (2016b) Millimeter-sized mesoporous carbon spheres for highly efficient catalytic oxidation of hydrogen sulfide at room temperature. Carbon 96:608–615. https://doi.org/10.1016/j.carbon.2015.10.001
Zhang H, Voroney RP, Price GW, White AJ (2017) Sulfur-enriched biochar as a potential soil amendment and fertilizer. Soil Res 55:93–99. https://doi.org/10.1071/SR15256
Zhang X, Cao L, Xiang W, Xu Y, Gao X (2022) Preparation and evaluation of fine-tuned micropore biochar by lignin impregnation for CO2 and VOCs adsorption. Sep Purif Technol 295:121295. https://doi.org/10.1016/j.seppur.2022.121295
Zhu H, Papurello D, Gandiglio M, Lanzini A, Akpinar I, Shearing PR, Manos G, Brett DJL, Zhang Y (2020) Study of H2S removal capability from simulated biogas by using waste-derived adsorbent materials. Processes 8:1030. https://doi.org/10.3390/pr8091030
Acknowledgements
We appreciate the financial support by the National Key R&D Program of China (2018YFC1902903), National Natural Science Foundation of China (22176005), and the Fundamental Research Funds for the Central Universities, Sun Yat-sen University (Project no. 22qntd2701).
Funding
This research was funded by the National Key R&D Program of China (2018YFC1902903), National Natural Science Foundation of China (22176005), and the Fundamental Research Funds for the Central Universities, Sun Yat-sen University (Project no. 22qntd2701).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by NW, XB and YC. The study was supervised and the draft was edited by QX. The first draft of the manuscript was written by DH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Supplementary Information
Additional file 1
: Table S1 Comparisons of biochars produced from different feedstocks and pyrolysis temperatures. Fig. S1 Proportions of sulfur products after H2S adsorption for DFW-BC.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, D., Wang, N., Bai, X. et al. The influencing mechanism of O2, H2O, and CO2 on the H2S removal of food waste digestate-derived biochar with abundant minerals. Biochar 4, 71 (2022). https://doi.org/10.1007/s42773-022-00199-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-022-00199-2