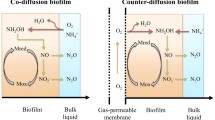
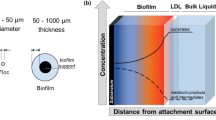
Abstract
Wastewater treatment plants are known to be important point sources for nitrous oxide (N2O) in the anthropogenic N cycle. Biofilm based treatment systems have gained increasing popularity in the treatment of wastewater, but the mechanisms and controls of N2O formation are not fully understood. Here, we review functional groups of microorganism involved in nitrogen (N) transformations during wastewater treatment, with emphasis on potential mechanism of N2O production in biofilms. Biofilms used in wastewater treatment typically harbour aerobic and anaerobic zones, mediating close interactions between different groups of N transforming organisms. Current models of mass transfer and biomass interactions in biofilms are discussed to illustrate the complex regulation of N2O production. Ammonia oxidizing bacteria (AOB) are the prime source for N2O in aerobic zones, while heterotrophic denitrifiers dominate N2O production in anoxic zones. Nitrosative stress ensuing from accumulation of NO2 − during partial nitrification or denitrification seems to be one of the most critical factors for enhanced N2O formation. In AOB, N2O production is coupled to nitrifier denitrification triggered by nitrosative stress, low O2 tension or low pH. Chemical N2O production from AOB intermediates (NH2OH, HNO, NO) released during high NH3 turnover seems to be limited to surface-near AOB clusters, since diffusive mass transport resistance for O2 slows down NH3 oxidation rates in deeper biofilm layers. The proportion of N2O among gaseous intermediates (NO, N2O, N2) in heterotrophic denitrification increases when NO or nitrous acid (HNO2) accumulates because of increasing NO2 −, or when transient oxygen intrusion impairs complete denitrification. Limited electron donor availability due to mass transport limitation of organic substrates into anoxic biofilm zones is another important factor supporting high N2O/N2 ratios in heterotrophic denitrifiers. Biofilms accommodating Anammox bacteria release less N2O, because Anammox bacteria have no known N2O producing metabolism and reduce NO2 − to N2, thereby lowering nitrosative stress to AOB and heterotrophs.




Similar content being viewed by others
References
Ahn JH, Kwan T, Chandran K (2011) Comparison of partial and full nitrification processes applied for treating high-strength Nitrogen wastewaters: microbial ecology through nitrous oxide production. Environ Sci Technol 45:2734–2740. doi:10.1021/es103534g
Anthonisen AC, Loehr RC, Prakasam TBS, Srinath EG (1976) Inhibition of nitrification by ammonia and nitrous-acid. J Water Pollut Control Fed 48:835–852
Arciero DM, Pierce BS, Hendrich MP, Hooper AB (2002) Nitrosocyanin, a red cupredoxin-like protein from Nitrosomonas europaea. Biochemistry 41:1703–1709. doi:10.1021/bi015908w
Arp DJ, Sayavedra-Soto LA, Hommes NG (2002) Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea. Arch Microbiol 178:250–255. doi:10.1007/s00203-002-0452-0
Beaumont HJE et al (2002) Nitrite reductase of Nitrosomonas europaea is not essential for production of gaseous nitrogen oxides and confers tolerance to nitrite. J Bacteriol 184:2557–2560. doi:10.1128/jb.184.9.2557-2560.2002
Beaumont HJE, Lens SI, Reijnders WNM, Westerhoff HV, van Spanning RJM (2004a) Expression of nitrite reductase in Nitrosomonas europaea involves NsrR, a novel nitrite-sensitive transcription repressor. Mol Microbiol 54:148–158. doi:10.1111/j.1365-2958.2004.04248.x
Beaumont HJE, van Schooten B, Lens SI, Westerhoff HV, van Spanning RJM (2004b) Nitrosomonas europaea expresses a nitric oxide reductase during nitrification. J Bacteriol 186:4417–4421. doi:10.1128/jb.186.13.4417-4421.2004
Beaumont HJE, Lens SI, Westerhoff HV, van Spanning RJA (2005) Novel nirK cluster genes in Nitrosomonas europaea are required for NirK-dependent tolerance to nitrite. J Bacteriol 187:6849–6851. doi:10.1128/jb.187.19.6849-6851.2005
Bergaust L, Mao Y, Bakken LR, Frostegård Å (2010) Denitrification response patterns during the transition to anoxic respiration and posttranscriptional effects of suboptimal pH on nitrogen oxide reductase in Paracoccus denitrificans. Appl Environ Microbiol 76:6387–6396. doi:10.1128/aem.00608-10
Bergaust L, van Spanning RJM, Frostegard A, Bakken LR (2012) Expression of nitrous oxide reductase in Paracoccus denitrificans is regulated by oxygen and nitric oxide through FnrP and NNR. Microbiology 158:826–834. doi:10.1099/mic.0.054148-0
Beyer S, Gilch S, Meyer O, Schmidt I (2009) Transcription of genes coding for metabolic key functions in Nitrosomonas europaea during aerobic and anaerobic growth. J Mol Microbiol Biotechnol 16:187–197. doi:10.1159/000142531
Blackburne R, Vadivelu VM, Yuan Z, Keller J (2007) Kinetic characterisation of an enriched Nitrospira culture with comparison to Nitrobacter. Water Res 41:3033–3042. doi:10.1016/j.watres.2007.01.043
Bonin P, Raymond N (1990) Effects of oxygen on denitrification in marine sediments. Hydrobiologia 207:115–122. doi:10.1007/BF00041447
Bothe H, Jost G, Schloter M, Ward BB, Witzel KP (2000) Molecular analysis of ammonia oxidation and denitrification in natural environments. FEMS Microbiol Rev 24:673–690. doi:10.1111/j.1574-6976.2000.tb00566.x
Bougard D, Bernet N, Chèneby D, Delgenès JP (2006) Nitrification of a high-strength wastewater in an inverse turbulent bed reactor: effect of temperature on nitrite accumulation. Process Biochem 41:106–113. doi:10.1016/j.procbio.2005.03.064
Bremner JM, Blackmer AM, Waring SA (1980) Formation of nitrous oxide and dinitrogen by chemical decomposition of hydroxylamine in soils. Soil Biol Biochem 12:263–269. doi:10.1016/0038-0717(80)90072-3
Brenzinger K, Dörsch P, Braker G (2015) pH-driven shifts in overall and transcriptionally active denitrifiers control gaseous product stoichiometry in growth experiments with extracted bacteria from soil. Front Microbiol. doi:10.3389/fmicb.2015.00961
Brockmann D, Rosenwinkel KH, Morgenroth E (2008) Practical identifiability of biokinetic parameters of a model describing two-step nitrification in biofilms. Biotechnol Bioeng 101:497–514. doi:10.1002/bit.21932
Campos JL, Arrojo B, Vázquez-Padín JR, Mosquera-Corral A, Méndez R (2009) N2O production by nitrifying biomass under anoxic and aerobic conditions. Appl Biochem Biotechnol 152:189–198. doi:10.1007/s12010-008-8215-2
Casciotti KL, Ward BB (2001) Dissimilatory nitrite reductase genes from autotrophic ammonia-oxidizing bacteria. Appl Environ Microbiol 67:2213–2221. doi:10.1128/aem.67.5.2213-2221.2001
Castro-Barros CM, Rodríguez-Caballero A, Volcke EIP, Pijuan M (2016) Effect of nitrite on the N2O and NO production on the nitrification of low-strength ammonium wastewater. Chem Eng J 287:269–276. doi:10.1016/j.cej.2015.10.121
Cema G, Plaza E, Trela J, Surmacz-Gorska J (2011) Dissolved oxygen as a factor influencing nitrogen removal rates in a one-stage system with partial nitritation and Anammox process. Water Sci Technol 64:1009–1015. doi:10.2166/wst.2011.449
Chain P et al (2003) Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J Bacteriol 185:2759–2773. doi:10.1128/jb.185.9.2759-2773.2003
Chandran K, Stein LY, Klotz MG, van Loosdrecht MC (2011) Nitrous oxide production by lithotrophic ammonia-oxidizing bacteria and implications for engineered nitrogen-removal systems. Biochem Soc Trans 39:1832–1837. doi:10.1042/bst20110717
Christensson M, Ekstrom S, Chan AA, Le Vaillant E, Lemaire R (2013) Experience from start-ups of the first ANITA Mox plants. Water Sci Technol 67:2677–2684. doi:10.2166/wst.2013.156
Ciudad G, Werner A, Bornhardt C, Muñoz C, Antileo C (2006) Differential kinetics of ammonia- and nitrite-oxidizing bacteria: a simple kinetic study based on oxygen affinity and proton release during nitrification. Process Biochem 41:1764–1772. doi:10.1016/j.procbio.2006.03.032
Colliver BB, Stephenson T (2000) Production of nitrogen oxide and dinitrogen oxide by autotrophic nitrifiers. Biotechnol Adv 18:219–232. doi:10.1016/s0734-9750(00)00035-5
Cua LS, Stein LY (2011) Effects of nitrite on ammonia-oxidizing activity and gene regulation in three ammonia-oxidizing bacteria. FEMS Microbiol Lett 319:169–175. doi:10.1111/j.1574-6968.2011.02277.x
Daims H et al (2015) Complete nitrification by nitrospira bacteria. Nature. doi:10.1038/nature16461
Derlon N, Coufort-Saudejaud C, Queinnec I, Paul E (2013) Growth limiting conditions and denitrification govern extent and frequency of volume detachment of biofilms. Chem Eng J 218:368–375. doi:10.1016/j.cej.2012.11.061
Dörsch P, Braker G, Bakken LR (2012) Community specific pH response of denitrification: experiments with cells extracted from organic soils. FEMS Microbiol Ecol 79:530–541
Dulekgurgen E, Dogruel S, Karahan Ö, Orhon D (2006) Size distribution of wastewater COD fractions as an index for biodegradability. Water Res 40:273–282
Ekama GA, Wentzel MC (2008) Nitrogen Removal. In: Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (eds) Biological wastewater treatment. IWA Publishing, London, pp 33–52
Eldyasti A, Nakhla G, Zhu J (2014) Influence of biofilm thickness on nitrous oxide (N2O) emissions from denitrifying fluidized bed bioreactors (DFBBRs). J Biotechnol 192(Part A):281–290. doi:10.1016/j.jbiotec.2014.10.008
Elenter D, Milferstedt K, Zhang W, Hausner M, Morgenroth E (2007) Influence of detachment on substrate removal and microbial ecology in a heterotrophic/autotrophic biofilm. Water Res 41:4657–4671. doi:10.1016/j.watres.2007.06.050
Freeman JP (1973) Less familiar reactions of oximes. Chem Rev 73:283–292. doi:10.1021/cr60284a001
Frunzke K, Zumft WG (1986) Inhibition of nitrous-oxide respiration by nitric oxide in the denitrifying bacterium Pseudomonas perfectomarina. Biochim Biophys Acta (BBA)-Bioenerg 852:119–125. doi:10.1016/0005-2728(86)90064-2
Fux C, Huang D, Monti A, Siegrist H (2004a) Difficulties in maintaining long-term partial nitritation of ammonium-rich sludge digester liquids in a moving-bed biofilm reactor (MBBR). Water Sci Technol 49:53–60
Fux C, Marchesi V, Brunner I, Siegrist H (2004b) Anaerobic ammonium oxidation of ammonium-rich waste streams in fixed-bed reactors. Water Sci Technol 49:77–82
Gujer W, Henze M, Mino T, van Loosdrecht M (1999) Activated sludge model no. 3. Water Sci Technol 39:183–193. doi:10.1016/s0273-1223(98)00785-9
Güven D et al (2005) Propionate oxidation by and Methanol inhibition of anaerobic ammonium-oxidizing bacteria. Appl Environ Microbiol 71:1066–1071. doi:10.1128/aem.71.2.1066-1071.2005
Hao X, Heijnen JJ, Van Loosdrecht MCM (2001) Sensitivity analysis of a biofilm model describing a one-stage completely autotrophic nitrogen removal (CANON) process. Biotechnol Bioeng 77:266–277. doi:10.1002/bit.10105
Harper WF, Terada A, Poly F, Le Roux X, Kristensen K, Mazher M, Smets BF (2009) The effect of hydroxylamine on the activity and aggregate structure of autotrophic nitrifying bioreactor cultures. Biotechnol Bioeng 102:714–724. doi:10.1002/bit.22121
Hellinga C, Schellen A, Mulder JW, van Loosdrecht MCM, Heijnen JJ (1998) The SHARON process: an innovative method for nitrogen removal from ammonium-rich waste water. Water Sci Technol 37:135–142. doi:10.1016/s0273-1223(98)00281-9
Hellinga C, Van Loosdrecht MCM, Heijnen JJ (1999) Model based design of a novel process for nitrogen removal from concentrated flows. Math Computer Model Dyn Syst 5:351–371. doi:10.1076/mcmd.5.4.351.3678
Henze M, Comeau Y (2008) Wastewater characterization. In: Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (eds) Biological wastewater treatment. IWA Publishing, London, pp 33–52
Henze M, Grady CPL, Gujer W, Marais GvR, Matsuo T (1987) Activated sludge model no. 1. IAWPRC Scientific and Technical Report No. 1. IAWPRC, London
Hooper AB (1968) A nitrite-reducing enzyme from Nitrosomonas europaea-preliminary characterization with hydroxylamine as electron donor. Biochim Biophys Acta 162:49–65. doi:10.1016/0005-2728(68)90213-2
Hooper AB, Terry KR (1979) Hydroxylamine oxidoreductase of nitrosomonas production of nitric-oxide from hydroxylamine. Biochim Biophys Acta 571:12–20. doi:10.1016/0005-2744(79)90220-1
Horn H, Reiff H, Morgenroth E (2003) Simulation of growth and detachment in biofilm systems under defined hydrodynamic conditions. Biotechnol Bioeng 81:607–617. doi:10.1002/bit.10503
Hu ZY, Lotti T, van Loosdrecht M, Kartal B (2013) Nitrogen removal with the anaerobic ammonium oxidation process. Biotechnol Lett 35:1145–1154. doi:10.1007/s10529-013-1196-4
Hyman MR, Arp DJ (1995) Effects of ammonia on the de novo synthesis of polypeptides in cells of Nitrosomonas europaea denied ammonia as an energy source. J Bacteriol 177:4974–4979
Itokawa H, Hanaki K, Matsuo T (2001) Nitrous oxide production in high-loading biological nitrogen removal process under low COD/N ratio condition. Water Res 35:657–664. doi:10.1016/s0043-1354(00)00309-2
Jason J, Cantera L, Stein LY (2007) Role of nitrite reductase in the ammonia-oxidizing pathway of Nitrosomonas europaea. Arch Microbiol 188:349–354. doi:10.1007/s00203-007-0255-4
Jetten MSM et al (1998) The anaerobic oxidation of ammonium. FEMS Microbiol Rev 22:421–437. doi:10.1111/j.1574-6976.1998.tb00379.x
Jia WL, Liang S, Zhang J, Ngo HH, Guo WS, Yan YJ, Zou YN (2013) Nitrous oxide emission in low-oxygen simultaneous nitrification and denitrification process: sources and mechanisms. Bioresour Technol 136:444–451. doi:10.1016/j.biortech.2013.02.117
Jiang QQ, Bakken LR (1999) Nitrous oxide production and methane oxidation by different ammonia-oxidizing bacteria. Appl Environ Microbiol 65:2679–2684
Jin RC, Yang GF, Yu JJ, Zheng P (2012) The inhibition of the Anammox process: a review. Chem Eng J 197:67–79. doi:10.1016/j.cej.2012.05.014
Kaelin D, Manser R, Rieger L, Eugster J, Rottermann K, Siegrist H (2009) Extension of ASM3 for two-step nitrification and denitrification and its calibration and validation with batch tests and pilot scale data. Water Res 43:1680–1692. doi:10.1016/j.watres.2008.12.039
Kampschreur MJ, Tan NCG, Picioreanu C, Jetten MSM, Schmidt I, van Loosdrecht MCM (2006) Role of nitrogen oxides in the metabolism of ammonia-oxidizing bacteria. Biochem Soc Trans 34:179–181
Kampschreur MJ, van der Star WRL, Wielders HA, Mulder JW, Jetten MSM, van Loosdrecht MCM (2008) Dynamics of nitric oxide and nitrous oxide emission during full-scale reject water treatment. Water Res 42:812–826. doi:10.1016/j.watres.2007.08.022
Kampschreur MJ et al (2009a) Emission of nitrous oxide and nitric oxide from a full-scale single-stage nitritation-anammox reactor. Water Sci Technol 60:3211–3217. doi:10.2166/wst.2009.608
Kampschreur MJ, Temmink H, Kleerebezem R, Jetten MSM, van Loosdrecht MCM (2009b) Nitrous oxide emission during wastewater treatment. Water Res 43:4093–4103. doi:10.1016/j.watres.2009.03.001
Kartal B, Kuypers MM, Lavik G, Schalk J, Op den Camp HJM, Jetten MSM, Strous M (2007a) Anammox bacteria disguised as denitrifiers: nitrate reduction to dinitrogen gas via nitrite and ammonium. Environ Microbiol 9:635–642. doi:10.1111/j.1462-2920.2006.01183.x
Kartal B et al (2007b) Candidatus “Anammoxoglobus propionicus” a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Syst Appl Microbiol 30:39–49. doi:10.1016/j.syapm.2006.03.004
Kartal B, Tan NCG, Van de Biezen E, Kampschreur MJ, Van Loosdrecht MCM, Jetten MSM (2010) Effect of nitric oxide on anammox bacteria. Appl Environ Microbiol 76:6304–6306. doi:10.1128/aem.00991-10
Kartal B et al (2011) Molecular mechanism of anaerobic ammonium oxidation. Nature 479:127–130. doi:10.1038/nature10453
Kartal B, de Almeida NM, Maalcke WJ, Op den Camp HJM, Jetten MSM, Keltjens JT (2013) How to make a living from anaerobic ammonium oxidation. FEMS Microbiol Rev 37:428–461. doi:10.1111/1574-6976.12014
Kester RA, De Boer W, Laanbroek HJ (1997) Production of NO and N2O by pure cultures of nitrifying and denitrifying bacteria during changes in aeration. Appl Environ Microbiol 63:3872–3877
Khan MZ, Mondal PK, Sabir S (2013) Aerobic granulation for wastewater bioremediation: a review. Can J Chem Eng 91:1045–1058. doi:10.1002/cjce.21729
Kim CH, Hollocher TC (1984) Catalysis of nitrosyl transfer reactions by a dissimilatory nitrite reductase (cytochrome c, d1). J Biol Chem 259:2092–2099
Kim SW, Miyahara M, Fushinobu S, Wakagi T, Shoun H (2010) Nitrous oxide emission from nitrifying activated sludge dependent on denitrification by ammonia-oxidizing bacteria. Bioresour Technol 101:3958–3963. doi:10.1016/j.biortech.2010.01.030
Kindaichi T, Ito T, Okabe S (2004a) Ecophysiological interaction between nitrifying bacteria and heterotrophic bacteria in autotrophic nitrifying biofilms as determined by microautoradiography-fluorescence in situ hybridization. Appl Environ Microbiol 70:1641–1650. doi:10.1128/aem.70.3.1641-1650.2004
Kindaichi T, Okabe S, Satoh H, Watanabe Y (2004b) Effects of hydroxylamine on microbial community structure and function of autotrophic nitrifying biofilms determined by in situ hybridization and the use of microelectrodes. Water Sci Technol 49:61–68
Kong Q, Zhang J, Miao M, Tian L, Guo N, Liang S (2013) Partial nitrification and nitrous oxide emission in an intermittently aerated sequencing batch biofilm reactor. Chem Eng J 217:435–441. doi:10.1016/j.cej.2012.10.093
Körner H, Zumft WG (1989) Expression of denitrification enzymes in response to the dissolved oxygen level and respiratory substrate in continuous culture of Pseudomonas stutzeri. Appl Environ Microbiol 55:1670–1676
Kostera J, Youngblut MD, Slosarczyk JM, Pacheco AA (2008) Kinetic and product distribution analysis of NO center dot reductase activity in Nitrosomonas europaea hydroxylamine oxidoreductase. J Biol Inorg Chem 13:1073–1083. doi:10.1007/s00775-008-0393-4
Kostera J, McGarry J, Pacheco AA (2010) Enzymatic interconversion of ammonia and nitrite: the right tool for the job. Biochemistry 49:8546–8553. doi:10.1021/bi1006783
Kozlowski JA, Price J, Stein LY (2014) Revision of N2O-producing pathways in the ammonia-oxidizing bacterium Nitrosomonas europaea ATCC 19718. Appl Environ Microbiol 80:4930–4935. doi:10.1128/aem.01061-14
Kuenen JG (2008) Anammox bacteria: from discovery to application. Nat Rev Microbiol 6:320–326
Lackner S, Horn H (2012) Comparing the performance and operation stability of an SBR and MBBR for single-stage nitritation-anammox treating wastewater with high organic load. Environ Technol 34:1319–1328. doi:10.1080/09593330.2012.746735
Law Y, Lant P, Yuan ZG (2011) The effect of pH on N2O production under aerobic conditions in a partial nitritation system. Water Res 45:5934–5944. doi:10.1016/j.watres.2011.08.055
Law Y, Lant P, Yuan Z (2013) The confounding effect of nitrite on N2O production by an enriched ammonia-oxidizing culture. Environ Sci Technol 47:7186–7194. doi:10.1021/es4009689
Law Y, Ni BJ, Lant P, Yuan ZG (2012a) N2O production rate of an enriched ammonia-oxidising bacteria culture exponentially correlates to its ammonia oxidation rate. Water Res 46:3409–3419. doi:10.1016/j.watres.2012.03.043
Law Y, Ye L, Pan Y, Yuan Z (2012b) Nitrous oxide emissions from wastewater treatment processes. Philos Trans R Soc Lond B Biol Sci 367:1265–1277. doi:10.1098/rstb.2011.0317
Liu B, Frostegård Å, Bakken LR (2014) Impaired reduction of N2O to N2 in acid soils is due to a posttranscriptional interference with the expression of nosZ. MBio. doi:10.1128/mBio.01383-14
Lo IW, Lo KV, Mavinic DS, Shiskowski D, Ramey W (2010) Contributions of biofilm and suspended sludge to nitrogen transformation and nitrous oxide emission in hybrid sequencing batch system. J Environ Sci 22:953–960. doi:10.1016/S1001-0742(09)60204-7
Lochmatter S, Gonzalez-Gil G, Holliger C (2013) Optimized aeration strategies for nitrogen and phosphorus removal with aerobic granular sludge. Water Res 47:6187–6197. doi:10.1016/j.watres.2013.07.030
Lotti T, van der Star WRL, Kleerebezem R, Lubello C, van Loosdrecht MCM (2012) The effect of nitrite inhibition on the anammox process. Water Res 46:2559–2569. doi:10.1016/j.watres.2012.02.011
Lotti T, Kleerebezem R, Lubello C, van Loosdrecht MCM (2014) Physiological and kinetic characterization of a suspended cell anammox culture. Water Res 60:1–14. doi:10.1016/j.watres.2014.04.017
Ma Y, Sundar S, Park H, Chandran K (2015) The effect of inorganic carbon on microbial interactions in a biofilm nitritation–anammox process. Water Res 70:246–254. doi:10.1016/j.watres.2014.12.006
Maia LB, Moura JJG (2014) How biology handles nitrite. Chem Rev 114:5273–5357. doi:10.1021/cr400518y
Morgenroth E (2008a) Modelling biofilm. In: Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (eds) Biological wastewater treatment. IWA Publishing, London, pp 457–492
Morgenroth E (2008b) Biofilm reactors. In: Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (eds) Biological wastewater treatment. IWA Publishing, London, pp 457–492
Morgenroth E, Wilderer PA (2000) Influence of detachment mechanisms on competition in biofilms. Water Res 34:417–426. doi:10.1016/S0043-1354(99)00157-8
Morley N, Baggs EM, Dörsch P, Bakken L (2008) Production of NO, N2O and N2 by extracted soil bacteria, regulation by NO2 − and O2 concentrations. FEMS Microbiol Ecol 65:102–112. doi:10.1111/j.1574-6941.2008.00495.x
Mulder A, Vandegraaf AA, Robertson LA, Kuenen JG (1995) Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiol Ecol 16:177–183. doi:10.1111/j.1574-6941.1995.tb00281.x
Ni B-J, Yuan Z (2013) A model-based assessment of nitric oxide and nitrous oxide production in membrane-aerated autotrophic nitrogen removal biofilm systems. J Membr Sci 428:163–171. doi:10.1016/j.memsci.2012.10.049
Ni B-J, Ruscalleda M, Pellicer-Nàcher C, Smets BF (2011) Modeling nitrous oxide production during biological nitrogen removal via nitrification and denitrification: extensions to the general ASM models. Environ Sci Technol 45:7768–7776. doi:10.1021/es201489n
Ni B-J, Smets BF, Yuan Z, Pellicer-Nàcher C (2013) Model-based evaluation of the role of anammox on nitric oxide and nitrous oxide productions in membrane aerated biofilm reactor. J Membr Sci 446:332–340. doi:10.1016/j.memsci.2013.06.047
Ni BJ, Peng L, Law Y, Guo J, Yuan Z (2014) Modeling of nitrous oxide production by autotrophic ammonia-oxidizing bacteria with multiple production pathways. Environ Sci Technol 48:3916–3924. doi:10.1021/es405592h
Okabe S, Satoh H, Watanabe Y (1999) In situ analysis of nitrifying biofilms as determined by in situ hybridization and the use of microelectrodes. Appl Environ Microbiol 65:3182–3191
Pan YT, Ni BJ, Yuan ZG (2013) Modeling electron competition among nitrogen oxides reduction and N2O accumulation in denitrification. Environ Sci Technol 47:11083–11091. doi:10.1021/es402348n
Park S, Bae W (2009) Modeling kinetics of ammonium oxidation and nitrite oxidation under simultaneous inhibition by free ammonia and free nitrous acid. Process Biochem 44:631–640. doi:10.1016/j.procbio.2009.02.002
Park KY, Inamori Y, Mizuochi M, Ahn KH (2000) Emission and control of nitrous oxide from a biological wastewater treatment system with intermittent aeration. J Biosci Bioeng 90:247–252. doi:10.1016/S1389-1723(00)80077-8
Peng L, Ni B-J, Erler D, Ye L, Yuan Z (2014) The effect of dissolved oxygen on N2O production by ammonia-oxidizing bacteria in an enriched nitrifying sludge. Water Res 66:12–21. doi:10.1016/j.watres.2014.08.009
Peng L, Ni B-J, Ye L, Yuan Z (2015a) The combined effect of dissolved oxygen and nitrite on N2O production by ammonia oxidizing bacteria in an enriched nitrifying sludge. Water Res 73:29–36. doi:10.1016/j.watres.2015.01.021
Peng L, Ni BJ, Ye L, Yuan Z (2015b) N2O production by ammonia oxidizing bacteria in an enriched nitrifying sludge linearly depends on inorganic carbon concentration. Water Res 74:58–66. doi:10.1016/j.watres.2015.02.003
Peng L, Liu Y, Ni B-J (2016a) Nitrous oxide production in completely autotrophic nitrogen removal biofilm process: a simulation study. Chem Eng J 287:217–224. doi:10.1016/j.cej.2015.11.026
Peng L, Ni B-J, Law Y, Yuan Z (2016b) Modeling N2O production by ammonia oxidizing bacteria at varying inorganic carbon concentrations by coupling the catabolic and anabolic processes. Chem Eng Sci 144:386–394. doi:10.1016/j.ces.2016.01.033
Pijuan M, Torà J, Rodríguez-Caballero A, César E, Carrera J, Pérez J (2014) Effect of process parameters and operational mode on nitrous oxide emissions from a nitritation reactor treating reject wastewater. Water Res 49:23–33. doi:10.1016/j.watres.2013.11.009
Poth M, Focht DD (1985) N-15 kinetic-analysis of N2O production by Nitrosomonas europaea-an examination of nitrifier dentrification. Appl Environ Microbiol 49:1134–1141
Poughon L, Dussap C-G, Gros J-B (2001) Energy model and metabolic flux analysis for autotrophic nitrifiers. Biotechnol Bioeng 72:416–433. doi:10.1002/1097-0290(20000220)72:4<416:AID-BIT1004>3.0.CO;2-D
Quinlan AV (1984) Prediction of the optimum pH for ammonia-n oxidation by Nitrosomonas europaea in well-aerated natural and domestic-waste waters. Water Res 18:561–566. doi:10.1016/0043-1354(84)90204-5
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous Oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125. doi:10.1126/science.1176985
Reichert P (1994) AQUASIM-a tool for simulation and data-analysis of aquatic systems. Water Sci Technol 30:21–30
Rezić T, Šantek B, Novak S, Marić V (2007) Heterotrophic cultivation of Paracoccus denitrificans in a horizontal rotating tubular bioreactor. World J Microbiol Biotechnol 23:987–996. doi:10.1007/s11274-006-9324-0
Richardson D, Felgate H, Watmough N, Thomson A, Baggs E (2009) Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle-could enzymic regulation hold the key? Trends Biotechnol 27:388–397. doi:10.1016/j.tibtech.2009.03.009
Rittmann BE, Regan JM, Stahl DA (1994) Nitrification as a source of soluble organic substrate in biological treatment. Water Sci Technol 30:1–8
Sabba F, Picioreanu C, Pérez J, Nerenberg R (2015) Hydroxylamine diffusion can enhance N2O emissions in nitrifying biofilms: a modeling study. Environ Sci Technol 49:1486–1494. doi:10.1021/es5046919
Schmid M et al (2000) Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst Appl Microbiol 23:93–106. doi:10.1016/S0723-2020(00)80050-8
Schmidt I (2008) Nitric oxide: interaction with the ammonia monooxygenase and regulation of metabolic activities in ammonia oxidizers. In: Cadenas E, Packer L (eds) Nitric oxide, Part F: oxidative and nitrosative stress in redox regulation of cell signaling, vol 440. Methods in Enzymology. Elsevier Academic Press Inc, San Diego, pp 121–135. doi:10.1016/s0076-6879(07)00807-5
Schmidt I (2009) Chemoorganoheterotrophic growth of Nitrosomonas europaea and Nitrosomonas eutropha. Curr Microbiol 59:130–138. doi:10.1007/s00284-009-9409-8
Schmidt I, Bock E (1997) Anaerobic ammonia oxidation with nitrogen dioxide by Nitrosomonas eutropha. Arch Microbiol 167:106–111. doi:10.1007/s002030050422
Schmidt I, Bock E (1998) Anaerobic ammonia oxidation by cell-free extracts of Nitrosomonas eutropha Antonie Van Leeuwenhoek. Int J Gen Mol Microbiol 73:271–278. doi:10.1023/a:1001572121053
Schmidt I, Bock E, Jetten MSM (2001a) Ammonia oxidation by Nitrosomonas eutropha with NO2 as oxidant is not inhibited by acetylene. Microbiology-Sgm 147:2247–2253
Schmidt I, Zart D, Bock E (2001b) Effects of gaseous NO2 on cells of Nitrosomonas eutropha previously incapable of using ammonia as an energy source Antonie Van Leeuwenhoek. Int J Gen Mol Microbiol 79:39–47. doi:10.1023/a:1010269331350
Schmidt I, Steenbakkers PJM, Op den Camp HJM, Schmidt K, Jetten MSM (2004a) Physiologic and proteomic evidence for a role of nitric oxide in biofilm formation by Nitrosomonas europaea and other ammonia oxidizers. J Bacteriol 186:2781–2788. doi:10.1128/jb.186.9.2781-2788.2004
Schmidt I, van Spanning RJM, Jetten MSM (2004b) Denitrification and ammonia oxidation by Nitrosomonas europaea wild-type, and NirK- and NorB-deficient mutants. Microbiology-Sgm 150:4107–4114. doi:10.1099/mic.0.27382-0
Schramm A, de Beer D, van den Heuvel JC, Ottengraf S, Amann R (1999) Microscale distribution of populations and activities of Nitrosospira and Nitrospira spp. along a macroscale gradient in a nitrifying bioreactor: quantification by in situ hybridization and the use of microsensors. Appl Environ Microbiol 65:3690–3696
Schreiber F, Loeffler B, Polerecky L, Kuypers MMM, de Beer D (2009) Mechanisms of transient nitric oxide and nitrous oxide production in a complex biofilm. ISME J 3:1301–1313. doi:10.1038/ismej.2009.55
Schreiber F, Wunderlin P, Udert KM, Wells GF (2012) Nitric oxide and nitrous oxide turnover in natural and engineered microbial communities: biological pathways, chemical reactions, and novel technologies. Front Microbiol 3:24. doi:10.3389/fmicb.2012.00372
Schulthess RV, Kühni M, Gujer W (1995) Release of nitric and nitrous oxides from denitrifying activated sludge. Water Res 29:215–226. doi:10.1016/0043-1354(94)E0108-I
Shaw LJ, Nicol GW, Smith Z, Fear J, Prosser JI, Baggs EM (2006) Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway. Environ Microbiol 8:214–222. doi:10.1111/j.1462-2920.2005.00882.x
Shen T, Stieglmeier M, Dai J, Urich T, Schleper C (2013) Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol Lett 344(2):121–129. doi:10.1111/1574-6968.12164
Sliekers AO, Haaijer SCM, Stafsnes MH, Kuenen JG, Jetten MSM (2005) Competition and coexistence of aerobic ammonium- and nitrite-oxidizing bacteria at low oxygen concentrations. Appl Microbiol Biotechnol 68:808–817. doi:10.1007/s00253-005-1974-6
Spieck E, Lipski A (2011) Chapter five-cultivation, growth physiology, and chemotaxonomy of Nitrite-Oxidizing bacteria. In: Martin GK (ed) Methods in enzymology, vol 486. Academic Press, London, pp 109–130. doi:10.1016/B978-0-12-381294-0.00005-5
Spott O, Russow R, Stange CF (2011) Formation of hybrid N2O and hybrid N2 due to codenitrification: first review of a barely considered process of microbially mediated N-nitrosation. Soil Biol Biochem 43:1995–2011. doi:10.1016/j.soilbio.2011.06.014
Starkenburg SR, Arp DJ, Bottomley PJ (2008) Expression of a putative nitrite reductase and the reversible inhibition of nitrite-dependent respiration by nitric oxide in Nitrobacter winogradskyi Nb-255. Environ Microbiol 10:3036–3042. doi:10.1111/j.1462-2920.2008.01763.x
Stein LY, Arp DJ (1998) Loss of ammonia monooxygenase activity in Nitrosomonas europaea upon exposure to nitrite. Appl Environ Microbiol 64:4098–4102
Strous M et al (1999a) Missing lithotroph identified as new planctomycete. Nature 400:446–449
Strous M, Kuenen JG, Jetten MSM (1999b) Key physiology of anaerobic ammonium oxidation. Appl Environ Microbiol 65:3248–3250
Stuven R, Bock E (2001) Nitrification and denitrification as a source for NO and NO2 production in high-strength wastewater. Water Res 35:1905–1914. doi:10.1016/s0043-1354(00)00471-1
Sundermeyer-Klinger H, Meyer W, Warninghoff B, Bock E (1984) Membrane-bound nitrite oxidoreductase of Nitrobacter: evidence for a nitrate reductase system. Arch Microbiol 140:153–158. doi:10.1007/BF00454918
Suzuki I, Dular U, Kwok SC (1974) Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J Bacteriol 120:556–558
Tallec G, Garnier J, Gousailles M (2006) Nitrogen removal in a wastewater treatment plant through biofilters: nitrous oxide emissions during nitrification and denitrification. Bioprocess Biosyst Eng 29:323–333. doi:10.1007/s00449-006-0081-0
Todt D, Dörsch P (2015) Nitrous oxide emissions in a biofilm loaded with different mixtures of concentrated household wastewater. Int J Environ Sci Technol. doi:10.1007/s13762-015-0778-1
Udert KM, Jenni S (2013) Biological nitrogen conversion processes. In: Larsen TA, Udert KM, Lienert J (eds) Source separation and decentralization for wastewater management. IWA Publishing, London, pp 291–305
Udert KM, Larsen TA, Gujer W (2005) Chemical nitrite oxidation in acid solutions as a consequence of microbial ammonium oxidation. Environ Sci Technol 39:4066–4075. doi:10.1021/es048422m
Upadhyay AK, Hooper AB, Hendrich MP (2006) NO reductase activity of the tetraheme cytochrome C(554) of Nitrosomonas europaea. J Am Chem Soc 128:4330–4337. doi:10.1021/ja055183+
van de Graaf AA, Mulder A, de Bruijn P, Jetten MS, Robertson LA, Kuenen JG (1995) Anaerobic oxidation of ammonium is a biologically mediated process. Appl Environ Microbiol 61:1246–1251
van Kessel MAHJ et al (2015) Complete nitrification by a single microorganism. Nature. doi:10.1038/nature16459
Wang CC, Lee PH, Kumar M, Huang YT, Sung SW, Lin JG (2010) Simultaneous partial nitrification, anaerobic ammonium oxidation and denitrification (SNAD) in a full-scale landfill-leachate treatment plant. J Hazard Mater 175:622–628. doi:10.1016/j.jhazmat.2009.10.052
Wanner O, Gujer W (1985) Competition in biofilms. Water Sci Technol 17:27–44
Wanner O, Gujer W (1986) A multispecies biofilm model. Biotechnol Bioeng 28:314–328. doi:10.1002/bit.260280304
Wanner O, Reichert P (1996) Mathematical modeling of mixed-culture biofilms. Biotechnol Bioeng 49:172–184
Wanner O, Eberl HJ, Morgenroth E, Noguera DR, Picioreanu C, Rittmann BE, van Loosdrecht MCM (2006) Mathematical modeling of biofilms. IWA Publishing, London
Whittaker M, Bergmann D, Arciero D, Hooper AB (2000) Electron transfer during the oxidation of ammonia by the chemolithotrophic bacterium Nitrosomonas europaea. Biochim Biophys Acta-Bioenerg 1459:346–355. doi:10.1016/s0005-2728(00)00171-7
Winkler MKH, Kleerebezem R, van Loosdrecht MCM (2012a) Integration of anammox into the aerobic granular sludge process for main stream wastewater treatment at ambient temperatures. Water Res 46:136–144. doi:10.1016/j.watres.2011.10.034
Winkler MKH, Yang J, Kleerebezem R, Plaza E, Trela J, Hultman B, van Loosdrecht MCM (2012b) Nitrate reduction by organotrophic anammox bacteria in a nitritation/anammox granular sludge and a moving bed biofilm reactor. Bioresour Technol 114:217–223. doi:10.1016/j.biortech.2012.03.070
Wood PM (1986) Nitrification as a bacterial energy source. In: Prosser JI (ed) Nitrification. Society for general microbiology. IRL Press, Oxford, pp 39–62
Wunderlin P, Mohn J, Joss A, Emmenegger L, Siegrist H (2012) Mechanisms of N2O production in biological wastewater treatment under nitrifying and denitrifying conditions. Water Res 46:1027–1037. doi:10.1016/j.watres.2011.11.080
Wunderlin P, Lehmann MF, Siegrist H, Tuzson B, Joss A, Emmenegger L, Mohn J (2013) Isotope signatures of N2O in a mixed microbial population system: constraints on N2O producing pathways in wastewater treatment. Environ Sci Technol 47:1339–1348. doi:10.1021/es303174x
Xiao Y, Wu S, Yang Z-H, Wang Z-J, Yan C-Z, Zhao F (2013) In situ probing the effect of potentials on the microenvironment of heterotrophic denitrification biofilm with microelectrodes. Chemosphere 93:1295–1300. doi:10.1016/j.chemosphere.2013.06.065
Yamanaka T, Shinra M (1974) Cytochrome C-552 and cytochrome C-554 derived from Nitrosomonas-Europaes-purification, properties, and their function in hydroxylamine oxidation. J Biochem 75:1265–1273
Yang JJ, Trela J, Plaza E, Tjus K (2013) N2O emissions from a one stage partial nitrification/anammox process in moving bed biofilm reactors. Water Sci Technol 68:144–152. doi:10.2166/wst.2013.232
Yoo H, Ahn K-H, Lee H-J, Lee K-H, Kwak Y-J, Song K-G (1999) Nitrogen removal from synthetic wastewater by simultaneous nitrification and denitrification (SND) via nitrite in an intermittently-aerated reactor. Water Res 33:145–154. doi:10.1016/S0043-1354(98)00159-6
Yoshinari T (1990) Emissions of N2O from various environments—the use of stable isotope composition of N2O as tracer for the studies of N2O biogeochemical cycling. In: Revsbech N, Sørensen J (eds) Denitrification in soil and sediment, vol 56. Federation of European microbiological societies symposium series. Springer, US, pp 129–150. doi:10.1007/978-1-4757-9969-9_8
Yu R, Chandran K (2010) Strategies of Nitrosomonas europaea 19718 to counter low dissolved oxygen and high nitrite concentrations. BMC Microbiol 10:11. doi:10.1186/1471-2180-10-70
Yu R, Kampschreur MJ, van Loosdrecht MCM, Chandran K (2010) Mechanisms and specific directionality of autotrophic nitrous oxide and nitric oxide generation during transient anoxia. Environ Sci Technol 44:1313–1319. doi:10.1021/es902794a
Zhang DJ, Cai Q, Zu B, Bai C, Zhang P (2010) The influence of trace NO(2) on the kinetics of ammonia oxidation and the characteristics of nitrogen removal from wastewater. Water Sci Technol 62:1037–1044. doi:10.2166/wst.2010.319
Zhou Y, Pijuan M, Yuan Z (2008a) Development of a 2-sludge, 3-stage system for nitrogen and phosphorous removal from nutrient-rich wastewater using granular sludge and biofilms. Water Res 42:3207–3217. doi:10.1016/j.watres.2008.04.012
Zhou Y, Pijuan M, Zeng RJ, Yuan Z (2008b) Free nitrous acid inhibition on nitrous oxide reduction by a denitrifying-enhanced biological phosphorus removal sludge. Environ Sci Technol 42:8260–8265. doi:10.1021/es800650j
Zhou Y, Oehmen A, Lim M, Vadivelu V, Ng WJ (2011) The role of nitrite and free nitrous acid (FNA) in wastewater treatment plants. Water Res 45:4672–4682. doi:10.1016/j.watres.2011.06.025
Zumft WG (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61:533–616
Acknowledgments
This study was supported by the Norwegian Research Council (Grant No skattefunn-226774) and Ecomotive AS, the present employer of the corresponding author. We thank Linda Hink (University of Aberdeen) for reviewing the chapter on AOB metabolism.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Todt, D., Dörsch, P. Mechanism leading to N2O production in wastewater treating biofilm systems. Rev Environ Sci Biotechnol 15, 355–378 (2016). https://doi.org/10.1007/s11157-016-9401-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-016-9401-2