Abstract
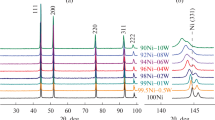
A method for the synthesis of microdispersed Fe–Pd (0–10 atom% Pd) alloys with a porous morphology has been proposed. The synthesis is based on reductive thermolysis of specially prepared single-source precursors. According to XRD data, the phase composition of the obtained alloys is represented by solid solutions based on a body-centered crystal lattice (bcc, Im-3 m) of iron. Samples of Fe–Pd alloys have a branched 3D microstructure formed by grains 0.5–1 µm in size interconnected by bridges. The effect of palladium concentration on the catalytic performance of the synthesized Fe–Pd alloys in the decomposition of saturated C2–C4 hydrocarbons to form carbon nanofibers (CNF) was explored. It was shown that Fe–Pd alloys exhibit a 10–40 times higher catalytic activity compared to pure iron. The carbon yield increases with increasing the palladium content. The produced nanomaterial, represented by carbon nanostructured fibers (CNFs) of 0.1–0.5 µm in diameter, has a low bulk density (40–60 g/L) and a high specific surface area (150–300 m2/g). The resulting metal–carbon material is prospective as a catalytic support, sorbent, or modifying additive in composite materials.










Similar content being viewed by others
Data Availability
Data is contained within the article.
References
Wang Z, Liu P, Han J et al (2017) Engineering the internal surfaces of three-dimensional nanoporous catalysts by surfactant-modified dealloying. Nat Commun 8:1066. https://doi.org/10.1038/s41467-017-01085-3
Xu Y, Zhang B (2014) Recent advances in porous Pt-based nanostructures: synthesis and electrochemical applications. Chem Soc Rev 43:2439. https://doi.org/10.1039/c3cs60351b
Hou C, Shi X-M, Zhao C-X et al (2014) SnO 2 nanoparticles embedded in 3D nanoporous/solid copper current collectors for high-performance reversible lithium storage. J Mater Chem A 2:15519. https://doi.org/10.1039/C4TA02604G
Zhao D, Xu C (2015) A nanoporous palladium-nickel alloy with high sensing performance towards hydrogen peroxide and glucose. J Colloid Interface Sci 447:50–57. https://doi.org/10.1016/j.jcis.2015.01.053
Lu L, Kang J (2018) Amperometric nonenzymatic sensing of glucose at very low working potential by using a nanoporous PdAuNi ternary alloy. Microchim Acta 185:111. https://doi.org/10.1007/s00604-017-2665-6
Solovyev AA, Rabotkin SV, Shipilova AV et al (2015) Solid oxide fuel cell with Ni–Al support. Int J Hydrogen Energy 40:14077–14084. https://doi.org/10.1016/j.ijhydene.2015.07.151
Wang X, Jia L, Li K et al (2018) Porous nickel-iron alloys as anode support for intermediate temperature solid oxide fuel cells: II. Cell performance and stability. Int J Hydrog Energy 43:21030–21036. https://doi.org/10.1016/j.ijhydene.2018.09.142
Lu L (2019) Nanoporous noble metal-based alloys: a review on synthesis and applications to electrocatalysis and electrochemical sensing. Microchim Acta 186:664. https://doi.org/10.1007/s00604-019-3772-3
Zhu W, Zhu G, Yao C et al (2020) Porous amorphous FeCo alloys as pre-catalysts for promoting the oxygen evolution reaction. J Alloys Compd 828:154465. https://doi.org/10.1016/j.jallcom.2020.154465
Xiao C, Li Y, Lu X, Zhao C (2016) Bifunctional porous NiFe/NiCo2O4 /Ni foam electrodes with triple hierarchy and double synergies for efficient whole cell water splitting. Adv Funct Mater 26:3515–3523. https://doi.org/10.1002/adfm.201505302
Li W, Hu Q, Liu Y et al (2020) Powder metallurgy synthesis of porous Ni-Fe alloy for oxygen evolution reaction and overall water splitting. J Mater Sci Technol 37:154–160. https://doi.org/10.1016/j.jmst.2019.06.021
Lin C, Zhang P, Wang S et al (2020) Engineered porous Co–Ni alloy on carbon cloth as an efficient bifunctional electrocatalyst for glucose electrolysis in alkaline environment. J Alloys Compd 823:153784. https://doi.org/10.1016/j.jallcom.2020.153784
Hu B, Yu J, Meng J et al (2022) Porous Ni–Cu alloy dendrite anode catalysts with high activity and selectivity for direct borohydride fuel cells. ACS Appl Mater Interfaces 14:3910–3918. https://doi.org/10.1021/acsami.1c15671
Avisar S, Shner Y, Abu-Reziq R et al (2022) Catalytically active nano-porous cobalt-palladium alloys. J Alloys Compd 891:161936. https://doi.org/10.1016/j.jallcom.2021.161936
Bauman YI, Mishakov IV, Rudneva YV et al (2020) Catalytic synthesis of segmented carbon filaments via decomposition of chlorinated hydrocarbons on Ni–Pt alloys. Catal Today 348:102–110. https://doi.org/10.1016/j.cattod.2019.08.015
Bauman YI, Rudneva YV, Mishakov IV et al (2019) Effect of Mo on the catalytic activity of Ni-based self-organizing catalysts for processing of dichloroethane into segmented carbon nanomaterials. Heliyon 5:e02428. https://doi.org/10.1016/j.heliyon.2019.e02428
Bauman YI, Mishakov IV, Vedyagin AA et al (2017) Promoting effect of Co, Cu, Cr and Fe on activity of Ni-Based alloys in catalytic processing of chlorinated hydrocarbons. Top Catal 60:171–177. https://doi.org/10.1007/s11244-016-0729-1
Rudneva YV, Shubin YV, Plyusnin PE et al (2019) Preparation of highly dispersed Ni1−xPdx alloys for the decomposition of chlorinated hydrocarbons. J Alloys Compd 782:716–722. https://doi.org/10.1016/j.jallcom.2018.12.207
Popov AA, Shubin YV, Bauman YI et al (2020) Preparation of porous Co-Pt alloys for catalytic synthesis of carbon nanofibers. Nanotechnology 31:495604. https://doi.org/10.1088/1361-6528/abb430
Ruiz-Cornejo JC, Sebastián D, Lázaro MJ (2020) Synthesis and applications of carbon nanofibers: a review. Rev Chem Eng 36:493–511. https://doi.org/10.1515/revce-2018-0021
Kablov EN, Kondrashov SV, Yurkov GY (2013) Prospects of using carbonaceous nanoparticles in binders for polymer composites. Nanotechnol Russ 8:163–185. https://doi.org/10.1134/S1995078013020080
Potylitsyna AR, Mishakov IV, Bauman YI et al (2022) Metal dusting as a key route to produce functionalized carbon nanofibers. Reac Kinet Mech Cat 135:1387–1404. https://doi.org/10.1007/s11144-022-02169-y
Bauman YI, Sigaeva SS, Mishakov IV et al (2017) Pyrolysis of 1,2-dichloroethane over Ni–Cr catalyst at resistive heating. Reac Kinet Mech Cat 120:691–701. https://doi.org/10.1007/s11144-017-1138-6
Grabke HJ (2003) Metal dusting Mater Corros 54:736–746. https://doi.org/10.1002/maco.200303729
Krishnankutty N, Rodriguez NM, Baker RTK (1996) Effect of copper on the decomposition of ethylene over an iron catalyst. J Catal 158:217–227. https://doi.org/10.1006/jcat.1996.0021
Rodriguez NM, Kim MS, Fortin F et al (1997) Carbon deposition on iron-nickel alloy particles. Appl Catal A Gen 148:265–282. https://doi.org/10.1016/S0926-860X(96)00142-1
Reshetenko TV, Avdeeva LB, Khasin AA et al (2004) Coprecipitated iron-containing catalysts (Fe-Al2O3, Fe-Co-Al2O3, Fe-Ni-Al2O3) for methane decomposition at moderate temperaturesI. Genesis of calcined and reduced catalysts. Appl Catal A Gen 268:127–138. https://doi.org/10.1016/j.apcata.2004.03.045
McCue I, Benn E, Gaskey B, Erlebacher J (2016) Dealloying and dealloyed materials. Annu Rev Mater Res 46:263–286. https://doi.org/10.1146/annurev-matsci-070115-031739
Kränzlin N, Niederberger M (2015) Controlled fabrication of porous metals from the nanometer to the macroscopic scale. Mater Horizons 2:359–377. https://doi.org/10.1039/c4mh00244j
Qiu X, Dai Y, Zhu X et al (2016) Template-engaged synthesis of hollow porous platinum–palladium alloy nanospheres for efficient methanol electro-oxidation. J Power Sour 302:195–201. https://doi.org/10.1016/j.jpowsour.2015.10.065
Malgras V, Ji Q, Kamachi Y et al (2015) Templated synthesis for nanoarchitectured porous materials. Bull Chem Soc Jpn 88:1171–1200. https://doi.org/10.1246/bcsj.20150143
Rebbecchi TA, Chen Y (2018) Template-based fabrication of nanoporous metals. J Mater Res 33:2–15. https://doi.org/10.1557/jmr.2017.383
Bauman YI, Kutaev NV, Plyusnin PE et al (2017) Catalytic behavior of bimetallic Ni–Fe systems in the decomposition of 1,2-dichloroethane. Effect of iron doping and preparation route. React Kinet Mech Catal 121:413–423. https://doi.org/10.1007/s11144-017-1180-4
Afonnikova SD, Popov AA, Bauman YI et al (2022) Porous Co-Pt nanoalloys for production of carbon nanofibers and composites. Materials (Basel) 15:7456. https://doi.org/10.3390/ma15217456
Gamal A, Eid K, El-Naas MH et al (2021) Catalytic methane decomposition to carbon nanostructures and COx-free hydrogen: a mini-review. Nanomaterials 11:1226. https://doi.org/10.3390/nano11051226
Zhou L, Enakonda LR, Harb M et al (2017) Fe catalysts for methane decomposition to produce hydrogen and carbon nano materials. Appl Catal B Environ 208:44–59. https://doi.org/10.1016/j.apcatb.2017.02.052
Venediktov AB, Korenev SV, Khranenko SP et al (2007) Properties of nitric acid palladium solutions with a high metal concentration. Russ J Appl Chem 80:695–704. https://doi.org/10.1134/S1070427207050023
PDF-2. Powder Diffraction Files Database (2003), International center for Diffraction Data: Newtown Square, Pennsylvania, USA. Accessed 19 Jan 2023
Kraus W, Nolze G (1996) POWDER CELL—a program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J Appl Crystallogr 29:301–303. https://doi.org/10.1107/S0021889895014920
Seto H, Noda Y, Yamada Y (1990) Precursor phenomena at martensitic phase transition in Fe–Pd Alloy. I. Two-Tetragonal-mixed phase and crest-riding-periodon. J Phys Soc Japan 59:965–977. https://doi.org/10.1143/JPSJ.59.965
Buschbeck J, Hamann S, Ludwig A et al (2010) Correlation of phase transformations and magnetic properties in annealed epitaxial Fe–Pd magnetic shape memory alloy films. J Appl Phys 107:113919. https://doi.org/10.1063/1.3383055
Hultgren R, Zapffe CA (1938) The crystal structures of the iron-palladium superlattices. Zeitschrift für Krist - Cryst Mater 99:509–512. https://doi.org/10.1524/zkri.1938.99.1.509
Jääskeläinen J, Suoninen E (1981) Structure and ordering of solid solutions of iron in palladium. Phys Status Solidi 63:241–245. https://doi.org/10.1002/pssa.2210630132
Vlasova NI, Gaviko VS, Popov AG et al (2010) Phase transformations in ferromagnetic nanostructured FePd alloy under severe plastic deformation and annealing. Solid State Phenom 168–169:392–395. https://doi.org/10.4028/www.scientific.net/SSP.168-169.392
Zhang SL, Sumiyama K, Nakamura Y (1988) Magnetic properties of nonequilibrium bcc and fcc Fe–Pd alloys produced by vapor quenching. J Magn Magn Mater 73:58–64. https://doi.org/10.1016/0304-8853(88)90168-0
Krumm S (1996) An Interactive windows program for profile fitting and size/strain analysis. Mater Sci Forum 228–231:183–190. https://doi.org/10.4028/www.scientific.net/MSF.228-231.183
Wojdyr M (2010) Fityk: a general-purpose peak fitting program. J Appl Crystallogr 43:1126–1128. https://doi.org/10.1107/S0021889810030499
Burton BP (1990) Binary Alloy Phase Diagrams, 2nd edn. ASM International, Materials Park, Ohio, pp. 1749–51.
Mishakov IV, Chesnokov VV, Buyanov RA, Pakhomov NA (2001) Decomposition of chlorinated hydrocarbons on iron-group metals. Kinet Catal 42:543–548. https://doi.org/10.1023/A:1010585808978
Mishakov IV, Bauman YI, Korneev DV, Vedyagin AA (2013) Metal Dusting as a route to produce active catalyst for processing chlorinated hydrocarbons into carbon nanomaterials. Top Catal 56:1026–1032. https://doi.org/10.1007/s11244-013-0066-6
Potylitsyna AR, Rudneva YV, Bauman YI et al (2023) Efficient production of segmented carbon nanofibers via catalytic decomposition of trichloroethylene over Ni–W catalyst. Materials 16:845. https://doi.org/10.3390/ma16020845
Vautard F, Ozcan S, Paulauskas F et al (2012) Influence of the carbon fiber surface microstructure on the surface chemistry generated by a thermo-chemical surface treatment. Appl Surf Sci 261:473–480. https://doi.org/10.1016/j.apsusc.2012.08.038
Couzi M, Bruneel J-L, Talaga D, Bokobza L (2016) A multi wavelength Raman scattering study of defective graphitic carbon materials: The first order Raman spectra revisited. Carbon N Y 107:388–394. https://doi.org/10.1016/j.carbon.2016.06.017
Dippel B, Jander H, Heintzenberg J (1999) NIR FT Raman spectroscopic study of flame soot. Phys Chem Chem Phys 1:4707–4712. https://doi.org/10.1039/a904529e
Acknowledgements
Characterization of the samples was performed using the equipment of the Center of Collective Use “National Center of Catalysts Research” and the Krasnoyarsk Regional Center of the Collective Use. The authors are grateful to A.N. Serkova for the performed SEM studies, M.N. Volochaev for the TEM results, B.A. Kolesov for the Raman spectroscopy data, T.E. Guselnikova and N.F. Beizel for performing the analysis by the ICP-AES method.
Funding
The work was financially supported by the Russian Science Foundation (project No. 21–13-00414, https://rscf.ru/project/21-13-00414/, NIIC SB RAS).
Author information
Authors and Affiliations
Contributions
IVM, AAV and YVS: Conceptualization AAP, SDA, ADV, YIB, and PEP: methodology AAP, SDA, ADV, YIB, and PEP: investigation YIB, and PEP: resources AAP, SDA, ADV: writing—original draft preparation YVS, IVM, and AAV: writing—review and editing AAP, SDA, and ADV: visualization YVS, and IVM: supervision YVS: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Popov, A.A., Afonnikova, S.D., Varygin, A.D. et al. Synthesis and catalytic activity of porous Fe–Pd alloys in the decomposition of C2–C4 hydrocarbons. Reac Kinet Mech Cat 137, 323–338 (2024). https://doi.org/10.1007/s11144-023-02549-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02549-y