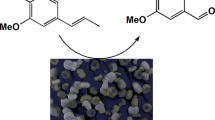
Abstract
Aldol condensation of 4-isopropylbenzaldehyde and propanal was carried out using functionalized MCM-41. The aldol condensations are usually performed using acidic or basic catalysts and due to this fact, the modification of MCM-41 was realized using post-grafting method by two acid groups (3-propylsulfoxy, 3-propylcarboxy) and one basic (the 3-(1,2-diethylamino)propyl) group. MCM-41 treated by sulfuric and nitric acid was also used. The prepared functionalized materials were characterized by BET, elemental analysis and UV–Vis spectroscopy. The optimal reaction conditions were found: the temperature of reaction mixture 100 °C, the molar ratio of reactants 4 isopropylbenzaldehyde:propanal = 1:2, the amount and the type of the catalyst: 50 wt% of MCM SO3H (compared to the amount of 4-isopropylbenzadehyde), the addition time of propanal 90 min and suitable solvent for the reaction was toluene. Using the optimal reaction conditions the yield of forcyclamenaldehyde was 45 %. The obtained results were compared to homogeneous catalysis with H2SO4 where the yield was 15 %.










Similar content being viewed by others
References
Huchel U, Bunn R, Materne M, Faber W, Smyrek H, Pierik T, Rittler F, Bauer A, Dischmann M, Sauf S, Preis-Amberger D (2010) DE102009001570A1, Henkel AG & Co
Shiaou SY, Ko A (2006) J Chin Chem Soc 54:1539–1545
Knorr A, Weissenborn A (1932) US1844013, Winthrop chemical company
Meuly WC (1937) US2102965A, E. I. du Pont de Nemours Company
Yang LM, Wang YJ, Luo GS, Dai YY (2005) Microporous Mesoporous Mater 84:275–282
Climent M (2001) J Catal 197:385–393
Climent M (1998) J Catal 175:70–79
Sharmaa SK, Parikhb PA, Jasraa RV (2010) Appl Catal A 386:34–42
Yadav GD, Aduri P (2012) J Mol Catal A: Chem 355:142–154
W.M. Van Rhijn, D.E. De Vos, B.F. Sels, W.D. Bossaert, A. Jacobs, Chemical Communications (1998) 317–318
Zhang Z, Pittman CU Jr, Sui S, Sun J, Wang Q (2013) Energies 6:1568–1589
Ziarani GM, Badoeib A, Abbasi A, Farahani Z (2009) Chin J Chem 27:1537–1542
Paterova I, Vyskocilova E, Cerveny L (2012) Top Catal 55(11–13):873–879
Mahdavinia GH, Amani AM, Sepehrian HC (2012) J. Chem 30:703–708
Maleki A, Javanshir S, Sharifi S (2014) Curr Chem Lett 3:125–132
Lusticka I, Vrbkova E, Vyskocilova E, Paterova I, Cerveny L (2013) Reac Kinet Mech Cat 108(1):205–212
Lusticka I, Vyskocilova-Leitmannova E, Cerveny L (2012) Chem Listy 107(2):114–120
Procopio A, De Luca G, Nardi M, Oliverio M, Paonessa R (2009) Green Chem 11:770–773
Pasqua L, Procopio A, Oliverio M, Paonessa R, Prete R, Nardi M, Casula MF, Testa F, Nagy JB (2013) J Porous Mater 20:865–873
Chen H, Wang Y (2002) Ceram Int 28:541–547
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Nature 359:710–712
Carpenter MS, E.W. M.(1959) US2875131A, The Givaudan Company, 1959
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vrbková, E., Vyskočilová, E. & Červený, L. Functionalized MCM-41 as a catalyst for the aldol condensation of 4-isopropylbenzaldehyde and propanal. Reac Kinet Mech Cat 114, 675–684 (2015). https://doi.org/10.1007/s11144-014-0811-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-014-0811-2