Abstract
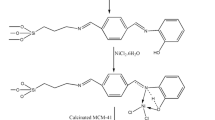
A new mixed ligand Ni complex of phenolic Schiff base and 2,2’ bipyridine was implanted on MCM-41 to prepare a simple and reusable catalyst. The designed heterogeneous catalyst has been characterized by FT-IR, PXRD analysis, N2 adsorption and desorption, SEM–EDX, TEM, ICP-OES and TGA. The synthesized Ni-MCM-41 is utilized as a potential catalyst against the Suzuki cross-coupling reaction of a wide range of aryl/alkyl/allyl halides with aryl boronic acid in DMF: water/water under microwave irradiation with the yield up to 95 / 86% of the catalytic product. Moreover, short reaction time, easy workup, eco-friendly, low cost and reusability of catalyst up to four times without a noteworthy loss of its activity are some of the merits of this method. The hot filtration test has been ratified that there was no leaching of metal during the catalytic reaction, indicating the heterogeneous manner of the catalyst.











Similar content being viewed by others
References
N. Kambe, T. Iwasaki, J. Terao, Chem. Soc. Rev. 40, 4937 (2011)
H. Doucet, Eur J Org Chem 2008(12), 2013 (2008)
F. Gonza’lez-Bobes, G.C. Fu, J. Am. Chem. Soc. 128, 5360 (2006)
S. Roslina, L.R. Odell, Chem. Commun 53, 6895 (2017)
J. Hassan, M. Sévignon, C. Gozzi, E. Schulz, M. Lemaire, Chem. Rev 102, 1359 (2002)
S.P. Stanforth, Tetrahedron 54, 263 (1998)
A. Suzuki, J. Organomet. Chem. 653, 83 (2002)
C.E. Tucker, J.G. de Vries, Top. Catal. 19, 111 (2002)
B.M. Choudary, S. Madhi, N.S. Chowdari, M.L. Kantam, B. Sreedhar, J. Am. Chem. Soc. 124, 14127 (2002)
F.S. Han, Chem. Soc. Rev. 42, 5270 (2013)
J.K. Ryan, M.T. Johne Meynard, D.S. Mark, K.V. Aaron, Organometallics 38, 2007 (2019)
Y.L. Zhao, Y. Li, S.M. Li, Y.G. Zhou, F.Y. Sun, L.X. Gao, F.S. Han, Adv. Synth. Catal. 353, 1543 (2011)
V.M. Chernyshev, V.P. Ananikov, ACS. Catal. 12, 1180 (2022)
V. Percec, J.Y. Bae, D.H. Hill, J. Org. Chem. 60, 1060 (1995)
D.G. Yu, M. Yu, B.T. Guan, B.J. Li, Y. Zheng, Z.H. Wu, Z.J. Shi, Org. Lett. 11, 3374 (2009)
P. Maity, D.M. Shacklady-Mcatee, G.P.A. Yap, E.R. Sirianni, M.P. Watson, J. Am. Chem. Soc. 135, 280 (2013)
M. Tobisu, T. Shimasaki, N. Chatani, Angew. Chem. Int. Ed. 120, 4944 (2008)
K.T. Sylvester, K. Wu, A.G. Doyle, J. Am. Chem. Soc. 134, 16967 (2012)
S.Q. Zhang, B.L.H. Taylor, C.L. Ji, Y. Gao, M.R. Harris, L.E. Hanna, E.R. Jarvo, K.N. Houk, X. Hong, J. Am. Chem. Soc. 139, 12994 (2017)
Z.Y. Xu, H.Z. Yu, Y. Fu, Chem. Asian J. 12, 1765 (2017)
M. Tobisu, T. Xu, T. Shimasaki, N. Chatani, J. Am. Chem. Soc. 133, 19505 (2011)
M.M. Beromi, A. Nova, D. Balcells, A.M. Brasacchio, G.W. Brudvig, L.M. Guard, N. Hazari, D.J. Vinyard, J. Am. Chem. Soc. 139, 922 (2017)
A.A. Finch, T. Blackburn, V. Snieckus, J. Am. Chem. Soc. 131, 17750 (2009)
J. Jo, Q. Tu, R. Xiang, G. Li, L. Zou, K.M. Maloney, H. Ren, J.A. Newman, X. Gong, X. Bu, Organometallics 38, 185 (2019)
P.K. Pandey, A.K. Sharma, S. Rani, G. Mishra, G. Kandasamy et al., ACS. Biomater. Sci. Eng. 8, 2860 (2018)
F.J. Carmona, I.J. Amezcua, S. Rojas, C.C. Romao, J.A.R. Navarro, C.R. Maldonado, E. Barea, Inorg. Chem. 56, 10474 (2017)
L. Lv, K. Wang, X.S. Zhao, J. Colloid. Interface. Sci. 305, 218 (2007)
J. Demel, M. Lamac, J. Cejka, P. Stepnicka, ChemSusChem 2, 442 (2009)
B. Munoz, A. Ramila, J.P. Pariente, I. Dıaz, M. Vallet-Regı, Chem. Mater 15, 500 (2003)
M.V. Regi, A. Ramila, R.P. Del Real, J.P. ariente, Chem. Mater 13, 308 (2001)
M. Yoshikazu, Y. Masanori, A. Eiichi, A. Sadao, T. Shunsuke, Ind. Eng. Chem. Res. 48, 938 (2009)
F. Havasi, A.G. Choghamarani, F. Nikpoura, New J. Chem. 39, 6504 (2015)
A. Corma, A. Martınez, V. Martınez-Soria, J. CataL 169, 480 (1997)
A. Bruckmann, A. Krebs, C. Bolm, Green. Chem. 10, 1131 (2008)
F.A. Bassyouni, S.M. Abu-Bakr, M. Abdel Rehim, Res. Chem. Intermed. 38, 283 (2012)
R.S. Varma, Green. Chem. 10, 1129 (2008)
A.R. Hajipour, F. Rafiee, Org. Prep. Proced. Int. 45, 465 (2013)
A. Cohen, M.D. Crozet, P. Rathelot, P. Vanelle, Green. Chem. 11, 1736 (2009)
D.L. Martins, H.M. Alvarez, L.C.S. Aguiar, Tetrahedron. Lett. 51, 6814 (2010)
K.L. Santos Castro, P.G. Lima, L.S.M. Miranda, R.O.M. Souza, Tetrahedron. Lett. 52, 4168 (2011)
N. Fresneau, M.A. Hiebel, L.A. Agrofoglio, S.B. Raboin, Molecules 17, 14409 (2012)
C. Chena, L. Ming Yang, Tetrahedron. Lett. 48, 2427 (2007)
M.M. Talukder, J.M.O. Cue, J.T. Miller, P.L. Gamage, A. Aslam et al., ACS Omega 37, 24018 (2020)
Y. Li, Y. Luo, L. Peng et al., Nat. Commun. 11, 417 (2020)
A. Ghorbani-Choghamarani, M. Mohammadi, Z. Taherinia, J. Iran. Chem. Soc. 16, 411 (2019)
M. Mohammadi, A. Ghorbani-Choghamarani, RSC. Adv. 6, 32653 (2016)
A. Ghorbani-Choghamarani, M. Mohammadi, R.H.E. Hudson, T. Tamoradi, Appl. Organomet. Chem. 33, e4977 (2019)
T. Tamoradi, S. Masoumeh Mousavi, M. Mohammadi, ChemistrySelect 5, 5077 (2020)
Q.X. Liu, K.Q. Cai, Z.X. Zhao, RSC. Adv. 5, 85568 (2015)
G. Vinoth, S. Indira, M. Bharathi, A. Nandhakumar, K. Sathishkumar, K. Shanmuga Bharathi, J. Coord. Chem. 71, 3934 (2018)
M. Bharathi, S. Indira, G. Vinoth, T. Mahalakshmi, E. Induja, K. Shanmuga Bharathi, J. Coord. Chem. 73, 653 (2020)
M. Mureseanu, V. Parvulescu, R. Ene, N. Cioatera, T.D. Pasatoiu, M. Andruh, J. Mater. Sci. 44, 6795 (2009)
M. Nikoorazm, A.G. Choghamarani, M. Khanmoradi, RSC. Adv. 6, 56549 (2016)
V.G. Deshmane, R.Y. Abrokwah, D. Kuila, Int. J. Hydrog. Energy 40, 10439 (2015)
S. Urus, M. Dolaz, M. Tumer, J. Inorg. Organomet. Polym. 20, 706 (2010)
M. Nikoorazm, A.G. Choghamarani, M. Khanmoradi, Appl. Organomet. Chem. 30, 236 (2016)
J. Wei, D.B. Ravn, L. Gram, P. Kingshott, Colloids. Surf. B. 32, 275 (2003)
M.S. Niasari, M. Bazarganipour, Appl. Surf. Sci. 255, 2963 (2008)
S. Wei, Z. Ma, P. Wang, Z. Dong, J. Ma, J. Mol. Catal. A: Chem. 370, 175 (2013)
P.P. Adrien, P. Luca Alessandro, C. Ilaria, G. Laurence, ACS. Catal. 8, 4812 (2018)
A.J.J. Lennox, G.C. Lloyd-Jones, Chem. Soc. Rev. 43, 412 (2014)
R. Nejat, A. Reza Mahjoub, Z. Hekmatian, T. Azadbakht, RSC. Adv. 5, 16029 (2015)
S. Das, S. Bhunia, T. Maity, S. Koner, J. Mol. Catal. A: Chem. 394, 188 (2014)
M. Nikoorazm, M. Khanmoradi, Z. Abdi, J. Iran. Chem. Soc. 17, 2577 (2020)
T. Mahamo, M.M. Mogorosi, J.R. Moss, S.F. Mapolie, J.C. Slootweg, K. Lammertsma, G.S. Smith, J. Organomet. Chem. 703, 34 (2012)
A. Zarnegaryan, Z. Dehbanipour, D. Elhamifar, Polyhedron 170, 530 (2019)
A. Ahmadi, T. Sedaghat, R. Azadi, H. Motamedi, Catal. Lett. 150, 112 (2020)
K. Dhara, K. Sarkar, D. Srimani, S. Kumar Saha, P. Chattopadhyay, A. Bhaumik, Dalton. Trans. 39, 6395 (2010)
A.R. Hajipour, K. Karami, A. Pirisedigh, Inorg. Chim. Acta. 370, 531 (2011)
G.G.F. Rojas, L.G. Sebastian, R.R. Martinez, B.A.A. Castillo, S.H. Ortega, D.M. Morales, Polyhedron 185, 114601 (2020)
Acknowledgements
The authors are grateful for the financial support from the University Grants Commission (UGC-BSR) F.30-319/2016 (BSR) Government of India, New Delhi, India. The first author acknowledges to University Grant Commission-Rajiv Gandhi National Fellowship (UGC-RGNF) (Grant No: RGNF-2015-17-SC-TAM-21024) research grant for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors openly express there is no conflict of interest in the submission of the current work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bharathi, M., Indira, S., Vinoth, G. et al. Implanted mixed ligand Ni complex of phenolic Schiff base and 2, 2’ bipyridine on MCM-41 as an efficient catalyst for Suzuki–Miyaura cross-coupling reactions: a greener approach. Res Chem Intermed 48, 3701–3719 (2022). https://doi.org/10.1007/s11164-022-04786-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04786-7