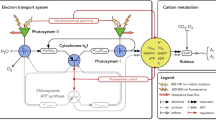
Abstract
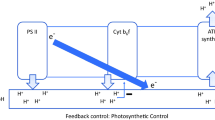
Life-long efforts of the Tartu photosynthesis research group have been summarized. The measurements were facilitated by self-designed instruments, distinct in multifunctionality and fastresponse time. The black-box type kinetical analysis on intact leaves has revealed several physiologically significant features of leaf photosynthesis. Rubisco studies reflected competition for the active site between the substrates and products, linearizing in vivo kinetics compared with the low-Km in vitro responses. Rubisco Activase usually activates only a small part of the Rubisco, making the rest of it a storage protein. Precisely quantifying absorbed photons and the responding transmittance changes, electron flow rates through cytochrome b6f, plastocyanin and photosystem I were measured, revealing competition between the proton-uncoupled cyclic electron flow from PSI to Cyt b6f to P700+ and the proton-coupled linear flow from PSII to Cyt b6f to P700+. Analyzing responses of O2 evolution and Chl fluorescence to ms-length light pulses we concluded that explanation of the sigmoidal fluorescence induction by excitonic connectivity between PSII units is a misconception. Each PSII processes excitation from its own antenna, but the sigmoidicity is caused by rise of the fluorescence yield of the QA-reduced PSII units after their QB site becomes occupied by reduced plastoquinone (or diuron). Unlike respiration, photosynthetic electrons must prepare their acceptor by coupled synthesis of 3ATP/4e−. Feedback regulation of this ratio leads to oscillations under saturating light and CO2, when the rate is Pi-limited. The slow oscillations (period 60s) indicate that the magnitudes of the deflections in the 3ATP/4e− ratio, corrected by regulating cyclic and alternative electron flow (including the Mehler type O2 reduction), are only a fraction of a per cent. The Pi limitation causes slip in the ATP synthase, slightly increasing the basic 12H+/3ATP requirement.
































Similar content being viewed by others
Abbreviations
- CET:
-
Cyclic electron transport
- ETC:
-
Electron transport chain
- ETR:
-
Electron transport rate
- FRL:
-
Far-red light
- LET:
-
Linear electron transport
- MTP:
-
Multiple-turnover pulses
- PC:
-
Plastocyanin
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- RPE:
-
Radical pair equilibrium
- (S)STF:
-
(Saturating) single-turnover flash
- wt:
-
Wild type
References
Badger MR, Collatz GJ (1978) Studies on the kinetic mechanism of ribulose-1,5-bisphosphate carboxylase and oxygenase reactions, with particular reference to to the effect of temperature on kinetic parameters. In: Carnegie Institution Year-Book. Department of Plant Biology, pp 355–361
Badger MR, Lorimer GH (1981) Interaction of sugar phosphates with the catalytic site of ribulose-1,5-bisphosphate carboxylase. Biochemistry 20(8):2219–2225
Baniulis D, Yamashita E, Zhang H, Hasan SS, Cramer WA (2008) Structure–function of the Cytochrome b6f complex. Photochem Photobiol 84:1349–1358
Breyton C, Nandha B, Johnson G, Joliot P, Finazzi G (2006) Redox modulation of cyclic electron flow around photosystem I in C3 plants. Biochemistry 45:13465–13475
Campbell WJ, Ogren WL (1990) Electron transport through photosystem I stimulates light activation of ribulose bisphosphate carboxylase/oxygenase (Rubisco) by rubisco activase. Plant Physiol 94:479–484
Christen G, Reifarth F, Renger G (1998) On the origin of the “35-µs kinetics” of P680+• reduction in photosystem II with an intact water oxidizing complex. FEBS Lett 429:49–52
Cleland WW, Andrews TJ, Gutteridge S, Hartman FC, Lorimer GH (1998) Mechanism of Rubisco: the carbamate as general base. Chem Rev 98:549–561
DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schünemann D, Finazzi G, Joliot P, Barbato R, Leister D (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132:273–285
De Causmaecker S, Douglass JS, Fantuzzi A, Nitschke W, Rutherford AW (2019) Energetics of the exchangeable quinone, QB, in photosystem II. Proc Natl Acad Sci USA 116:19458–19463
Dilley RA, Schreiber U (1984) Correlation between membrane-localized protons and flash-driven ATP formation in chloroplast thylakoids. J Bioenerg Biomembr 16(3):173–193
Dilley RA, Theg SM, Beard WA (1981) Membrane-proton interactions in chloroplast bioenergetics: localized proton domains. Ann Rev Plant Physiol 38:347–389
Eichelmann H, Laisk A (1999) Ribulose-1,5-bisphosphate carboxylase/oxygenase content, assimilatory charge and mesophyll conductance in leaves. Plant Physiol 119:179–189
Eichelmann H, Oja V, Rasulov B, Padu E, Bichele I, Pettai H, Niinemets Ü, Laisk A (2004) Development of leaf photosynthetic parameters in Betula pendula Roth leaves: correlations with photosystem I density. Plant Biol 6:307–318
Eichelmann H, Oja V, Rasulov B, Padu E, Bichele I, Pettai H, Mänd P, Kull O, Laisk A (2005) Adjustment of leaf photosynthesis to shade in a natural canopy: reallocation of nitrogen. Plant Cell Env 28:389–401
Eichelmann H, Talts E, Oja V, Padu E, Laisk A (2009) Rubisco in planta kcat is regulated in balance with photosynthetic electron transport. J Exp Bot 60:4077–4088
Emerson R, Lewis CM (1943) The dependence of quantum yield of Chlorella photosynthesis on wave length of light. Am J Bot 30:165–178
Farquhar GD (1979) Models describing the kinetics of ribulose bisphosphate carboxylase-oxygenase. Arch Biochem Biophys 2:456–468
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Hess JL, Tolbert NE (1966) Glycolate, glycine, serine and glycerate formation during photosynthesis by tobacco leaves. J Biol Chem 241:5705–5711
Hind G, Crowther D, Shahak Y, Slovacek RE (1981) The function and mechanism of cyclic electron transport. Photosynthesis II. Electron Transport and Photophosphorylation. Balaban International Science Services, Philadelphia
Johnson G (2003) Thiol regulation of the thylakoid electron transport chain - a missing link in the regulation of photosynthesis. Biochemistry 42:3040–3044
Jordan DB, Chollet R, Ogren WL (1983) Binding of phosphorylated effectors by active and inactive forms of ribulose-1,5-bisphosphate carboxylase. Biochemistry 22:3410–3418
Junge W, McLaughlin S (1987) The role of fixed and mobile buffers in the kinetics of proton movement. Biochim Biphys Acta 890:1–5
Kirchhoff H (2008) Molecular crowding and order in photosynthetic membranes. Trends Plant Sci 13:201–207
Kirchhoff H, Schöttler MA, Maurer J, Weis E (2004) Plastocyanin redox kinetics in spinach chloroplasts: evidence for disequilibrium in the high potential chain. Biochim Biophys Acta 1659:63–72
Kolber Z, Prasil O, Falkowski PG (1998) Measurements of variable chlorophyll fluorescence using fast repetition rate technique. I. Defining methodology and experimental protocols. Biochim Biophys Acta 1367:88–106
Laisk A (1970) A model of leaf photosynthesis and photorespiration. In: Shetlik I (ed) Prediction and measurement of photosynthetic productivity. PUDOC, Wageningen, pp 295–306
Laisk A (ed) (1977) Kinetics of photosynthesis and photorespiration in C3 plants. Nauka, Moscow
Laisk A (1982) Correspondence of a photosynthetic system to environmental conditions (in Russian). In: Nichiporovitch AA (ed) Physiology of photosynthesis. Nauka, Moscow
Laisk A, Loreto F (1996) Determining photosynthetic parameters from leaf CO2 exchange and chlorophyll fluorescence: Rubisco specificity factor, dark respiration in the light, excitation distribution between photosystems, alternative electron transport and mesophyll diffusion resistance. Plant Physiol 110:903–912
Laisk A, Oja V (1971) A three-channel apparatus for detailed investigation of the leaf CO2 exchange. In: Frey T (ed) Estonian Contributions to the International Biological Programme, II. Institute of Zoology and Botany, Acad. Sci. of Estonian SSR, Tartu, pp 113–128
Laisk A, Oja V (1972) A mathematical model of leaf photosynthesis and photorespiration. II. Experimental verification. In: Nitchiporovitch AA (ed) Theoretical foundations of the photosynthetic productivity. Nauka, Moscow, pp 362–368 ((in Russian))
Laisk A, Oja V (1974) Leaf photosynthesis in short pulses of CO2. The carboxylation reaction in vivo. Fiziologija Rastenij 21(6):1123–1131 (in Russian)
Laisk A, Oja V (1994) Range of the photosynthetic control of postillumination P700 reduction rate in sunflower leaves. Photosynth Res 39:39–50
Laisk A, Oja V (1995) Coregulation of electron transport through PS I by Cyt b6f, excitation capture by P700 and acceptor side reduction. Time kinetics and electron transport requirement. Photosynth Res 45:11–19
Laisk A, Oja V (1998) Dynamics of leaf photosynthesis. Rapid-response measurements and their interpretations. CSIRO, Collingwood
Laisk A, Oja V (2000) Alteration of PSII properties with non-photochemical excitation quenching. Philos Trans R Soc Lond B 355:1405–1418
Laisk A, Oja V (2013) Thermal phase and excitonic connectivity in fluorescence induction. Photosynth Res 117:431–448. https://doi.org/10.1007/s11120-013-9915-1
Laisk A, Oja V (2018) Kinetics of photosystem II electron transport: a mathematical analysis based on chlorophyll fluorescence induction. Photosynth Res 136:63–82. https://doi.org/10.1007/s11120-017-0439-y
Laisk A, Oja V (2020) Variable fluorescence of closed photochemical reaction centers. Photosyth Res. https://doi.org/10.1007/s11120-020-00712-3):335-346
Laisk A, Oja V, Rahi M (1970) Diffusion resistances as related to the leaf anatomy. Fiziologija Rastenij (sov Plant Physiol) 17(1):40–48
Laisk A, Oja V, Kiirats O (1984) Assimilatory power (post-illumination CO2 uptake) in leaves - measurement, environmental dependencies and kinetic properties. Plant Physiol 76:723–729
Laisk A, Kiirats O, Eichelmann H, Oja V (1987) Gas exchange studies of carboxylation kinetics in intact leaves. Progress in Photosynthesis Research. Martinus Nijhoff Publishers, Dordrecht.
Laisk A, Oja V, Kiirats O, Raschke K, Heber U (1989) The state of photosynthetic apparatus in leaves as analyzed by rapid gas exchange and optical methods: the pH of the chloroplast stroma and activation of enzymes in vivo. Planta 177:350–358
Laisk A, Oja V, Walker D, Heber U (1992) Oscillations in photosynthesis and reduction of Photosystem I acceptor side in sunflower leaves. Functional Cyt B/f-PSI-FNR complexes. Photosynthetica 27(4):465–479
Laisk A, Oja V, Rasulov B, Rämma H, Eichelmann H, Kasparova I, Pettai H, Padu E, Vapaavuori E (2002) A computer-operated routine of gas exchange and optical measurements to diagnose photosynthetic apparatus in leaves. Plant Cell Environ 25:923–943
Laisk A, Eichelmann H, Oja V, Peterson RB (2005a) Control of cytochrome b6f at low and high light intensity and cyclic electron transport in leaves. Biochim Biophys Acta 1708:79–90
Laisk A, Eichelmann H, Oja V, Rasulov B, Padu E, Bichele I, Pettai H, Kull O (2005b) Adjustment of leaf photosynthesis to shade in natural canopy: rate parameters. Plant Cell Env 28:375–388
Laisk A, Eichelmann H, Oja V (2006a) C3 photosynthesis in silico. Photosynth Res 90:45–66
Laisk A, Eichelmann H, Oja V, Rasulov B, Rämma H (2006b) Photosystem II cycle and alternative electron flow in leaves. Plant Cell Physiol 47(7):972–983
Laisk A, Eichelmann H, Oja V, Talts E, Scheibe R (2007) Rates and roles of cyclic and alternative electron flow in potato leaves. Plant Cell Physiol 48(11):1575–1588
Laisk A, Talts E, Oja V, Eichelmann H, Peterson R (2010) Fast cyclic electron transport around photosystem I in leaves under far-red light: a proton-uncoupled pathway? Photosynth Res 103:79–95
Laisk A, Eichelmann H, Oja V (2012) Oxygen evolution and chlorophyll fluorescence from multiple turnover light pulses: charge recombination in photosystem II in sunflower leaves. Photosynth Res 113:145–155
Laisk A, Oja V, Eichelmann H, Dall’Osto L (2014) Action spectra of photosystems II and I and quantum yield of photosynthesis in leaves in State 1. Biochim Biophys Acta 1837:315–325
Laisk A, Eichelmann H, Oja V (2015) Oxidation of plastohydroquinone by photosystem II and by dioxygen in leaves. Biochim Biophys Acta 1847:565–575
Laisk A, Oja V, Eichelmann H (2016) Kinetics of plastoquinol oxidation by the Q-cycle in leaves. Biochim Biophys Acta 1857:819–830
Ley AC, Mauzerall DC (1986) The extent of energy transfer among photosystem II reaction centers in chlorella. Biochem Biophys Acta 850:234–248
Lorimer GH, Andrews TJ, Tolbert NE (1973) Ribulose diphosphate oxygenase. II. Further proof of reaction products and mechanism of action. Biochemistry 12:18–23
Mani K, Zournas A, Dismukes GC (2022) Bridging the gap between Kok-type and kinetic models of photosynthetic electron transport within Photosystem II. Photosynth Res 151:83–102
Ogren WL, Bowes G (1971) Ribulose diphosphate carboxylase regulates soybean photorespiration. Nature New Biol 230(13):159–160
Oja V, Laisk A (2000) Oxygen yield from single turnover flashes in leaves:non-photochemical excitation quenching and the number of active PSII. Biochim Biophys Acta 1460(2–3):291–301
Oja V, Laisk A (2012) Photosystem II antennae are not energetically connected: evidence based on flash-induced O2 evolution and chlorophyll fluorescence in sunflower leaves. Photosynth Res 114:15–28
Oja V, Laisk A (2020) Time- and reduction-dependent rise of photosystem II fluorescence during microseconds-long inductions in leaves. Photosynth Res. https://doi.org/10.1007/s11120-020-00783-2
Oja V, Laisk A, Heber U (1986) Light-induced alkalization of the chloroplast stroma in vivo as estimated from the CO2 capacity of intact sunflower leaves. Biochim Biophys Acta 849:355–365
Oja V, Eichelmann H, Peterson RB, Rasulov B, Laisk A (2003) Decyphering the 820 nm signal: redox state of donor side and quantum yield of photosystem I in leaves. Photosynth Res 78:1–15
Oja V, Eichelmann H, Anijalg A, Rämma H, Laisk A (2010) Equilibrium or disequilibrium? A dual-wavelength investigation of photosystem I donors. Photosynth Res. https://doi.org/10.1007/s11120-010-9534-z
Oja V, Eichelmann H, Laisk A (2011a) Oxygen evolution from single- and multiple-turnover light pulses: temporal kinetics of electron transport through PSII in sunflower leaves. Photosynth Res 110:99–109
Oja V, Eichelmann H, Laisk A (2011b) The size of the lumenal proton pool in leaves during induction and steady-state photosynthesis. Photosynth Res 110:73–88
Portis AR Jr (1992) Regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase activity. Annu Rev Plant Physiol Plant Mol Biol 43:415–437
Prášil O, Kolber ZS, Falkowski PG (2018) Control of the maximal chlorophyll fluorescence yield by the QB binding site. Photosynthetica 56:150–162
Ross J (1981) The radiation regime and architecture of plant stands. Junk Publishers, London
Schansker G, To’th S, Kova’cs L, Holzwarth AR, Garab G (2011) Evidence for a fluorescence yield change driven by a light-induced conformational change within photosystem II during the fast chlorophyll a fluorescence rise. Biochim Biophys Acta 1807:1032–1043
Schreiber U, Klughammer C (2013) Wavelength-dependent photodamage to Chlorella investigated with a new type of multi-color PAM chlorophyll fluorometer. Photosynth Res 114:165–177
Seelert H, Dencher NA, Müller DJ (2003) Fourteen protomers compose the oligomer II of the proton-rotor in spinach chloroplast ATP synthase. J Mol Biol 333:337–344
Sharkey TD, Stitt M, Heineke D, Gerhardt R, Raschke K, Heldt HW (1986) Limitation of photosynthesis by carbon metabolism. II. O2-intensitive CO2 uptake results from limitation of triose phosphate utilization. Plant Physiol 81:1123–1129
Simkin AJ, Kapoor L, Doss CGP, Hofmann TA, Lawson T, Ramamoorthy S (2022) The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth Res 152:23–42
Sipka G, Müller P, Brettel K, Magyar M, Kovács L, Zhu Q, Xiao Y, Han G, Lambrev PH, Shen J-R, Garab G (2019) Redox transients of P680 associated with the incremental chlorophyll-a fluorescence yield rises elicited by a series of saturating flashes in diuron-treated photosystem II core complex of Thermosynechococcus vulcanus. Physiol Plantarum 166:22–32
Sipka G, Magyar M, Mezzetti A, Akhtar P, Zhu Q, Xiao Y, Han G, Santabarbara S, Shen J-R, Lambrev PH, Garab G (2021) Light-adapted charge-separated state of photosystem II: structural and functional dynamics of the closed reaction center. Plant Cell 33:1286–1302
Szczepaniak M, Sander J, Nowaczyk M, Müller MG, Rögner M, Holzwarth AR (2009) Charge separation, stabilization, and protein relaxation in photosystem II core particles with closed reaction center. Biophys J 96:621–631
Talts E, Oja V, Rämma H, Rasulov B, Anijalg A, Laisk A (2008) Dark inactivation of ferredoxin-NADP reductase and cyclic electron transport under far-red light in sunflower leaves. In: Allen JF, Gantt E, Golbeck JH, Osmond B (eds) Photosynthesis. Energy from the Sun: 14th International Congress on Photosynthesis. Springer, pp 687--690
Tcherkez G (2016) The mechanism of Rubisco-catalysed oxygenation. Plant Cell Environ 39:983–997
Walker DA (1992) Concerning oscillations. Photosynth Res 34:387–395
Viil J, Laisk A, Oja V, Pärnik T (1972) Positive influence of oxygen on photosynthesis. Doklady AN SSSR 204(5):1269–1271 ((in Russian))
Viil J, Laisk A, Oja V, Pärnik T (1977) Enchancement of photosynthesis caused by oxygen under saturating irradiance and high CO2 concentrations. Photosynthetica 11(3):251–259
Yokota A, Kitaoka S (1985) Correct pK values for dissociation constant of carbonic acid lower the reported Km values of ribulose bisphosphate carboxylase by half. Presentation of a nomograph and an equation for determining the pK values. Biochem Biophys Res Commun 131(3):1075–1079
Zelitch I (1971) Photosynthesis, photorespiration and plant productivity. Academy Press, New York
Acknowledgements
I am thankful to my wife Tiiu Laisk for the sixty-two years of love and trust, allowing me to fully devote to science. The work was mainly financed by Estonian Republic (Soviet Union until 1991) via Tartu University and Academy of Science. Mine and Vello’s visits to Australian National University, Würzburg University, Sheffield University, Umeå University, Washington State University were financed by the hosts. Significant was the support by G. Soros Foundation immediately after Estonia became independent in 1991, and a grant by the European Commission ten years later. Once again I wish to mention the names Barry Osmond, Ulrich Heber, David Walker, Gerry Edwards, Richard Peterson, whose role has been decisive in my scientific career. And my cordial thanks to Reviewer #2 for the supportive attitude to publish this text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In memory of Vello Oja (04.02.1943–22.12. 2020).
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Laisk, A. Prying into the green black-box. Photosynth Res 154, 89–112 (2022). https://doi.org/10.1007/s11120-022-00960-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-022-00960-5