Abstract
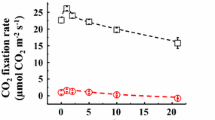
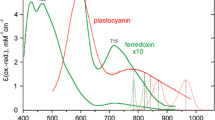
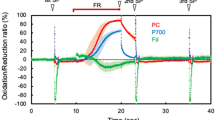
Fast cyclic electron transport (CET) around photosystem I (PS I) was observed in sunflower (Helianthus annuus L.) leaves under intense far-red light (FRL) of up to 200 μmol quanta m−2 s−1. The electron transport rate (ETR) through PS I was found from the FRL-dark transmittance change at 810 and 950 nm, which was deconvoluted into redox states and pool sizes of P700, plastocyanin (PC) and cytochrome f (Cyt f). PC and P700 were in redox equilibrium with K e = 35 (ΔE m = 90 mV). PS II ETR was based on O2 evolution. CET [(PS I ETR) − (PS II ETR)] increased to 50–70 μmol e− m−2 s−1 when linear electron transport (LET) under FRL was limited to 5 μmol e− m−2 s−1 in a gas phase containing 20–40 μmol CO2 mol−1 and 20 μmol O2 mol−1. Under these conditions, pulse-saturated fluorescence yield F m was non-photochemically quenched; however, F m was similarly quenched when LET was driven by low green or white light, which energetically precluded the possibility for active CET. We suggest that under FRL, CET is rather not coupled to transmembrane proton translocation than the CET-coupled protons are short-circuited via proton channels regulated to open at high ΔpH. A kinetic analysis of CET electron donors and acceptors suggests the CET pathway is that of the reversed Q-cycle: Fd → (FNR) → Cyt cn → Cyt bh → Cyt bl → Rieske FeS → Cyt f → PC → P700 →→ Fd. CET is activated when PQH2 oxidation is opposed by high ΔpH, and ferredoxin (Fd) is reduced due to low availability of e− acceptors. The physiological significance of CET may be photoprotective, as CET may be regarded as a mechanism of energy dissipation under stress conditions.














Similar content being viewed by others
Abbreviations
- BPGA:
-
Bisphosphoglyceric acid
- CET:
-
Cyclic electron transport
- Chl:
-
Chlorophyll
- Cyt:
-
Cytochrome
- E m :
-
Midpoint redox potential
- ETR:
-
Electron transport rate
- Fd:
-
Ferredoxin
- FNR:
-
Ferredoxin-NADP reductase
- FRL:
-
Far-red light
- g H :
-
Proton conductivity of ATPase
- LET:
-
Linear electron transport
- NPQ:
-
Non-photochemical quenching
- PC:
-
Plastocyanin
- PGA:
-
3-Phosphoglyceric acid
- PAD:
-
Photon absorption flux density
- PFD:
-
Incident photon flux density
- PS II:
-
Photosystem II
- PS I:
-
Photosystem I
- P700:
-
Donor pigment of PS I
- PQ:
-
Plastoquinone
- PQH2 :
-
Plastoquinol
- RuBP:
-
Ribulose-1,5-bisphosphate
- WL:
-
White light
References
Alric J, Pierre Y, Picot D, Lavergne J, Rappaport F (2005) Spectral and redox characterization of the heme ci of the cytochrome b6f complex. Proc Natl Acad Sci USA 102:15860–15865
Arnon DI (1959) Conversion of light into chemical energy in photosynthesis. Nature 184:10–21
Arnon DI, Allen MB, Whatley FR (1956) Photosynthesis by isolated chloroplasts IV. General concept and comparison of three photochemical reactions. Biochim Biophys Acta 20:449–461
Arnon DI, Tsujimoto HY, McSwain BD (1967) Ferredoxin and photosynthetic phosphorylation. Nature 214:562–566
Avenson TJ, Cruz JA, Kramer DM (2004) Modulation of energy-dependent quenching of excitons in antennae of higher plants. Proc Natl Acad Sci USA 101:5530–5535
Avenson TJ, Cruz JA, Kanazawa A, Kramer D (2005a) Regulating the proton budget of higher plant photosynthesis. Proc Natl Acad Sci USA 102:9709–9713
Avenson TJ, Kanazawa A, Crus JA, Takizawa K, Ettinger WE, Kramer DM (2005b) Integrating the proton circuit into photosynthesis: progress and challenges. Plant Cell Environ 28:97–109
Baniulis D, Yamashita E, Zhang Z, Hasan SS, Cramer WA (2008) Structure-function of the cytochrome b6f complex. Biochem Photobiol 84:1349–1358
Berry EA, Kuras MG, Huang L, Crofts AR (2000) Structure and function of cytochrome bc complexes. Annu Rev Biochem 69:1005–1075
Breyton C, Nandha B, Johnson G, Joliot P, Finazzi G (2006) Redox modulation of cyclic electron flow around photosystem I in C3 plants. Biochemistry 45:13465–13475
Bukhov NG, Wiese C, Neimanis S, Heber U (1999) Heat sensitivity of chloroplasts and leaves: leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth Res 59(1):81–93
Bukhov N, Egorova E, Carpentier R (2002) Electron flow to photosystem I from stromal reductants in vivo: the size of the pool of stromal reductants controls the rate of electron donation to both rapidly and slowly reducing photosystem I units. Planta 215:812–820
Cha Y, Mauzerall DC (1992) Energy storage of linear and cyclic electron flows in photosynthesis. Plant Physiol 100:1869–1877
Clarke JE, Johnson GN (2001) In vivo temperature dependence of cyclic and pseudocyclic electron transport in barley. Planta 212:808–816
Cruz JA, Avenson TJ, Kanazawa A, Takizawa K, Edwards GE, Kramer DM (2005) Plasticity in light reactions of photosynthesis for energy production and photoprotection. J Exp Bot 56:395–406
Fan D-Y, Nie Q, Hope AB, Hillier W, Pogson BJ, Chow WS (2007) Quantification of cyclic electron flow around Photosystem I in spinach leaves during photosynthetic induction. Photosynth Res 94:347–357
Feniouk BA, Mulkidjanian AY, Junge W (2005) Proton slip in the ATP synthase of Rhodobacter capsulatus: induction, proton conduction, and nucleotide dependence. Biochim Biophys Acta 1706:184–194
Golbeck JH (1987) Structure, function and organization of the photosystem I reaction center complex. Biochim Biophys Acta 895:167–204
Golbeck JH, Bryant DA (1991) Photosystem I. Curr Top Bioenerg 16:83–177
Golding AJ, Johnson GN (2003) Down-regulation of linear and activation of cyclic electron transport during drought. Planta 218:107–114
Harbinson J, Hedley CL (1989) The kinetics of P 700+ reduction in leaves: a novel in situ probe of thylakoid functioning. Plant Cell Environ 12:357–369
Havaux M (1992) Photoacoustic measurements of cyclic electron flow around photosystem I in leaves adapted to light-states 1 and 2. Plant Cell Physiol 33(6):799–803
Heber U, Bukhov NG, Neimanis S, Kobayashi Y (1995a) Maximum H+/v PSI stoichiometry of proton transport during cyclic electron flow in intact chloroplasts is at least two, but probably higher than two. Plant Cell Physiol 36:1639–1647
Heber U, Gerst U, Krieger A, Neimanis S, Kobayashi Y (1995b) Coupled cyclic electron transport in intact chloroplasts and leaves of C3 plants: does it exist? if so, what is its function? Photosynth Res 46:269–275
Herbert S, Fork DC, Malkin S (1990) Photoacoustic measurements in vivo of energy storage by cyclic electron flow in algae and higher plants. Plant Physiol 94:926–934
Hind G, Crowther D, Shahak Y, Slovacek RE (1981) The function and mechanism of cyclic electron transport. In: Akoyunoglou G (ed) Photosynthesis II. Electron transport and photophosphorylation. Balaban International Science Services, Philadelphia, PA, pp 87–97
Hosler JP, Yocum CF (1985) Evidence for two cyclic photophosphorylation reactions concurrent with ferrodoxin-catalyzed non-cyclic electron transport. Biochim Biophys Acta 808:21–31
Hosler JP, Yocum CF (1987) Regulation of cyclic photophosphorylation during ferredoxin-mediated electron transport. Plant Physiol 83:965–969
Joët T, Cournac L, Peltier G, Havaux M (2002) Cyclic electron flow around photosystem I in C3 plants. In vivo control by the redox state of chloroplasts and involvement of the NADH-dehydrogenase complex. Plant Physiol 128:760–769
Johnson GN (2005) Cyclic electron transport in C3 plants: fact or artefact? J Exp Bot 56:407–416
Joliot P, Joliot A (2002) Cyclic electron transfer in plant leaf. Proc Natl Acad Sci USA 99:10209–10214
Joliot P, Joliot A (2005) Quantification of cyclic and linear flows in plants. Proc Natl Acad Sci USA 102:4913–4918
Joliot P, Joliot A (2006) Cyclic electron flow in C3 plants. Biochim Biophys Acta 1757:362–368
Joliot P, Béal D, Joliot A (2004) Cyclic electron flow under saturating excitation of dark-adapted Arabidopsis leaves. Biochim Biophys Acta 1656:166–176
Kanazawa A, Kramer DM (2002) In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc Natl Acad Sci USA 99:12789–12794
Kirchhoff H, Schöttler MA, Maurer J, Weis E (2004) Plastocyanin redox kinetics in spinach chloroplasts: evidence for disequilibrium in the high potential chain. Biochim Biophys Acta 1659:63–72
Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192:261–268
Kramer DM, Avenson TJ, Edwards GE (2004) Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci 9:349–357
Laisk A, Edwards GE (2000) A mathematical model of C4 photosynthesis: the mechanism of concentrating CO2 in NADP-malic enzyme type species. Photosynth Res 66:199–224
Laisk A, Oja V (1994) Range of the photosynthetic control of postillumination P700 reduction rate in sunflower leaves. Photosynth Res 39:39–50
Laisk A, Oja V (1995) Coregulation of electron transport through PS I by Cyt b6f, excitation capture by P700 and acceptor side reduction. Time kinetics and electron transport requirement. Photosynth Res 45:11–19
Laisk A, Oja V (1998) Dynamic gas exchange of leaf photosynthesis. Measurement and interpretation. CSIRO Publishing, Collingwood, Australia
Laisk A, Oja V, Rasulov B, Rämma H, Eichelmann H, Kasparova I, Pettai H, Padu E, Vapaavuori E (2002) A computer-operated routine of gas exchange and optical measurements to diagnose photosynthetic apparatus in leaves. Plant Cell Environ 25:923–943
Laisk A, Eichelmann H, Oja V, Peterson RB (2005) Control of cytochrome b6f at low and high light intensity and cyclic electron transport in leaves. Biochim Biophys Acta 1708:79–90
Laisk A, Eichelmann H, Oja V, Talts E, Scheibe R (2007) Rates and roles of cyclic and alternative electron flow in potato leaves. Plant Cell Physiol 48:1575–1588
Makino A, Miyake C, Yokota A (2002) Physiological functions of the water-water cycle (Mehler reaction) and the cyclic electron flow around PSI in rice leaves. Plant Cell Physiol 43:1017–1026
Miyake C, Shinzaki Y, Miyata M, Tomizawa K (2004) Enhancement of cyclic electron flow around PSI at high light and its contribution to the induction of non-photochemical quenching of Chl fluorescence in intact leaves of tobacco plants. Plant Cell Physiol 45:1426–1433
Miyake C, Horiguchi S, Makino A, Shinzaki Y, Yamamoto H, Tomizawa KI (2005a) Effects of light intensity on cyclic electron flow around PSI and its relationship to non-photochemical quenching of Chl fluorescence in tobacco leaves. Plant Cell Physiol 46:1819–1830
Miyake C, Miyata M, Shinzaki Y, Tomizawa K (2005b) CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves—relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol 46:629–637
Munekage Y, Hashimoto M, Miyake C, Tomizawa K-I, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429:579–582
Munne-Bosch S, Shikanai T, Asada K (2005) Enhanced ferredoxin-dependent cyclic electron flow around photosystem I and alpha-tocopherol quinone accumulation in water-stressed ndhB inactivated tobacco mutants. Planta 222:502–511
Oja V, Eichelmann H, Peterson RB, Rasulov B, Laisk A (2003) Decyphering the 820 nm signal: redox state of donor side and quantum yield of photosystem I in leaves. Photosynth Res 78:1–15
Oja V, Bichele I, Hüve K, Rasulov B, Laisk A (2004) Reductive titration of photosystem I and differential extinction coefficient of P700+ at 810–950 nm in leaves. Biochim Biophys Acta 1658:225–234
Oja V, Eichelmann H, Laisk A (2008) Equilibrium or disequilibrium? A dual-wavelength investigation of photosystem I donors. In: Allen JF, Gantt E, Golbeck JH, Osmond B (eds) Photosynthesis. energy from the Sun: 14th international congress on photosynthesis. Springer, Berlin, pp 687–690
Pan R-S, Dilley RA (2000) Influence of Ca2+ on the thylakoid lumen violaxanthin de-epoxidase activity through Ca2+ gating of H+ flux at the CFoH+ channel. Photosynth Res 65(2):141–154
Peterson RB, Oja V, Laisk A (2001) Chlorophyll fluorescence at 680 and 730 nm and leaf photosynthesis. Photosynth Res 70:185–196
Pettai H, Oja V, Freiberg A, Laisk A (2005) Photosynthetic activity of far-red light in green plants. Biochim Biophys Acta 1708:311–321
Sacksteder CA, Kramer DM (2000) Dark-interval relaxation kinetics (DIRK) of absorbance changes as a quantitative probe of steady-state electron transfer. Photosynth Res 66:145–158
Sacksteder CA, Kanazawa A, Jacoby ME, Kramer DM (2000) The proton to electron stoichiometry of steady-state photosynthesis in living plants: a proton-pumping Q cycle is continuously engaged. Proc Natl Acad Sci USA 97:14283–14288
Siggel U (1974) The control of electron transport by two pH-sensitive sites. In: Avron M (ed) Proceedings of the 3rd international congress on photosynthesis. Elsevier, Amsterdam, pp 645–654
Tagawa K, Tsujimoto HY, Arnon DI (1963) Role of chloroplast ferredoxin in the energy conversion process of photosynthesis. Proc Natl Acad Sci USA 49:567–572
Talts E, Oja V, Rämma H, Rasulov B, Anijalg A, Laisk A (2007) Dark inactivation of ferredoxin-NADP reductase and cyclic electron flow under far-red light in sunflower leaves. Photosynth Res 94:109–120
Vallon O, Bulte L, Dainese P, Olive J, Bassi R, Wollman F-A (1991) Lateral redistribution of cytochrome b6/f complexes along thylakoid membranes upon state transitions. Proc Natl Acad Sci USA 88:8262–8266
Yamamoto H, Kato H, Shinazaki Y, Horiguchi S, Shikanai T, Hase T, Endo T, Nishioka M, Makino A, Tomizawa K, Miyake C (2006) Ferredoxin limits cyclic electron flow around PSI (CEF-PSI) in higher plants—stimulation of CEF-PSI enhances non-photochemical quenching of Chl fluorescence in transplastomic tobacco. Plant Cell Physiol 47:1355–1371
Yin X, Harbinson J, Struik PC (2006) Mathematical review of literature to assess alternative electron transports and interphotosystem excitation partitioning of steady-state C3 photosynthesis under limiting light. Plant Cell Environ 29:1771–1782
Zhang H, Whitelegge JP, Cramer WA (2001) Ferredoxin:NADP+ oxidoreductase is a subunit of the chloroplast cytochrome b6f complex. J Biol Chem 276:38159–38165
Acknowledgments
This study was supported by Targeted Financing Theme SF 0180045s08 from Estonian Ministry of Education and Science, and by Grants 6607 and 6611 from Estonian Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laisk, A., Talts, E., Oja, V. et al. Fast cyclic electron transport around photosystem I in leaves under far-red light: a proton-uncoupled pathway?. Photosynth Res 103, 79–95 (2010). https://doi.org/10.1007/s11120-009-9513-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-009-9513-4