Abstract
Aims
The composition of soil fungal communities is known to impact tree performance. However, fungal communities differ among soils with different precipitation histories and may change during drought. This study aimed to determine the influence of soil origin and associated climate adaptation of fungal communities on European beech seedlings’ drought responses.
Methods
Seedlings were established from the same seed source and grown in three soils with different precipitation histories but similar water retention properties. One year after establishment, half of the seedlings were exposed to a two-month drought with predawn leaf water potentials of about –1.5 MPa, the other half remained well-watered (control). Before and during the drought, soil and root fungal community composition, root architecture, seedling growth, carbon allocation and leaf physiology were determined.
Results
The drought effect on the fungal community composition was the lowest in dry region soils, suggesting a natural adaptation of the fungal communities to dry environments. Nevertheless, contrary to our expectations, the seedlings grown in dry region soils with respective adapted fungal communities were most affected by drought. This was evidenced by a lower predawn water potential, probably due to shorter root systems with higher root branching compared to those grown in moist region soils where a greater taproot length was observed.
Conclusion
Beech seedlings´ drought responses depend largely on their different rooting patterns and less on the soil fungal communities that are adapted to long-term precipitation conditions. Yet, microbial effects cannot be excluded. Future research should focus more on the role of specific microbial species on plant root growth and drought responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In European temperate forests, European beech (Fagus sylvatica (L.)) is a predominant tree species with a known drought sensitivity in both, the seedling (Lendzion and Leuschner 2008; Gebauer et al. 2020; Beloiu et al. 2022) and the mature tree stage (Arend et al. 2022; Frei et al. 2022; Schmied et al. 2023), which raises concerns about the species’ future role in forests (Rennenberg et al. 2004; Geßler et al. 2007; Leuschner 2020).
Change in stomatal aperture (closure) is generally seen as a first quick physiological response to drought (Choat et al. 2018), however, beech is considered a more anisohydric species that will maintain its photosynthetic activity under increasing drought, which makes it more prone to water loss (Leuschner 2020). Other slower responses relate to shifts in carbon (C) allocation to increased belowground structural growth (Meier and Leuschner 2008a; Hommel et al. 2016) and increase in non-structural carbon (NSC) concentrations (Blessing et al. 2015; Chuste et al. 2020), leading to osmotic adjustments at the cellular and organ level (Tomasella et al. 2018; Leuschner et al. 2019). This preferential allocation belowground can generally lead to an increase in root to shoot ratios (as observed at global scale, Poorter et al. 2012; Tumber-Dávila et al. 2022)). While root morphological characteristics such as architecture and rooting depth play significant roles for water uptake and can potentially mitigate drought effects (Brunner et al. 2015; Brinkmann et al. 2019; Clément et al. 2019; Germon et al. 2020; Kahmen et al. 2022), increased solute concentrations, such as soluble sugars, in the root system can keep the water potential of roots lower than that of soil and therefore maintain root water uptake under drought (Meier et al. 1992; Aaltonen et al. 2017; Nikolova et al. 2020). In beech seedlings, root biomass decreased during drought (Meier and Leuschner 2008b; Gebauer et al. 2020) and fine root growth, lifespan and rooting depth were reduced (Meier and Leuschner 2008a). Furthermore, a trend towards smaller root diameter and lower specific root length was observed in response to drought (Meier and Leuschner 2008a).
Previous studies have also investigated the variation in drought responses and the potential adaptation of beech selected from different provenances (Leuschner 2020; Petrik et al. 2022), or along climatic gradients in mature forests (Nahm et al. 2007; Meier and Leuschner 2008a). Typically, although not always, provenances of beech from dry regions appeared to be more adapted to dry conditions, e.g. less mortality and less growth reduction under drought, compared to those from moister regions (Leuschner 2020 and references therein). All these studies, however, focused only on the plant response and did not explore possible linkages with edaphic conditions of the soils from these locations. Soil properties such as soil type (Contran et al. 2013; Thiel et al. 2014; Buhk et al. 2016; Liu et al. 2017) and microbial communities (Fitzpatrick et al. 2018; Meisner et al. 2018) can have tremendous direct and indirect impacts on plants and their responses. For example, it has been shown that seedlings inoculated with microbial communities originating from arid regions had higher drought tolerance (Allsup et al. 2023). Ectomycorrhizal (ECM) fungi are known to improve the plant hydraulic function as well as increase the storage of non-structural carbohydrate (NSC) (Wang et al. 2021) and transfer water between trees (Egerton-Warburton et al. 2007). However, little is known about the linkage between naturally drought adapted soil microbiomes and the drought resistance of plants (Körner 2011).
Recent studies highlight the importance of soil and root-associated fungi for plant performance (Gundale et al. 2014; Anthony et al. 2022) particularly under stress conditions (Lehto and Zwiazek 2011; Kivlin et al. 2013; Lata et al. 2018; Porter et al. 2020; Outamamat et al. 2022; Allsup et al. 2023). Fungal species can be assigned to different trophic modes: saprotrophic, symbiotrophic, and pathotrophic (sensu Nguyen et al. 2016a), or as fungi with unknown ecological role (Tedersoo and Smith 2013; Nguyen et al. 2016a; Unuk et al. 2019) as well as animal- or mycoparasites (Nguyen et al. 2016a; Põlme et al. 2020). Saprotrophic fungi are often referred to as “free-living fungi” (e.g. Castaño et al. 2018) because, unlike ECM fungi, they do not form symbiotic relationships with host plants and obtain their nutrients by decomposing organic material in the soil (e.g. Nguyen et al. 2016b). Hence, they are suspected to be more sensitive to environmental changes such as drought (Castaño et al. 2018). However, some saprotrophic fungal species can additionally establish interactions with plants, for example by living as root endophytes (Põlme et al. 2020) or by forming hyphal mantle and Hartig net-like structures on the roots similar to ECM fungi (Smith et al. 2017) and thus compete for niches with ECM fungi. Ectomycorrhizal fungi can grow extraradical hyphae from the hyphal mantle, which are classified by their extension into the surrounding soil as contact, short-distance, medium-distance, and long-distance exploration types (Agerer 2001; Weigt et al. 2012). Under drought, several studies showed that the abundance of ECM fungi decreased (Lozano et al. 2021; Castaño et al. 2023) and their composition changed (Shi et al. 2002; Swaty et al. 2004; Richard et al. 2011; Nickel et al. 2018). Thus, abundances of short- and medium-distance explorers decreased, while the number of long-distance explorers increased (Nickel et al. 2018), which enlarge the water absorbing area and explore the soil for water with their rhizomorphs (Mohan et al. 2014; Brunner et al. 2015; Nickel et al. 2018; Castaño et al. 2023). Precipitation history has been shown to alter the fungal community structure leading to more drought-resistant fungal species in dry regions that were not present in more humid regions (Cavender-Bares et al. 2009). However, it is still poorly understood how drought events affect fungal communities in soils with different precipitation histories and how those subsequently impact specific plant drought responses.
Therefore, the present study focused on soils with different precipitation histories and their effect on (i) root-associated fungal communities and (ii) drought responses of beech seedlings. We hypothesized that a fungal community associated with soils from dry regions is better adapted to drought and thus less affected (fungal community shows less change) to a subsequent drought event compared to that from moist regions. Further, we hypothesized that seedlings that were established and grown in a soil from a dry region show better acclimation to drought at the fine root level than those grown in a moist region soil, i.e., longer taproot length and/or higher root to shoot ratio, and that this acclimation of the root system and the adaptation of the associated fungi collectively mitigate drought effects on beech.
Methods
Experimental setup
Soils from three regions in Bavaria, Germany, were collected in mixed beech (Fagus sylvatica L.)—spruce (Picea abies (L.) KARST.) stands along a natural precipitation gradient ranging from a dry region (DR) in the north (Gramschatz Forest close to Arnstein: 49.903° (N), 9.977° (E); 330 m.a.s.l) with average rainfall of 310 mm (1971–2000) during the growing season between May and October, over an intermediate region (IR) (Kranzberg Forest close to Freising: 48.419° (N), 11.661° (E); 490 m.a.s.l.) with average rainfall of 480 mm, to a moist region (MR) in the south (Großhaager Forest close to Wasserburg: 48.142° (N), 12.073° (E); 620 m.a.s.l.) with average rainfall of 640 mm during the growing season (Pretzsch et al. 2014). All locations have a long-term average temperature of 13.8–14.0 °C during the growing season (1971–2000) (Pretzsch et al. 2014). Soil characteristics of the different regions are listed in Table S1 in supplements. At each site, 20 cm of the top layer excluding litter were taken, sieved to 1 cm. The soils were then mixed with 30 vol% quartzite sand to equalize soil water retention properties, which enabled us to expose seedlings grown in the three different region soils to the same drought stress. To ensure that the soil-sand-mixture (from here onwards termed “soil”) included enough nutrients for the seedlings throughout the experiment phase, soil nutrient content was analysed (Table S2). The nitrogen concentration of all soils (0.1–0.2%) showed no deficiency (> 0.08%). Soil organic matter before the drought treatment (Table S2) was determined by physical destruction using the widely used loss-on-ignition method. Therefore, 5 g of soil of each region (n = 3) were weighted first after drying at 70 °C for 72 h and then again after burning the soil organic matter for 3 h at 500 °C.
In spring 2019, beech (Fagus sylvatica L.) was seeded (4 seeds, seed source HkG 81024 Alpenvorland, a moist region) in 20–40 pots per soil region (17 × 17 × 20 cm (depth), in total 90 pots) and grown for one year under well-watered conditions in an unheated greenhouse. For that, all pots were regularly watered to field capacity (equivalent to c. 21.5% volumetric soil water content (SWC)). In spring 2020, 20 pots from each region were selected and 10 pots with 4 seedlings each were assigned to either a control or a drought treatment (n = 10) according to similar seedling sizes. Within each region, control and drought treatment pots had similar root collar diameter and height, thus similar initial seedling biomass (see Fig. S1 for initial biomass). Two months before applying the drought treatment (spring 2020), all pots were transferred to another greenhouse with UV transparent glass and temperature-controlled conditions to keep the experiment conditions as natural as possible. Greenhouse temperature was synchronized with outside temperature conditions during the experiment and climatic variables were measured automatically four times per hour, including air humidity and photosynthetic photon flux density (PPFD). The mean temperature during the study period (May, 22nd 2020–August, 31st 2020) was 20 ± 6 °C, vapour pressure deficit was 0.82 ± 0.8 kPa and mean PPFD during the daytime (6 am–8 pm) was 208 µmol m−2 s−1 (Fig. S2). The maximum PPFD was reached between 10–11 am with up to 1500 µmol m−2 s−1. The pots were randomly arranged and re-arranged every two weeks to minimize potential spatial differences in air circulation, light, temperature and neighbouring effects in the greenhouse. No fertilizer or plant protection agents were used during the experiment.
Pre-drought experiments
Relationship between soil water potential and SWC was established for each region soil (Fig. S3) using a pressure plate method (Richards 1941; Wang et al. 2015), whereby the curves did not significantly differ among region soils (tested after logarithmic transformation of soil water potential). The average field capacity determined was 21.6%, 21.8%, and 21.1% SWC, for DR, IR, and MR soil, respectively.
In addition, the relationship between predawn plant water potential (ΨPD) and SWC was measured with extra pots that were not selected for the drought treatment, using a Scholander pressure chamber (mod. 1505D, PMS Instrument Co., Albany, OR, USA) and Time Domain Reflectometry (TDR100, Campbell Scientific, Logan, CT, USA, measured at the center of the pot using 10 cm probes), respectively. According to the relationship between ΨPD and the SWC (Fig. S3), the target SWC of each pot was defined as 20% (close to the field capacity) for control and 12% (expecting predawn plant water potentials of –1 to –1.5 MPa) for drought treatment for all three region soils. Prior to the drought treatment, the initial weight of each pot at the SWC of 20% (-0.006 MPa, based on the relationship between soil water potential and SWC) was noted and SWC was maintained in all pots by watering them every other day to their respective initial weight.
Drought treatment
At the end of May, after the beech had flushed and the shoots had expanded, the drought treatment was superimposed on the drought group. The drought treatment lasted for eight weeks. To make the intensive harvest work at the end of the study manageable, pots were randomly assigned to one of three groups staggered with different drought starting times one week apart (drought start on May 25th, June 1st, and June 8th, 2020, respectively). All treatment combinations were equally distributed in each time group and the phenological stages of the seedlings were not different among the different starting times. The control pots were watered every other day to maintain a SWC of 20%. For drought treatment, the pot weight was recorded every other day and subsequently watered by adding half the weight lost from the day before (Galvez et al. 2011) to gradually dry the soil to 12% SWC within 3–4 weeks (Fig. 1a). SWC of drought pots was also measured with TDR sensors before every watering. After reaching 12% SWC, the pots were weighted again and watered to this target weight every other day. On hot days SWC was measured daily and seedlings were additionally watered off schedule with about half the amount of water loss to avoid a lethal drought.
a Soil water content (SWC, in vol.%) of drought pots with dry region (DR, purple), intermediate region (IR, yellow) and moist region (MR, green) soil during the drought treatment of 56 days (8 weeks). b SWC of control (blue) and drought (red) pots at the harvest. c Predawn plant water potential (ΨPD) at the day of harvest. d Mean net carbon assimilation rates (A) and e) stomatal conductance (gs) during 4th and 7th week. Lowercase letters indicate significant differences among the groups according to post-doc test
Leaf gas exchange
CO2 assimilation rates (A) at a CO2 concentration of 400 ppm and leaf stomatal conductance to water vapour (gs) were measured before applying the drought and during the 4th and 7th week of the drought treatment, using an open gas exchange system LI-6800 (Li-Cor Inc., Lincoln, NE, USA). One fully expanded leaf was randomly selected for each plant and the same leaf was measured consistently, excluding newly produced leaves during the drought treatment. During the measurement, the PPFD was set to 500 μmol m−2 s−1, the leaf temperature to 25 °C, and the relative humidity to around 60%.
13C labelling
In the last (8th) week of the drought treatment, three days before harvest, all pots (n = 10 i.e. 60 pots in total) were labelled with 13C-CO2 for three consecutive days (2020 July 27th-29th, August 3rd-5th, and August 10th-12th, for each group with stagged drought starting day, respectively). Leaf samples for the pre-labelling background were collected from randomly chosen three pots per treatment (n = 3) before the labelling (Table S3). Pots were then located in a transparent tent with a volume of c. 1300 L. The target C isotope composition in the tent during the labelling was set to 20,000‰ to make sure that the expected natural difference in the isotopic signature among organs (leaf, stem, coarse root, and fine root) (~ 1 ‰, Ruehr et al. 2009) would be negligible (< 1% of the isotopic signature in the samples after labelling), since pre-labelling sampling was only possible for leaves to avoid destructive samplings. To achieve the target isotopic value in the tent, labelled (20 atom%) 13CO2 and normal CO2 (-3.41‰) were mixed and continuously provided to the tent via a mass flow controller with max 10 ml/min each. According to the isotopic values in the tent, the mix ratio of these two gases were regulated. The labelling started at 4 am and ended at 8 pm each day. LED lights were used from 6 am to 4 pm to ensure photosynthetic activity (c. 350 μmol m−2 s−1 PPFD). Four electronic fans were used for homogeneous distribution of labelled air in the tent. The atmospheric CO2 concentration and δ13C in the tent were continuously monitored during the labelling using cavity ring-down spectroscopy (CRDS, ESP-1000; PICARRO, see Table S4). Direct diffusion of 13CO2 into the soil likely happened but was not relevant for the study since only plant bulk tissues were considered. Temperature, Vapour Pressure Deficit, and PPFD were also monitored throughout the labelling days (Table S4).
Soil and seedling sampling
Soil samples were taken before (initial) and after the drought treatment using a soil corer (diameter: 5 mm, length: 20 cm). At each measurement time, three soil cores were randomly taken per pot, combined and instantly frozen at -80 °C.
At the end of the study (directly after the three days of 13C labelling), ΨPD was measured before sunrise (2 am–5 am CET). Immediately after, SWC of each pot was recorded with TDR sensors, and the seedlings were harvested on July 30th, Aug 6th, and Aug 13th, respectively. Seedlings were then separated into leaves, stem, coarse roots (> 2mm), and fine roots (< 2mm) and their fresh weights were recorded. Prior to drying, the leaf samples were scanned (Epson Perfection 4990 Photo, Epson Deutschland GmbH, Meerbusch, Germany) to determine the leaf area and calculate the specific leaf area (SLA) based on the leaf dry weight. All vital fine root tips (defined as not shrunken, distorted or dark brown (Pena et al. 2023)) were counted under a stereomicroscope and classified as mycorrhizal or non-mycorrhizal. The mycorrhizal colonisation was then determined as follows (Danzberger et al. 2023; Pena et al. 2023):
Each root system was photographed on graph paper. Two thirds of the fine roots were directly frozen at –80 °C for DNA extraction (see below). The remaining fine root samples and the other leaf, stem and coarse root samples were oven dried at 64 °C for 72 h to determine their dry weight, and ground for later analyses using a ball mill (MM400, Retsch, Haan, Germany). The dry weight of the fine roots that were frozen for DNA extraction was calculated using their fresh weight and the ratio of the dry to fresh weight of the respective fine roots (determined from the remaining one third of the fine roots).
Growth and biomass
Basal stem diameter and height of each plant were determined before the onset of the drought treatment and at the end of the study directly before the harvest. Basal stem diameter was recorded twice perpendicular to each other, and the average was used. To ensure measurement consistency the stem was marked 1 cm above the soil.
Since initial destructive seedling samples could not be obtained before the onset of drought due to sample limitations, we estimated the initial biomass for each seedling using allometric functions based on the final harvest data of the control seedlings (nls function in R, package: stats, version: 3.6.1, Fig. S1). First, an allometric function for estimating stem biomass was developed using stem diameter and height as input parameters individually for each soil. The calculated stem biomass corresponded well to the measured stem biomass according to linear regression (p < 0.001, R2 = 0.84).
whereby a and b are the coefficient determined by the function. Using these coefficients together with stem diameter and height before the onset of the drought treatment, the initial stem biomass was calculated. Then, using final harvest data of the control seedlings, relationship between total root biomass (i.e. combined coarse and fine roots) and stem biomass was developed with a linear regression (lm function in R). The calculated root biomass agreed well with the measured root biomass according to linear regression (calculated against measured root biomass at the harvest, p < 0.001, R2 = 0.61). It was not possible to determine the initial biomass of coarse and fine roots separately, because no or only weak relationships existed between both root types and stem biomass. Finally, initial total root biomass before the onset of the drought was calculated from the initial stem biomass using the relationship.
Relative stem growth during the drought treatment of 8 weeks were calculated for each seedling by subtracting the estimated initial stem biomass from the measured final stem biomass at the harvest.
Taproot length and branching intensity
The taproot length expresses the length from the stem base to the root tip at the end of the taproot. Therefore, the root systems from images taken at the harvest were measured using the program ImageJ (version 1.53a, National Institute of Health, USA). In parallel, the number of all branches (McCormack et al. 2015) was counted and divided by the total length of the respective fourth-order root to gain a comparative parameter (“branching intensity”).
Analysis of stable C isotopic composition (δ13C) and allocation of newly assimilated C
Stable C isotopic composition (δ13C) of leaf, stem, coarse root and fine root samples were determined with an isotope ratio mass spectrometer (IRMS, delta V Advantage; Thermo Fisher Scientific) coupled to an Elemental Analyzer (Euro EA; Eurovector).
13C derived from the newly assimilated C (13Cexcess in μg) was calculated for each plant organ using δ13C converted to atom%, C content, and biomass at the harvest (Ruehr et al. 2009).
whereby atom%s and atom%pre are atom% of the samples at the harvest and before the labeling, respectively. B gives the biomass at the harvest (mg), and C% the percentage of carbon in the sample. Finally, 13Cexcess in each organ was summed up for each plant and expressed as a relative allocation among the organs.
Non-structural carbohydrates (NSC)
Total soluble sugars were extracted from ground leaf, stem, coarse root and fine root samples in 80% hot ethanol, followed by a phenol–sulfuric assay to determine their concentration colorimetrically (Landhäusser et al. 2018). Six seedlings from 6 different pots were randomly chosen for each treatment (n = 6). Starch concentration was determined after an α-amylase and amyloglucosidase digestion followed by a peroxide-glucose oxidase/o-dianisidine reaction and concentrations were expressed as percent of sample dry weight. Using the concentration data and the biomass of leaves, stems, coarse and fine roots at harvest, sugar and starch content for each sample tissue were calculated. Finally, after combining the content of sugar and starch for coarse and fine roots, it was expressed as a ratio of the sugar and starch content in roots (belowground) to the total content (leaf, stem, coarse, and fine roots all combined) for each seedling.
DNA extraction, library preparation, sequencing and bioinformatics
Frozen fine root systems (n = 10 per soil and treatment) were ground in liquid nitrogen. DNA of 250 mg root powder per sample as well as 250 mg soil (n = 6 per soil, treatment and sampling time) were extracted according to the DNeasy PowerSoil Pro Kit (Qiagen, Hilden, Germany) protocol and diluted to 5 ng / µL. For high-throughput sequencing, a polymerase chain reaction (PCR) was performed in triplicates with primer mixtures for the ITS2 rDNA as described by Tedersoo et al. (2015), containing Illumina dual adapter sequences for Miseq sequencing (protocol Part # 15044223; Illumina, San Diego, CA, USA, Table S5). Library preparation was performed as described in Danzberger et al. (2023) and Method S1 in supplements.
The sequence data from the sequencing was provided in FASTQ format and prepared for further analysis using the fungal analysis pipeline PIPITS v2.7 (Gweon et al. 2015). In a first step, read pairs were joined and the sequences were filtered for quality based on the pipeline’s default settings. Afterwards, the fungal ITS2 sequences were extracted using ITSx (Bengtsson-Palme et al. 2013) and sequences below 100 bp excluded. The remaining sequences were assigned to OTUs based on 97% sequence identity using VSEARCH (Rognes et al. 2016) and chimeras removed using the UNITE CHIME reference dataset (Nilsson et al. 2015). Taxonomic classification relied on RDP Classifier (Wang et al. 2007) comparing sequences with those in the UNITE database (Kõljalg et al. 2013). Further fungal traits such as “fungal trophic mode”, “ectomycorrhizal exploration types” (Agerer 2001), and saprotrophic “primary” and “secondary” lifestyle were assigned by matching the genus level of the taxonomic output with the FungalTraits database (Põlme et al. 2020).
Statistical analysis
For statistics and graphic illustration, the software R (version 4.0.2, R Core Team 2021) and RStudio (version 1.4.1717, RStudio Inc.) were used. Sequencing data were randomly rarefied (average of 1.000 repetitions) using “Rarefy” in GUniFrac (Chen et al. 2012) to a depth of 6.000 (soil samples) and 5.000 (root samples) sequences per sample. Taxa that occurred less than 10 times were removed. Bray–Curtis dissimilarities between samples were calculated using “vegdist” in the R package vegan (Oksanen et al. 2019). The function “diversity” in the same package was applied to determine the Shannon–Wiener Diversity Index (“Shannon Index”) and Simpson’s Index of Diversity (“Simpson Index”), and “specnumber” to determine species richness. Thereupon, evenness was calculated by Shannon Index/log (species count).
All the datasets were analysed using a linear mixed model using treatment (control and drought) and region soil (DR, IR, and MR) as fixed effects and start of the drought (separated into three groups) as a random effect (package: nlme, version: 3.1–151). For the data of biomass, growth, fine root architecture, and allocation of newly assimilated C, average values of each pot were calculated before applying linear-mixed model (n = 10). Normality of the residuals (Shapiro test/ qq-plots) and homogeneity of variances (Levene test) were tested for every model. If any fixed factor was significant, post-hoc test with Tukey correction (package: emmeans, version: 2.30–0) was performed. Correlations of fine root parameters were determined with Pearson correlation (“cor.test” in stats). For sequence data, multivariate homogeneity of group dispersions was tested using the function “betadisper” in vegan. To test the effects of treatment and soil region, soil- and root-community data were analysed comparatively with a permutational multivariate analysis of variance (PERMANOVA) using “adonis” (package vegan), whereby all models were run with 9.999 permutations. If significant differences between the levels of a factor occurred, a multilevel pairwise comparisons of permutational multivariate analysis of variance (pairwise PERMANOVA; pairwise.adonis; (Martinez Arbizu 2020)) with Bonferroni p-value correction was applied. The functions “prune_taxa” and “transform_sample_counts” from the package phyloseq (version 1.36.0; McMurdie and Holmes 2013) were used to assess relative abundances of fungal trophic modes and exploration types. Data in text and tables are given as the mean ± 1SE, unless stated otherwise.
Results
Efficacy of the drought treatment
Soil moisture in the drought treatment was equally low for all region soils (Fig. 1a). Soil water content (SWC) of drought pots gradually decreased after the start of the drought treatment and reached the target SWC of 12 vol.-% after 3–4 weeks. The mean SWC at the end of the drought treatment was 11.8 ± 0.3 vol.-% in DR, 11.3 ± 0.2 in IR, and 10.9 ± 0.3 in MR soil (Fig. 1b), which was significantly lower than that of controls with around 20% (treatment p < 0.001, Table S6).
While SWC of drought pots was significantly lower than that of controls in all region soils, ΨPD of drought seedlings was only significantly lower in DR and IR soils (treatment x region soil p < 0.001, Fig. 1c). Despite the similar SWC and its relationship with soil water potential in of all three region soils, drought treated seedlings growing in MR soil showed similar ΨPD to the controls, and thus significantly higher ΨPD (-0.84 ± 0.10 MPa) than drought seedlings growing in DR and IR soil (with c. -1.90 MPa), while ΨPD of control seedlings were similar in all region soils (-0.56 MPa on average).
Net carbon assimilation rates and stomatal conductance showed similar patterns as ΨPD (Fig. 1d,e). Drought treatment significantly reduced both parameters, while the extent of the decrease was different among region soils (treatment x region soil p < 0.001 Table S6). While assimilation rates and stomatal conductance of DR and IR seedlings were reduced by > 75% under drought compared to controls, the decrease in MR seedlings were only 20% for assimilation rates and 47% for stomatal conductance.
Fungal community composition
10 root samples and 13 soil samples have been excluded from further analyses due to a low sequencing depth. For further analysis, 2571 OTUs originating from root samples, and 2299 OTUs from soil samples were left. Because soil fungal community composition did not significantly change between pre-drought and post-drought controls during the two-month drought treatment (p = 0.34, R2 = 0.02, PERMANOVA), the pre-drought samples have been excluded and only the post-drought samples in both treatments have been compared.
The soil fungal community composition only differed significantly between the three different region soils (p < 0.001, R2 = 0.62, PERMANOVA) but was not significantly influenced by drought treatment within the different region soils. All alpha-diversity metrics were highest in DR soil (Table S7). Saprotrophic fungi were the most abundant group in all region soils (Fig. 2a) and their abundance increased from DR to IR to MR soil with no significant effect of drought treatment. The composition of the saprotrophic fungal community differed significantly between the different region soils (p < 0.001, R2 = 0.71, PERMANOVA). Similarly to saprotrophs, the ECM fungal community was significantly affected by the soil region (p < 0.001, R2 = 0.34, PERMANOVA) but not by drought treatment. The proportion of ECM fungi within the soil fungal community was higher in DR and IR compared to MR soil (Fig. 2a).
In contrast to the soil fungal community composition, the root-associated fungal community composition was affected by both, region soil (p < 0.001, R2 = 0.50, PERMANOVA) and drought (p = 0.002, R2 = 0.06, PERMANOVA). None of the diversity metrics of root-associated fungi were affected by drought treatment, but Shannon diversity, species richness and Evenness were significantly influenced by region soil (Table S7). PERMANOVA indicated that region soil and treatment significantly affected both, root-associated saprotrophic (region soil p = 0.0001, R2 = 0.48; drought p = 0.0031, R2 = 0.05) and ECM fungal communities (region soil p = 0.0001, R2 = 0.26; drought p = 0.0031, R2 = 0.05). In controls, saprotrophic and ECM fungi made up around 50% each in IR and MR roots, while in DR roots the abundance of saprotrophs was slightly higher than of ECM fungi (Fig. 2b). The relative abundance of saprotrophs increased with drought treatment in IR and MR roots accompanying a decrease of ECM fungi. In DR roots, however, abundance changes were minimal. For both soil and root-associated fungal communities, “region soil” had a higher effect size compared to “drought treatment”.
The most abundant soil saprotrophic species differed between the region soils but were not affected by drought treatment in any region soil (Table S8). Among the ECM species (Table S9), it was noticeable that in both, DR and IR, drought tolerant Melanogaster sp. was by far the most abundant species, which did not change during drought. In MR, however, the abundance of fungal species changed with drought treatment. While Lactarius sp., Amanita sp. and Hebeloma radicosum were predominant in controls, Amanita sp. and Hydnotrya tulasnei, were the most common species in the drought treatment. Lactarius sp., on the other hand, only accounted for 4% in the drought treatment compared to 24.2% in controls.
Among root-associated saprotrophs (Table S10), Oidiodendron sp., a species with primary saprotrophic and secondary root-endophytic lifestyle, was highly abundant in each region soil in controls and under drought. With drought, the abundance of Penicillium sp. increased in root samples from DR and IR, but not in MR soils. Among ECM fungi (Table S11), Melanogaster sp. was by far the most abundant species in DR and IR roots in both, controls and drought. In MR, however, the abundance of species changed drastically with drought. While Sebacina sp. made up 96% of all ECM species in MR controls, it decreased to only 0.1% under drought. The abundance of drought tolerating Amanita sp. in MR on the other hand increased from 0.4% in controls to 78% under drought, and Theleophora terrestris, another drought tolerating species, made up 15% of all ECM species in MR during drought, while it was not detected in controls.
ECM exploration types (Fig. S4) were dominated by long- and medium-distance types in DR and IR soils and roots in both controls and during drought, whereby a high share of long-distance explorers was covered by Melanogaster sp.. In MR soils and roots, however, short- and medium distance explorers were predominating in controls, while during drought, the strong increase of Amanita sp. and Hydnotrya tulasnei represented a dominance of contact types.
Biomass and growth of seedlings
Initial plant biomass (Fig. S1) and biomass of control plants at the harvest (Fig. 3a) were the highest in IR soil and the lowest in the MR soil (region soil p < 0.001, Table S6). Drought treatment significantly reduced the relative stem growth (Fig. 3b, treatment p < 0.05, Table S6), which led to a significantly lower biomass of drought treated seedlings at the harvest compared to the controls (treatment p < 0.01, Fig. 3a, Table S6). Reduction of the relative stem growth under drought was higher in DR (from 40 ± 6% in control to 20 ± 4% in drought seedlings, p = 0.07 according to post-hoc test, Fig. 3b) and in IR seedlings (from 25 ± 3% in control to 10 ± 4% in drought, p = 0.4) compared to MR seedlings (from 32 ± 7% in control to 28 ± 3% in drought, p > 0.9). Root to shoot ratio (Fig. 3c) significantly decreased under drought (treatment p < 0.01, Table S6), while the ratio was significantly higher in IR and MR seedlings compared to DR seedlings (region soil p < 0.01, Table S6). Specific leaf area (SLA) was not affected by drought treatment, although it was significantly higher in IR compared to DR and MR seedlings (region soil p < 0.05, Table S6, Fig. S5).
a Biomass at the harvest separately in leaf, stem, coarse root, and fine root under control (blue) and drought (red) treatment. b Relative stem growth during the drought treatment of 8 weeks. c Ratio of root to shoot biomass at the harvest. Dry region (DR), intermediate region (IR) and moist region (MR) soil. Asterisks indicate significant differences of total biomass among treatments and region soils. ***; p < 0.001, **; p < 0.01, n.s.; not significant. Lowercase letters indicate significant differences among the groups according to post-hoc test
Fine root architecture
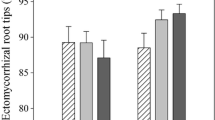
The root architecture described by taproot length, branching intensity, vital tips, and mycorrhizal colonisation differed between the treatments and except vital tips between region soils (Table S6). With drought, seedlings in DR and MR were rooting significantly longer (Fig. 4a) and had a lower branching intensity (Fig. 4b). Furthermore, branching intensity was negatively correlated with taproot length (p < 0.001, r = -0.5) indicating that as taproot length increased, the number of branches per cm declined. The number of vital tips tended to decrease in DR and IR, but not significantly (Fig. 4c). The mycorrhizal colonisation, however, declined with drought in IR and MR soils whereby the decrease was not significant in MR (Fig. 4d). Nevertheless, a decline of mycorrhization in MR soil was supported by a low number of sequence reads in MR drought samples.
Fine root architecture including taproot length (a), branching intensity (b), percentage of vital root tips (c) and mycorrhizal colonisation (d). Blue boxes represent controls and red boxes drought treatment. Dry region (DR), intermediate region (IR) and moist region (MR) soil. Lowercase letters indicate significant differences among the groups according to post-hoc test
Allocation of newly assimilated C
Control seedlings allocated similar amounts of newly assimilated C to belowground organs in all region soils (47 ± 3% of total newly assimilated C, Fig. 5), while drought effects were different among region soils (treatment x region soil p < 0.05, Table S6). Drought significantly reduced the belowground allocation of newly assimilated C in DR and IR seedlings. Here, more newly assimilated C remained in the leaves (54 ± 5% and 53 ± 3% of total newly assimilated C) compared to control plants with 36 ± 5% and 30 ± 2% in the leaves, respectively. In contrast, similar amounts of newly assimilated C were allocated belowground in MR seedlings under both control and drought treatments (46 ± 3%).
Allocation of newly assimilated C (Cnew) in leaf, stem, coarse roots and fine roots after three days of labelling under control (blue) and drought (red) treatments. Dry region (DR), intermediate region (IR) and moist region (MR) soil. The asterisk indicates significant differences in the ratio of belowground to total Cnew between control and drought plants according to post-hoc test: **; p < 0.01, *; p < 0.05, n.s.; not significant
NSC concentration and pools
Sugar concentration of control seedlings was similar in all organs among the three region soils (Fig. S6, except for coarse root, where DR seedlings showed lower sugar concentration compared to the IR seedlings). Under drought, sugar concentration significantly increased in all organs of DR and IR seedlings (Fig. S6), while MR seedlings only increased their sugar concentration in the stem. Starch concentration in the leaf significantly increased in IR seedlings under drought, while it decreased in MR seedlings (Fig. S7, treatment x region soil p < 0.05, Table S6). Starch concentration in stems and coarse roots displayed only an effect of region soil in DR seedlings showing lower concentrations than the IR and MR seedlings (p < 0.05, Table S6). Starch concentration in fine roots was similar in all seedlings under both control and drought treatments.
Ratios of belowground to total sugar pools varied between 40–45% in the seedlings growing in different region soils (Fig. 6a); however, the ratio did not differ among seedlings growing in the different region soils and were not affected by drought (Table S6). For starch the ratio of the belowground to the total starch pool was also not affected by drought (Table S6), but plants grown in DR soil had a significantly higher ratio compared to seedlings grown in MR soil (region soil p < 0.05 Table S6; Fig. 6b).
Ratio of belowground (root) sugar and starch content to the total sugar (a) and starch (b) pools (leaf, stem, coarse and fine roots all combined), in dry region (DR, purple), intermediate region (IR, yellow) and moist region (MR, green) soil. Lowercase letters indicate significant differences among the groups according to post-hoc test
Discussion
The present study aimed to elucidate the effects of soils with different long-term precipitation histories on soil and root-associated fungal community composition and beech seedling performance in response to a drought.
Fungal communities in dry region soil were less affected by drought
Before the onset of drought (soil) and in controls (soil and root-associated), fungal communities differed among the region soils, suggesting an adaptation of fungal communities to soil moisture over time, which has previously been shown for ECM fungi along a soil moisture gradient (Cavender-Bares et al. 2009). Likewise, Canarini et al. (2021) stated that soil microbial communities were more drought tolerant after repeated long-term drought, which could enhance the resilience of ecosystem functioning. A more in-depth look at the species composition reinforced the presumption of community adaptation. Among the saprotrophs in soil and root samples, Penicillium sp., Mortierella sp., and Oidiodendron sp. were dominating. Of these, Penicillium sp. was found in earlier studies mainly when low soil moisture prevailed (Ridout et al. 2017). Mortierella sp. and Oidiodendron sp. have been classified as species with secondary root-associated or root-endophytic lifestyle (Põlme et al. 2020), that may help the fungi surviving dry periods (Smith et al. 2017). While a high occurrence of drought-tolerant ECM fungal species such as Melanogaster (Izzo et al. 2005; Frey et al. 2021), have been detected in DR and IR in both controls and during drought, this was not the case in MR.
Whereas there was no effect of the drought treatment on the soil fungal communities, there was a drought effect on the root-associated fungal communities. This indicates that soil fungal communities might be more stable compared to root-associated fungal communities during a short-term drought event (de Vries et al. 2012, 2018; Fu et al. 2022). This is in line with several other studies which found that soil fungal communities are largely unaffected by droughts of different durations and severities (Bastida et al. 2019; Liu et al. 2020; Wilhelm et al. 2022). A possible explanation could be that root-associated fungi responded to the seedlings’ reaction to drought (Lozano et al. 2021) such as increased exudation (Karst et al. 2017; Williams and de Vries 2020; Brunn et al. 2022) or changed root architecture (Nikolova et al. 2020). Soil fungi, on the other hand, are not in direct contact with the root and might therefore be less influenced by these responses.
With drought, the root-associated fungal community composition changed most in MR and least in DR soils when considering the abundance of different trophic modes, fungal exploration types and species abundances, which support a natural adaptation of fungal communities to soil moisture deficits in the DR soil. In root samples (Fig. 2b), the relative abundance of saprotrophic and ECM fungi in controls was around 50% each in all region soils, but with drought the relative abundance of saprotrophs increased in IR and the most in MR roots. This supports our hypothesis for root associated fungi that fungal communities in DR are least affected and is in line with a study by Lozano et al. (2021), who found that the abundance of saprotrophic fungi increased during drought, while it decreased for mutualist fungi. Furthermore, some saprotrophic species have been identified to have a secondary, root-associated lifestyle (Tedersoo and Smith 2013; Smith et al. 2017), which enables them to occupy multiple ecological niches (Selosse et al. 2018) and thus compete with ECM fungi. Different adaptations within the three region soils to drought was also reflected at the species level of root-associated fungi. While in DR and IR root-associated communities Melanogaster sp. made up the vast majority in both control and drought treatments, there was a drastic change in species abundance in MR. Here, the abundance of Amanita sp., a drought-tolerant fungus (Querejeta et al. 2003) classified as contact exploration type (Agerer 2001), increased extremely under drought compared to controls. This complies with an increase in the abundance of contact types under prolonged drought (Castaño et al. 2018), and may be explained by their lower carbon costs to sustain (Lehto and Zwiazek 2011). In this experiment, however, no significant difference in the supply of new photoassimilates to roots was detected between controls and drought treated plants in MR soil (Fig. 5).
In total, the fungal communities in DR soil roots were least affected by drought treatment, while those in MR soil roots underwent the greatest changes, confirming our first hypothesis that fungal communities in DR soil are less affected by drought. In soil fungal communities, however, no changes have been observed.
Beech grown in dry region soil were less acclimated to drought at fine root level
While fungal communities in DR soil appear to be better adapted to drought compared to those in MR soil, seedlings grown in DR soil did not show any better acclimation in fine root architecture, contrasting with our second hypothesis. Rather, seedlings grown in MR soil had a longer root system and lower branching intensity than those grown in DR and IR soil (Fig. 4a,b). This may result from slight differences in soil texture with a higher sand proportion in MR compared to DR soil, since fine root growth rate is higher in sand than in clay soil for beech (Weemstra et al. 2017). Potentially, seed origin (Alpenvorland, moist region) could have genetically contributed to differences in drought responses based on the different selection pressures of habitats, i.e. possible advantages in MR soil compared to in DR soil. However, this is unlikely the main effect in our study, since our seed source when grown in moist as well as dry region soils showed a similar plasticity in fine roots under drought. This is supported by a previous study across Europe reporting no effect of local adaptation on survival of beech seedlings (Muffler et al. 2021). Under drought, seedlings in both DR and MR soils significantly increased taproot length with a parallel decrease in branching, whereby the fine roots in MR soil rooted longest compared to the plants growing in IR and DR soils. Longer and deeper growing roots with less branching have been identified as active drought response in seedlings (Meijer et al. 2011; Asefa et al. 2022). As observed in mature European beech forests (Hodge 2004; Wambsganss et al. 2021), beech seedlings showed a high morphological plasticity in roots (towards faster foraging strategy) to cope with drought. Furthermore, although the root to shoot ratio decreased under drought in all region soils (Fig. 3c), contrary to the global observations (Poorter et al. 2012; Tumber-Dávila et al. 2022), it was significantly higher in MR compared to DR seedlings.
All in all, these results provide no support for our second hypothesis, that seedlings grown in DR soil would be better acclimated to dry conditions at the fine root level compared to those grown in MR soil.
Acclimation in fine root rather than adaptation in fungal community composition mitigated drought effect on beech seedlings
It is often discussed that a mycorrhizal symbiosis can improve the drought performance in tree seedlings (e.g. Querejeta et al. 2003; Marjanović et al. 2005; Bréda et al. 2006; Wang et al. 2021). In the present study, however, even though the root fungal communities appear to be adapted to low SWC, a relative improvement of seedling growth during drought was not observed in DR soil. Seedlings growing in MR soil were least stressed during the drought while having the least adapted fungal community and lowest mycorrhizal colonisation, indicating that mycorrhization only had a minor contribution on seedling plant water uptake (Steudle and Heydt 1997; Buchenau et al. 2022). Rather, the longer and deeper root architecture may have contributed more to the better performance of the MR seedlings under drought than the fungal community composition. These results are similar to the findings by Moser et al. (2015), that root architecture rather than ECM colonisation is crucial for drought resistance of Pinus sylvestris. Kipfer et al. (2012) also found that a symbiosis with ECM fungi did not provide additional support during desiccation. Allsup et al. (2023) found that an inoculation of seedlings with fungal communities from dry regions enhances the plant drought survival, but this applied only to arbuscular mycorrhizal fungi while ECM fungi had no significant effect on the seedlings’ drought performance. ECM fungi influence root growth by regulating fungal and host growth hormones (Fitter 1987; Hetrick 1991; Navarro-Ródenas et al. 2013; Calvo-Polanco et al. 2019; de Freitas Pereira et al. 2023). Therefore, it is possible that the more sparsely fungus-colonised roots in MR were less inhibited by fungi in their growth and were thus able to grow longer into the soil. As a result, these seedlings in MR showed a higher ΨPD during drought (Fig. 1c), leading to their better photosynthesis and growth (Figs. 1 and 3). We are aware that the average taproot length was longer than the depth of the pots (> 20 cm) and we don´t know how the taproots were growing inside pots. However, we assume that the taproots after 20 cm were growing at the bottom of the pots where the highest water availability can be expected. Therefore, it is likely that the longest taproot length of MR seedlings contributed to their better water uptake and higher ΨPD by larger soil exploration. Differences in soil texture are unlikely the cause for the different ΨPD, since the relationship between soil water potential and the SWC were similar among three region soils (Fig. S3). Furthermore, smaller initial biomass and leaf area of MR seedlings (Fig. S1) are probably caused by the higher C/N ratio of MR soil compared to the other region soils and can be a potential cause for less drought effects through less water use, in addition to the root architecture. However, this is unlikely since SWC measurements before the regular watering showed similar values (Fig. 1a) and the amount of added water was similar in all three region soils. Allocation of newly assimilated C to belowground sinks decreased under drought following the decrease in ΨPD, as observed in previous studies in beech seedlings (Ruehr et al. 2009; Zang et al. 2014) and other species (Poorter et al. 2012). Although allocation of newly assimilated C belowground is important for the maintenance and growth of roots (Blessing et al. 2016; Hommel et al. 2016; Hikino et al. 2022), less C was transferred there in DR and IR seedlings under drought, thus no acclimation was observed in C allocation contrary to our second hypothesis. This is likely due to delayed sugar export from leaves and/or reduced phloem transport (Zang et al. 2014; Hesse et al. 2018). Although roots are important for long-term reserve storage (Blessing et al. 2016; Wiley et al. 2019; Montague et al. 2022) and root growth (Wang et al. 2018; Tang et al. 2022; Domingo et al. 2023), allocation to NSC reserve pools did not shift to belowground organs under drought in our study contrary to observations in other species (Galvez et al. 2011, 2013; Chuste et al. 2020). This might be due to a shorter duration or lower intensity of drought in the present study compared to their studies. Although the ratio of belowground to total starch pool was significantly higher in DR compared to MR plants (Fig. 6a), these reserves were not used during drought and thus did likely not contribute to the mitigation of drought, maintaining or even increasing reserves in roots (Galvez et al. 2011, 2013).
Above all, the results indicate that MR seedlings, which showed some acclimation to drought at the fine root level, experienced the least drought stress during this experiment. Additionally, the fungal community associated with MR seedlings significantly changed towards more drought-tolerant species. These findings support our third hypothesis, stating that the root system including root and associated fungi can mitigate drought effects on plants.
Conclusion
A changing climate with more frequent and severe droughts challenges global forests. Hence, it is essential to evaluate which factors can positively impact tree survival. The results of this study suggest that the drought stress response of beech seedlings appears to depend more strongly on the plasticity of root architecture rather than on fungal community composition even if soil fungal communities are adapted to drought conditions. However, a fungal contribution to the seedlings’ drought performance cannot be excluded completely and needs to be studied further by e.g. including other groups of microorganisms and their interactions with root-associated fungi, as well as specific root-fungus interactions. Specifically, inoculation of sterilized seedlings with single drought tolerant fungal strains from our study could separate the direct effect of root-fungus interaction from the effect of root architecture. Since this study explores these relationships on seedlings in a rather artificial environment, we cannot preclude differing responses that will occur under natural conditions and that vary with tree age (Hartmann et al. 2018). Nevertheless, this study pinpoints the need to evaluate the effects of drought on trees in the context of root associated microorganisms.
Data availability
The raw sequencing data presented in this study are openly available in in the NCBI Sequence Read Archive and included in the BioProject with accession number PRJNA1063582. All other data presented in this study are available in this article and the respective supplementary information.
References
Aaltonen H, Linden A, Heinonsalo J et al (2017) Effects of prolonged drought stress on Scots pine seedling carbon allocation. Tree Physiol 37:418–427. https://doi.org/10.1093/treephys/tpw119
Agerer R (2001) Exploration types of ectomycorrhizae. Mycorrhiza 11:107–114. https://doi.org/10.1007/s005720100108
Allsup CM, George I, Lankau RA (2023) Shifting microbial communities can enhance tree tolerance to changing climates. Science 380:835–840. https://doi.org/10.1126/science.adf2027
Anthony MA, Crowther TW, van der Linde S et al (2022) Forest tree growth is linked to mycorrhizal fungal composition and function across Europe. ISME J 1–10. https://doi.org/10.1038/s41396-021-01159-7
Arend M, Link RM, Zahnd C et al (2022) Lack of hydraulic recovery as a cause of post-drought foliage reduction and canopy decline in European beech. New Phytol 234:1195–1205. https://doi.org/10.1111/nph.18065
Asefa M, Worthy SJ, Cao M et al (2022) Above- and below-ground plant traits are not consistent in response to drought and competition treatments. Ann Bot 1–12. https://doi.org/10.1093/aob/mcac108
Bastida F, López-Mondéjar R, Baldrian P et al (2019) When drought meets forest management: effects on the soil microbial community of a Holm oak forest ecosystem. Sci Total Environ 662:276–286. https://doi.org/10.1016/j.scitotenv.2019.01.233
Beloiu M, Stahlmann R, Beierkuhnlein C (2022) Drought impacts in forest canopy and deciduous tree saplings in Central European forests. For Ecol Manag 509. https://doi.org/10.1016/j.foreco.2022.120075
Bengtsson-Palme J, Ryberg M, Hartmann M et al (2013) Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol Evol 4:914–919. https://doi.org/10.1111/2041-210X.12073
Blessing CH, Werner RA, Siegwolf R, Buchmann N (2015) Allocation dynamics of recently fixed carbon in beech saplings in response to increased temperatures and drought. Tree Physiol 35:585–598. https://doi.org/10.1093/treephys/tpv024
Blessing CH, Barthel M, Gentsch L, Buchmann N (2016) Strong coupling of shoot assimilation and soil respiration during drought and recovery periods in beech as indicated by natural abundance δ13 C measurements. Front Plant Sci 7:1–12. https://doi.org/10.3389/fpls.2016.01710
Bréda N, Huc R, Granier A, Dreyer E (2006) Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann for Sci 63:625–644. https://doi.org/10.1051/forest:2006042
Brinkmann N, Eugster W, Buchmann N, Kahmen A (2019) Species-specific differences in water uptake depth of mature temperate trees vary with water availability in the soil. Plant Biol 21:71–81. https://doi.org/10.1111/plb.12907
Brunn M, Hafner BD, Zwetsloot MJ et al (2022) Carbon allocation to root exudates is maintained in mature temperate tree species under drought. New Phytol 965–977. https://doi.org/10.1111/nph.18157
Brunner I, Herzog C, Dawes MA et al (2015) How tree roots respond to drought. Front Plant Sci 6:547. https://doi.org/10.3389/fpls.2015.00547
Buchenau N, van Kleunen M, Wilschut RA (2022) Direct and legacy-mediated drought effects on plant performance are species-specific and depend on soil community composition. Oikos 2022. https://doi.org/10.1111/oik.08959
Buhk C, Kämmer M, Beierkuhnlein C et al (2016) On the influence of provenance to soil quality enhanced stress reaction of young beech trees to summer drought. Ecol Evol 6:8276–8290. https://doi.org/10.1002/ece3.2472
Calvo-Polanco M, Armada E, Zamarreño AM et al (2019) Local root ABA/cytokinin status and aquaporins regulate poplar responses to mild drought stress independently of the ectomycorrhizal fungus Laccaria bicolor. J Exp Bot 70:6437–6446. https://doi.org/10.1093/jxb/erz389
Canarini A, Schmidt H, Fuchslueger L et al (2021) Ecological memory of recurrent drought modifies soil processes via changes in soil microbial community. Nat Commun 12:1–14. https://doi.org/10.1038/s41467-021-25675-4
Castaño C, Lindahl BD, Alday JG et al (2018) Soil microclimate changes affect soil fungal communities in a Mediterranean pine forest. New Phytol 220:1211–1221. https://doi.org/10.1111/nph.15205
Castaño C, Suarez-Vidal E, Zas R, et al (2023) Ectomycorrhizal fungi with hydrophobic mycelia and rhizomorphs dominate in young pine trees surviving experimental drought stress. Soil Biol Biochem 178. https://doi.org/10.1016/j.soilbio.2022.108932
Cavender-Bares J, Izzo A, Robinson R, Lovelock CE (2009) Changes in ectomycorrhizal community structure on two containerized oak hosts across an experimental hydrologic gradient. Mycorrhiza 19:133–142. https://doi.org/10.1007/s00572-008-0220-3
Chen J, Bittinger K, Charlson ES et al (2012) Associating microbiome composition with environmental covariates using generalized UniFrac distances. Bioinformatics 28:2106–2113. https://doi.org/10.1093/bioinformatics/bts342
Choat B, Brodribb TJ, Brodersen CR et al (2018) Triggers of tree mortality under drought. Nature 558:531–539. https://doi.org/10.1038/s41586-018-0240-x
Chuste PA, Maillard P, Bréda N et al (2020) Sacrificing growth and maintaining a dynamic carbohydrate storage are key processes for promoting beech survival under prolonged drought conditions. Trees - Struct Funct 34:381–394. https://doi.org/10.1007/s00468-019-01923-5
Clément C, Pierret A, Maeght JL et al (2019) Linking tree-rooting profiles to leaf phenology: a first attempt on Tectona Grandis Linn F. Trees - Struct Funct 33:1491–1504. https://doi.org/10.1007/s00468-019-01876-9
Contran N, Günthardt-Goerg MS, Kuster TM et al (2013) Physiological and biochemical responses of Quercus pubescens to air warming and drought on acidic and calcareous soils. Plant Biol 15:157–168. https://doi.org/10.1111/j.1438-8677.2012.00627.x
Danzberger J, Werner R, Mucha J et al (2023) Drought legacy effects on fine-root-associated fungal communities are modulated by root interactions between tree species. Front Glob Change 6:1–16. https://doi.org/10.3389/ffgc.2023.1197791
de Freitas PM, Cohen D, Auer L et al (2023) Ectomycorrhizal symbiosis prepares its host locally and systemically for abiotic cue signaling. Plant J. https://doi.org/10.1111/tpj.16465
de Vries FT, Liiri ME, Bjørnlund L et al (2012) Land use alters the resistance and resilience of soil food webs to drought. Nat Clim Change 2:276–280. https://doi.org/10.1038/nclimate1368
de Vries FT, Griffiths RI, Bailey M et al (2018) Soil bacterial networks are less stable under drought than fungal networks. Nat Commun 9:3033. https://doi.org/10.1038/s41467-018-05516-7
Domingo G, Vannini C, Marsoni M et al (2023) A multifaceted approach to reveal the very-fine root’s response of Fagus sylvatica seedlings to different drought intensities. Physiol Plant 175:1–15. https://doi.org/10.1111/ppl.13934
Egerton-Warburton LM, Querejeta JI, Allen MF (2007) Common mycorrhizal networks provide a potential pathway for the transfer of hydraulically lifted water between plants. J Exp Bot 58:1473–1483. https://doi.org/10.1093/jxb/erm009
Fitter AH (1987) An architectural approach to the comparative ecology of plant root systems. New Phytol 106:61–77. https://doi.org/10.1111/j.1469-8137.1987.tb04683.x
Fitzpatrick CR, Copeland J, Wang PW et al (2018) Assembly and ecological function of the root microbiome across angiosperm plant species. Proc Natl Acad Sci U S A 115:E1157–E1165. https://doi.org/10.1073/pnas.1717617115
Frei ER, Gossner MM, Vitasse Y et al (2022) European beech dieback after premature leaf senescence during the 2018 drought in northern Switzerland. Plant Biol 24:1132–1145. https://doi.org/10.1111/plb.13467
Frey B, Walthert L, Perez-Mon C, et al (2021) Deep soil layers of drought-exposed forests harbor poorly known bacterial and fungal communities. Front Microbiol 12. https://doi.org/10.3389/fmicb.2021.674160
Fu W, Chen B, Jansa J et al (2022) Contrasting community responses of root and soil dwelling fungi to extreme drought in a temperate grassland. Soil Biol Biochem 169:108670. https://doi.org/10.1016/j.soilbio.2022.108670
Galvez DA, Landhäusser SM, Tyree MT (2011) Root carbon reserve dynamics in aspen seedlings: does simulated drought induce reserve limitation? Tree Physiol 31:250–257. https://doi.org/10.1093/treephys/tpr012
Galvez DA, Landhäusser SM, Tyree MT (2013) Low root reserve accumulation during drought may lead to winter mortality in poplar seedlings. New Phytol 198:139–148. https://doi.org/10.1111/nph.12129
Gebauer R, Plichta R, Urban J et al (2020) The resistance and resilience of European beech seedlings to drought stress during the period of leaf development. Tree Physiol 40:1147–1164. https://doi.org/10.1093/treephys/tpaa066
Germon A, Laclau JP, Robin A, Jourdan C (2020) Tamm review: deep fine roots in forest ecosystems: why dig deeper? For Ecol Manag 466. https://doi.org/10.1016/j.foreco.2020.118135
Geßler A, Keitel C, Kreuzwieser J et al (2007) Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees - Struct Funct 21:1–11. https://doi.org/10.1007/s00468-006-0107-x
Gundale MJ, Kardol P, Nilsson MC et al (2014) Interactions with soil biota shift from negative to positive when a tree species is moved outside its native range. New Phytol 202:415–421. https://doi.org/10.1111/nph.12699
Gweon HS, Oliver A, Taylor J et al (2015) PIPITS: An automated pipeline for analyses of fungal internal transcribed spacer sequences from the Illumina sequencing platform. Methods Ecol Evol 6:973–980. https://doi.org/10.1111/2041-210X.12399
Hartmann H, Adams HD, Hammond WM et al (2018) Identifying differences in carbohydrate dynamics of seedlings and mature trees to improve carbon allocation in models for trees and forests. Environ Exp Bot 152:7–18. https://doi.org/10.1016/j.envexpbot.2018.03.011
Hesse BD, Goisser M, Hartmann H, Grams TEE (2018) Repeated summer drought delays sugar export from the leaf and impairs phloem transport in mature beech. Tree Physiol 39:192–200. https://doi.org/10.1093/treephys/tpy122
Hetrick BAD (1991) Mycorrhizas and root architecture. Experientia 47:355–362. https://doi.org/10.1007/BF01972077
Hikino K, Danzberger J, Riedel VP et al (2022) Dynamics of initial C allocation after drought release in mature Norway spruce ‐ Increased belowground allocation of current photoassimilates covers only half of the C used for fine‐root growth. Glob Change Biol 1–17. https://doi.org/10.1111/gcb.16388
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24. https://doi.org/10.1111/j.1469-8137.2004.01015.x
Hommel R, Siegwolf R, Zavadlav S et al (2016) Impact of interspecific competition and drought on the allocation of new assimilates in trees. Plant Biol Stuttg Ger 18:785–796. https://doi.org/10.1111/plb.12461
Izzo AD, Meyer M, Trappe JM et al (2005) Hypogeous ectomycorrhizal fungal species on roots and in small mammal diet in a mixed-conifer forest. For Sci 51:243–254
Kahmen A, Basler D, Hoch G et al (2022) Root water uptake depth determines the hydraulic vulnerability of temperate European tree species during the extreme 2018 drought. Plant Biol 24:1224–1239. https://doi.org/10.1111/plb.13476
Karst J, Gaster J, Wiley E, Landhäusser SM (2017) Stress differentially causes roots of tree seedlings to exude carbon. Tree Physiol 37:154–164. https://doi.org/10.1093/treephys/tpw090
Kipfer T, Wohlgemuth T, van der Heijden MGA et al (2012) Growth response of drought-stressed pinus sylvestris seedlings to single- and multi-species inoculation with ectomycorrhizal fungi. PLoS ONE 7:e35275. https://doi.org/10.1371/journal.pone.0035275
Kivlin SN, Emery SM, Rudgers JA (2013) Fungal symbionts alter plant responses to global change. Am J Bot 100:1445–1457. https://doi.org/10.3732/ajb.1200558
Kõljalg U, Nilsson RH, Abarenkov K et al (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277. https://doi.org/10.1111/mec.12481
Körner C (2011) The Grand Challenges in Functional Plant Ecology. Front Plant Sci 2. https://doi.org/10.3389/fpls.2011.00001
Landhäusser SM, Chow PS, Dickman LT et al (2018) Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiol 38:1764–1778. https://doi.org/10.1093/treephys/tpy118
Lata R, Chowdhury S, Gond SK, White JF (2018) Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol 66:268–276. https://doi.org/10.1111/lam.12855
Lehto T, Zwiazek JJ (2011) Ectomycorrhizas and water relations of trees: a review. Mycorrhiza 21:71–90. https://doi.org/10.1007/s00572-010-0348-9
Lendzion J, Leuschner C (2008) Growth of European beech (Fagus sylvatica L.) saplings is limited by elevated atmospheric vapour pressure deficits. For Ecol Manag 256:648–655. https://doi.org/10.1016/j.foreco.2008.05.008
Leuschner C, Wedde P, Lübbe T (2019) The relation between pressure–volume curve traits and stomatal regulation of water potential in five temperate broadleaf tree species. Ann For Sci 76. https://doi.org/10.1007/s13595-019-0838-7
Leuschner C (2020) Drought response of European beech (Fagus sylvatica L.)—A review. Perspect Plant Ecol Evol Syst 47:125576. https://doi.org/10.1016/j.ppees.2020.125576
Liu JF, Arend M, Yang WJ et al (2017) Effects of drought on leaf carbon source and growth of European beech are modulated by soil type. Sci Rep 7:1–9. https://doi.org/10.1038/srep42462
Liu Y, Li X, Kou Y (2020) Ectomycorrhizal fungi: participation in nutrient turnover and community assembly pattern in forest ecosystems. Forests 11. https://doi.org/10.3390/F11040453
Lozano YM, Aguilar-Trigueros CA, Roy J, Rillig MC (2021) Drought induces shifts in soil fungal communities that can be linked to root traits across 24 plant species. New Phytol 232:1917–1929. https://doi.org/10.1111/nph.17707
Marjanović Ž, Nehls U, Hampp R (2005) Mycorrhiza formation enhances adaptive response of hybrid poplar to drought. Ann N Y Acad Sci 1048:496–499. https://doi.org/10.1196/annals.1342.080
Martinez Arbizu P (2020) pairwiseAdonis: pairwise multilevel comparison using adonis. R package version 0.4
McCormack ML, Dickie IA, Eissenstat DM et al (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207:505–518. https://doi.org/10.1111/nph.13363
McMurdie PJ, Holmes S (2013) Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8:e61217. https://doi.org/10.1371/journal.pone.0061217
Meier IC, Leuschner C (2008a) Belowground drought response of European beech: fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob Change Biol 14:2081–2095. https://doi.org/10.1111/j.1365-2486.2008.01634.x
Meier IC, Leuschner C (2008b) Genotypic variation and phenotypic plasticity in the drought response of fine roots of European beech. Tree Physiol 28:297–309. https://doi.org/10.1093/treephys/28.2.297
Meier CE, Newton RJ, Puryear JD, Sen S (1992) Physiological responses of loblolly pine (Pinus taeda L.) seedlings to drought stress: osmotic adjustment and tissue elasticity. J Plant Physiol 140:754–760. https://doi.org/10.1016/S0176-1617(11)81034-5
Meijer SS, Holmgren M, Van Der Putten WH (2011) Effects of plant-soil feedback on tree seedling growth under arid conditions. J Plant Ecol 4:193–200. https://doi.org/10.1093/jpe/rtr011
Meisner A, Jacquiod S, Snoek BL et al (2018) Drought legacy effects on the composition of soil fungal and prokaryote communities. Front Microbiol 9:294. https://doi.org/10.3389/fmicb.2018.00294
Mohan JE, Cowden CC, Baas P et al (2014) Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: mini-review. Fungal Ecol 10:3–19. https://doi.org/10.1016/j.funeco.2014.01.005
Montague MS, Landhäusser SM, McNickle GG, Jacobs DF (2022) Preferential allocation of carbohydrate reserves belowground supports disturbance-based management of American chestnut (Castanea dentata). For Ecol Manag 509. https://doi.org/10.1016/j.foreco.2022.120078
Moser B, Kipfer T, Richter S et al (2015) Drought resistance of Pinus sylvestris seedlings conferred by plastic root architecture rather than ectomycorrhizal colonisation. Ann for Sci 72:303–309. https://doi.org/10.1007/s13595-014-0380-6
Muffler L, Schmeddes J, Weigel R et al (2021) High plasticity in germination and establishment success in the dominant forest tree Fagus sylvatica across Europe. Glob Ecol Biogeogr 30:1583–1596. https://doi.org/10.1111/geb.13320
Nahm M, Matzarakis A, Rennenberg H, Geßler A (2007) Seasonal courses of key parameters of nitrogen, carbon and water balance in European beech (Fagus sylvatica L.) grown on four different study sites along a European North-South climate gradient during the 2003 drought. Trees - Struct Funct 21:79–92. https://doi.org/10.1007/s00468-006-0098-7
Navarro-Ródenas A, Bárzana G, Nicolás E et al (2013) Expression analysis of aquaporins from desert truffle mycorrhizal symbiosis reveals a fine-tuned regulation under drought. Mol Plant Microbe Interact 26:1068–1078. https://doi.org/10.1094/MPMI-07-12-0178-R
Nguyen NH, Song Z, Bates ST et al (2016a) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248. https://doi.org/10.1016/j.funeco.2015.06.006
Nguyen NH, Williams LJ, Vincent JB et al (2016b) Ectomycorrhizal fungal diversity and saprotrophic fungal diversity are linked to different tree community attributes in a field-based tree experiment. Mol Ecol 25:4032–4046. https://doi.org/10.1111/mec.13719
Nickel UT, Weikl F, Kerner R et al (2018) Quantitative losses vs. qualitative stability of ectomycorrhizal community responses to 3 years of experimental summer drought in a beech–spruce forest. Glob Change Biol 24:e560–e576. https://doi.org/10.1111/gcb.13957
Nikolova PS, Bauerle TL, Häberle KH et al (2020) Fine-root traits reveal contrasting ecological strategies in European beech and Norway spruce during extreme drought. Front Plant Sci 11. https://doi.org/10.3389/fpls.2020.01211
Nilsson RH, Tedersoo L, Ryberg M et al (2015) A comprehensive, automatically updated fungal ITS sequence dataset for reference-based chimera control in environmental sequencing efforts. Microbes Environ 30:145–150. https://doi.org/10.1264/jsme2.ME14121
Oksanen AJ, Blanchet FG, Friendly M, Kindt R, Legendre P, Mcglinn D et al (2019) Vegan. In: Kaplan DM (ed) Encyclopedia of food and agricultural ethics. Springer Netherlands, Dordrecht, pp 2395–2396. https://doi.org/10.1007/978-94-0241179-9_301576
Outamamat E, El Mrabet S, Dounas H et al (2022) Symbiotic interactions between a newly identified native mycorrhizal fungi complex and the endemic tree Argania spinosa mediate growth, photosynthesis, and enzymatic responses under drought stress conditions. Can J Res 52:335–345. https://doi.org/10.1139/cjfr-2020-0351
Pena R, Bluhm SL, Ammerschubert S et al (2023) Mycorrhizal C/N ratio determines plant-derived carbon and nitrogen allocation to symbiosis. Commun Biol 6:1–10. https://doi.org/10.1038/s42003-023-05591-7
Petrik P, Petek-Petrik A, Kurjak D et al (2022) Interannual adjustments in stomatal and leaf morphological traits of European beech ( Fagus sylvatica L.) demonstrate its climate change acclimation potential. Plant Biol 24:1287–1296. https://doi.org/10.1111/plb.13401
Põlme S, Abarenkov K, Nilsson RH et al (2020) FungalTraits: a user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers 105. https://doi.org/10.1007/s13225-020-00466-2
Poorter H, Niklas KJ, Reich PB et al (2012) Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol 193:30–50. https://doi.org/10.1111/j.1469-8137.2011.03952.x
Porter SS, Bantay R, Friel CA et al (2020) Beneficial microbes ameliorate abiotic and biotic sources of stress on plants. Funct Ecol 34:2075–2086. https://doi.org/10.1111/1365-2435.13499
Pretzsch H, Rötzer T, Matyssek R et al (2014) Mixed Norway spruce (Picea abies [L.] Karst) and European beech (Fagus sylvatica [L.]) stands under drought: from reaction pattern to mechanism. Trees - Struct Funct 28:1305–1321. https://doi.org/10.1007/s00468-014-1035-9
Querejeta JI, Egerton-Warburton LM, Allen MF (2003) Direct nocturnal water transfer from oaks to their mycorrhizal symbionts during severe soil drying. Oecologia 134:55–64. https://doi.org/10.1007/s00442-002-1078-2
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rennenberg H, Seiler W, Matyssek R et al (2004) Die Buche (Fagus sylvatica L.) - ein Waldbaum ohne Zukunft im südlichen Mitteleuropa? Allgemeine Forst- und Jagdzeitung 175:210–224
Richard F, Roy M, Shahin O et al (2011) Ectomycorrhizal communities in a Mediterranean forest ecosystem dominated by Quercus ilex: seasonal dynamics and response to drought in the surface organic horizon. Ann for Sci 68:57–68. https://doi.org/10.1007/s13595-010-0007-5
Richards LA (1941) A pressure-membrane extraction apparatus for soil solution. Soil Sci 51(5):377e
Ridout M, Houbraken J, Newcombe G (2017) Xerotolerance of Penicillium and Phialocephala fungi, dominant taxa of fine lateral roots of woody plants in the intermountain Pacific Northwest, USA. Rhizosphere 4:94–103. https://doi.org/10.1016/j.rhisph.2017.09.004
Rognes T, Flouri T, Nichols B et al (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 2016:1–22. https://doi.org/10.7717/peerj.2584
Ruehr NK, Offermann CA, Gessler A et al (2009) Drought effects on allocation of recent carbon: from beech leaves to soil CO2 efflux. New Phytol 184:950–961. https://doi.org/10.1111/j.1469-8137.2009.03044.x
Schmied G, Hilmers T, Mellert KH et al (2023) Nutrient regime modulates drought response patterns of three temperate tree species. Sci Total Environ 868:161601. https://doi.org/10.1016/j.scitotenv.2023.161601
Selosse MA, Schneider-Maunoury L, Martos F (2018) Time to re-think fungal ecology? Fungal ecological niches are often prejudged. New Phytol 217:968–972. https://doi.org/10.1111/nph.14983
Shi L, Guttenberger M, Kottke I, Hampp R (2002) The effect of drought on mycorrhizas of beech (Fagus sylvatica L.): changes in community structure, and the content of carbohydrates and nitrogen storage bodies of the fungi. Mycorrhiza 12:303–311. https://doi.org/10.1007/s00572-002-0197-2
Smith GR, Finlay RD, Stenlid J et al (2017) Growing evidence for facultative biotrophy in saprotrophic fungi: data from microcosm tests with 201 species of wood-decay basidiomycetes. New Phytol 215:747–755. https://doi.org/10.1111/nph.14551
Steudle E, Heydt H (1997) Water transport across tree roots. In: Rennenberg H, Eschrich W, Ziegler H (eds) Trees - contributions to modern tree physiology. Backhuys Publishers, Leiden, The Netherlands, pp 239–255
Swaty RL, Deckert RJ, Whitham TG, Gehring CA (2004) Ectomycorrhizal abundance and community composition shifts with drought: predictions from tree rings. Ecology 85:1072–1084. https://doi.org/10.1890/03-0224
Tang Y, Schiestl-Aalto P, Saurer M et al (2022) Tree organ growth and carbon allocation dynamics impact the magnitude and δ13C signal of stem and soil CO2fluxes. Tree Physiol 42:2404–2418. https://doi.org/10.1093/treephys/tpac079
Tedersoo L, Smith ME (2013) Lineages of ectomycorrhizal fungi revisited: foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol Rev 27:83–99. https://doi.org/10.1016/j.fbr.2013.09.001
Tedersoo L, Anslan S, Bahram M et al (2015) Shotgun metagenomes and multiple primer pair-barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. MycoKeys 10:1–43. https://doi.org/10.3897/mycokeys.10.4852
Thiel D, Kreyling J, Backhaus S et al (2014) Different reactions of central and marginal provenances of fagus sylvatica to experimental drought. Eur J Res 133:247–260. https://doi.org/10.1007/s10342-013-0750-x
Tomasella M, Beikircher B, Häberle KH et al (2018) Acclimation of branch and leaf hydraulics in adult Fagus sylvatica and Picea abies in a forest through-fall exclusion experiment. Tree Physiol 38:198–211. https://doi.org/10.1093/treephys/tpx140
Tumber-Dávila SJ, Schenk HJ, Du E, Jackson RB (2022) Plant sizes and shapes above and belowground and their interactions with climate. New Phytol 235:1032–1056. https://doi.org/10.1111/nph.18031
Unuk T, Martinović T, Finžgar D et al (2019) Root-associated fungal communities from two phenologically contrasting silver Fir (Abies alba Mill.) groups of trees. Front Plant Sci 10:214. https://doi.org/10.3389/fpls.2019.00214
Wambsganss J, Beyer F, Freschet GT et al (2021) Tree species mixing reduces biomass but increases length of absorptive fine roots in European forests. J Ecol 109:2678–2691. https://doi.org/10.1111/1365-2745.13675
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. https://doi.org/10.1128/AEM.00062-07
Wang M, Kong L, Zang M (2015) Effects of sample dimensions and shapes on measuring soil-water characteristic curves using pressure plate. J Rock Mech Geotech Eng 7:463–468. https://doi.org/10.1016/j.jrmge.2015.01.002
Wang C, Chen Z, Yin H et al (2018) The responses of forest fine root biomass/necromass ratio to environmental factors depend on mycorrhizal type and latitudinal region. J Geophys Res Biogeosciences 123:1769–1788. https://doi.org/10.1029/2017JG004308
Wang J, Zhang H, Gao J et al (2021) Effects of ectomycorrhizal fungi (Suillus variegatus) on the growth, hydraulic function, and non-structural carbohydrates of Pinus tabulaeformis under drought stress. BMC Plant Biol 21:1–13. https://doi.org/10.1186/s12870-021-02945-3
Weemstra M, Sterck FJ, Visser EJW et al (2017) Fine-root trait plasticity of beech (Fagus sylvatica) and spruce (Picea abies) forests on two contrasting soils. Plant Soil 415:175–188. https://doi.org/10.1007/s11104-016-3148-y
Weigt RB, Raidl S, Verma R, Agerer R (2012) Exploration type-specific standard values of extramatrical mycelium - a step towards quantifying ectomycorrhizal space occupation and biomass in natural soil. Mycol Prog 11:287–297. https://doi.org/10.1007/s11557-011-0750-5
Wiley E, King CM, Landhäusser SM (2019) Identifying the relevant carbohydrate storage pools available for remobilization in aspen roots. Tree Physiol 39:1109–1120. https://doi.org/10.1093/treephys/tpz051
Wilhelm RC, Munoz-Ucros J, Weikl F et al (2022) The effects of mixed-species root zones on the resistance of soil bacteria and fungi to experimental and natural reductions in soil moisture. J Ecol 873:162266. https://doi.org/10.1016/j.scitotenv.2023.162266
Williams A, de Vries FT (2020) Plant root exudation under drought: implications for ecosystem functioning. New Phytol 225:1899–1905. https://doi.org/10.1111/nph.16223
Zang U, Goisser M, Grams TEE et al (2014) Fate of recently fixed carbon in European beech (Fagus sylvatica) saplings during drought and subsequent recovery. Tree Physiol 34:29–38. https://doi.org/10.1093/treephys/tpt110
Acknowledgements
We would like to thank Sarah Kristen and Isabella Pitzen for their support with watering plants, daily SWC measurements and root architecture analysis, and Thomas Feuerbach for setting up and maintaining the measurement and 13C labelling equipment. We also appreciate supports during the intensive harvests by Elke Gerstner, Barbara Groß and Joseph Heckmair. Furthermore, we thank Tina Kiedeisch for a helping hand during DNA extraction and sequencing preparation. We also thank Pak Chow for NSC quantification measurements, Franziska Bucka for a support with pressure plates, and Uwe Blum for performing soil nutrient measurements. We also thank Benjamin D. Hafner for his generous pre-submission review.
Funding
Open Access funding enabled and organized by Projekt DEAL. The project was funded by the German Research Foundation (DFG) through grants GR 1881/5-1, MA1763/10-1, PR555/2-1, PR292/22-1 and by the Bavarian State Ministries of the Environment and Consumer Protection as well as Food, Agriculture and Forestry (W047/Kroof II).
Author information
Authors and Affiliations
Contributions
KP and TEEG originally designed the experiment, and JD and KH finalized the experimental design. All authors contributed to the sample and the data collection. JD and KH analysed, interpreted the data, and wrote the manuscript with support from all authors. All authors revised and edited the manuscript. JD and KH contributed equally.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Responsible Editor: Susan Schwinning.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jasmin Danzberger and Kyohsuke Hikino share the first authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Danzberger, J., Hikino, K., Landhäusser, S.M. et al. Drought acclimation of beech seedlings depends largely on their rooting patterns and less on the fungal communities in soils. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06784-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06784-7