Abstract
Background and aims
Iron (Fe) plaque on rice roots is a mixture of Fe oxide and oxyhydroxide minerals thought to protect rice from high levels of arsenic (As) in flooded paddy soils. Silicon (Si), phosphorus (P), and selenium (Se) also exist as oxyanions in rice paddies, but the impacts of Fe plaque on uptake of these nutrients are unknown.
Methods
We used natural variation in paddy soil chemistry to test how Si, P, As, and Se move from porewater to plaque to plant via multiple techniques. In a pot study, we monitored Fe plaque deposition and porewater chemistry in 5 different soils over time and measured plaque/plant chemistry and Fe plaque mineralogy at harvest. We normalized oxyanion concentrations by Fe to determine the preferential retention on plaque or plant uptake.
Results
Low phosphorus availability increased root Fe-oxidizing activity, while Fe, Si, P, As, and Se concentrations in plaque were strongly correlated with porewater. Plaque did not appreciably retain Si and Se, and the oxyanions did not compete for adsorption sites on the Fe plaque.
Conclusion
Root Fe plaque seems to protect rice from As uptake, does not interfere with Si and Se uptake, and roots adapt to maintain P nutrition even with retention of porewater P on plaque.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is a unique crop that has adapted to grow in flooded soils. Development of aerenchyma tissues provides oxygen to the roots and also results in radial oxygen loss (ROL) into the rhizosphere soil (Evans 2004; Colmer and Voesenek 2009). This ROL is highest at the root tip where the Casparian bands are not yet suberized and 20–40 mm behind the tip where lateral roots are emerging (Armstrong et al. 2000; Kotula and Steudle 2009). While aerenchyma formation is constitutive in rice, aerenchyma size and porosity are controlled by soil redox potential (Colmer 2003; Colmer and Voesenek 2009; Mongon et al. 2014). ROL oxidizes soluble Fe2+ to insoluble Fe3+ precipitates on the outside of roots, primarily at root tips and lateral root junctions (Hansel et al. 2001, 2002; Seyfferth et al. 2017; Limmer et al. 2021). This Fe plaque is composed primarily of the Fe oxyhydroxides (FeOx) ferrihydrite (Fe(OH)3∙nH2O, “FHY”), lepidocrocite (γ-FeOOH, “LEP”), and goethite (α-FeOOH, “GOE”) (Hansel et al. 2001; Seyfferth et al. 2011; Amaral et al. 2017; Limmer et al. 2018). FHY is a short-range ordered, highly-reactive mineral which forms first and is predominant in the rhizosphere (Voegelin et al. 2007) but can transform over time into the more crystalline LEP and GOE (Schwertmann et al. 1999; Hansel et al. 2005; Cornell and Schwertmann 2003a). The high points of zero charge of FeOx (>7) allow them to form strong inner-sphere adsorption complexes with many anions (Cornell and Schwertmann 2003b), which generally weaken as pH increases (Fuller et al. 1993; Liu et al. 2001; Øgaard et al. 2006; Shi et al. 2009; Zhu et al. 2011; Jacobson and Fan 2019). Anions adsorb onto edge and corner sites of FeOx, with FHY having more of the stronger edge sites than LEP and GOE (Manceau and Charlet 1994; Manceau 1995; Cornell and Schwertmann 2003a; Hiemstra and Van Riemsdijk 2009). This, along with the large surface area (>200 m2/g vs. ~50 m2/g) of FHY causes it to be the most effective FeOx for anion adsorption (Dzombak and Morel 1990; Jackson and Miller 2000; Liu et al. 2001; Ona-Nguema et al. 2005; Cornell and Schwertmann 2003b; Zhu et al. 2011; Das et al. 2013; Couture et al. 2013; Jacobson and Fan 2019; Zhang et al. 2021; Sabur et al. 2022).
Reducing conditions in flooded paddies can also affect cycling of contaminants and nutrients. Such conditions mobilize As, which can result in toxicity to rice plants and humans consuming rice (Gilmour and Wells 1980; Abedin et al. 2002; Meharg and Rahman 2003; Williams et al. 2006; Rahman et al. 2008). Arsenite (As3+, H3AsO3) enters rice roots through silicon transporters, and thus returning silicon-rich rice residues can decrease As uptake in suboxic soils (Ma et al. 2006, 2008; Seyfferth and Fendorf 2012; Seyfferth et al. 2013, 2016; Teasley et al. 2017; Limmer et al. 2018; Seyfferth et al. 2019a; Linam et al. 2021). Because FeOx adsorb anions so effectively, Fe plaque is also thought to prevent phytotoxicity from the high porewater As concentrations, mainly As3+, found in flooded paddy soils (Hansel et al. 2001, 2002; Inskeep et al. 2002; Guo et al. 2007; Deng et al. 2010; Frommer et al. 2011; Lee et al. 2013; Syu et al. 2013; Yamaguchi et al. 2014; Amaral et al. 2017; Yang et al. 2020). Reduction of arsenate (As5+, H3AsO4) to As3+ decreases its adsorption strength, while reduction of selenate (Se6+, H2SeO4) to selenite (Se4+, H2SeO3) increases its adsorption strength (Hayes et al. 1987; Manning et al. 1998; McLaughlin et al. 1999; Favorito et al. 2018). Relative to As, there have been significantly fewer studies of how Fe plaque impacts the plant uptake of other oxyanion forming elements such as silicon, phosphorus, and selenium, which are found as H4SiO40, H2PO4−, HSeO3−, and SeO42− at circum-neutral pH. These elements are key plant nutrients (Girling 1984; Savant et al. 1997; McLaughlin et al. 1999; Alloway 2008) and in their inorganic forms, soil P, As, and Se mobility is largely controlled by adsorption reactions with FeOx (Girling 1984; Hinsinger 2001; Prietzel et al. 2016a, b; Gérard 2016; Dorau et al. 2019; Koch et al. 2019). Furthermore, adsorbed Si, P, As, and organic matter hinder the transformation of FHY (Fuller et al. 1993; Manceau 1995; Waychunas et al. 1995; Gálvez 1999; Chen et al. 2015; Seyfferth 2015; Teasley et al. 2017; Limmer et al. 2018; Thomasarrigo et al. 2018; Schulz et al. 2022). Silicic acid is also unique in that it can polymerize on FeOx surfaces over time with high loadings (Pokrovski et al. 2003; Eick et al. 2009; Christl et al. 2012; Kanematsu et al. 2018). Collectively, this suggests the potential for complicated interactions between oxyanion adsorbates and FeOx in rice root Fe plaque.
Sharing adsorption sites means that inorganic forms of Si, P, As, and Se can compete for adsorption on FeOx. Competition is not truly reciprocal or reversible due to encapsulation of anions and changing surface charge (Barrow et al. 2005; Sabur et al. 2022). While As generally outcompetes P for adsorption sites, P does decrease As5+ and As3+ adsorption (Jain and Loeppert 2000; Liu et al. 2001; Smith et al. 2002; Goh and Lim 2004; Chen et al. 2005; Barrow et al. 2005; Zhang et al. 2008; Zhu et al. 2011; Jessen et al. 2012; Mei et al. 2023). Phosphate very strongly outcompetes Si, Se4+, and Se6+ for adsorption on FeOx and soils (Goh and Lim 2004; Liu et al. 2004; Barrow et al. 2005; Eich-Greatorex et al. 2010; Hiemstra 2018; Sabur et al. 2022). Se4+ and As5+ compete somewhat (Zhu et al. 2011; Jacobson and Fan 2019; Wang et al. 2022), and Si competes with As3+, though there is a strong dependence on order of adsorption/aging (Luxton et al. 2006, 2008; Jessen et al. 2012; Hiemstra 2018). All of these anion adsorption studies have been conducted with synthesized minerals in pure solutions, and it is unclear whether adsorption competition plays a role in anion mobility in soils, particularly in uncontaminated paddy soils.
In this work, we sought to use natural variation in soil chemistry to determine how Fe plaque impacts rice uptake of Si, P, As, and Se; use of the term “oxyanions” throughout will refer exclusively to these elements. We used vinyl films to capture the Fe-oxidizing activity of roots in situ, measured root plaque mineral composition using Fe EXAFS, and determined the associations of anions with Fe minerals on the root plaque using selective dissolutions in 5 different soils in a pot study. We compared these root plaque data to plant, porewater, and soil chemistry. We hypothesized that:
-
1.
Rice roots will deposit more Fe plaque which will be less crystalline in silty soils compared to clayey soils
-
2.
Oxyanion (Si, P, As, and Se) concentration in Fe plaque will be directly correlated to porewater concentrations and will decrease with plaque crystallinity
-
3.
Fe plaque will decrease plant uptake of As but not Si, P, or Se
-
4.
Higher plaque Si and P will compete with and decrease adsorption of As to Fe plaque
-
5.
Return of rice husk residue will increase plant and plaque Si
Materials and methods
Soil descriptions
We collected five diverse U.S. soils for this study. Four soils are from working rice paddy fields in Lonoke and Poinsett counties, AR, USA. Two of these soils are classified as vertisols: one is a Perry silty clay (VR1) and the other is a Sharkey-Steele silty clay (VR2), both formed from alluvium. The third soil is a Herbert silt loam alfisol/stagnosol formed from alluvium and loess (ST), and the fourth is a Henry silt loam alfisol/planosol formed from loess (PL). The final soil is a highly weathered, alluvial Elsinboro silt loam ultisol/acrisol (AC) from the rice paddy mesocosms on the University of Delaware farm in Newark, DE, USA. Soil was collected from the fields to 25 cm depth, topsoil (A horizon) and subsoil (E and/or B horizons) were kept separate, and the soil was stored moist in sealed bins. Three small cores (25 cm depth) of the collected soil were composited, dried, and ground for soil characterization. Soil texture (hydrometer), nutrient status (Mehlich-III), and soil organic matter (SOM; loss on ignition at 360 °C) were measured following standard procedures at the University of Delaware soil testing laboratory. Electrical conductivity was measured in 1:2 w:w soil-water slurry. Elemental analysis was measured in triplicate alongside standard reference soils via microwave-assisted HNO3 extraction (EPA 3051A). Finally, soil As pools were determined via a 5 step sequential extraction (Wenzel et al. 2001) which divides soil As into non-specifically adsorbed (F1), specifically-adsorbed (F2), amorphous (hydr)oxide-bound (F3), crystalline (hydr)oxide-bound (F4), and residual (F5) fractions. We note that F2 was an ammonium phosphate extraction, so P was not analyzed in the sequential extraction due to interferences by entrapped solution.
Pot study
We grew rice in a greenhouse pot study to monitor the formation and chemistry of Fe plaque in the different soils. The greenhouse maintained 14-hour day length and temperatures from 25 to 35 °C. The equivalent of 8 kg dry soil was loosely packed into 8 L plastic pots with topsoil over subsoil and not sieved to maintain soil structure as much as possible. Fertilizers (N, P, and K; Table S1) were mixed into the topsoil at recommended rates (Hardke 2021). A total of 8 pots were packed with each soil. Half the pots had rice husk mixed into the topsoil at low rates (equivalent to ~2 years of production, or ~ 4 t/ha) to test whether ~annual rice husk return affected oxyanion concentration in plaque. Rice seedlings (‘Jefferson’) were germinated in potting soil then transplanted into the pots ~2 weeks after emergence, 1 plant per pot. The soils were then flooded (using deionized water) for the remainder of the experiment until they were drained 5 days prior to harvest. We collected porewater samples every two weeks in evacuated HDPE bottles using Rhizon samplers (Rhizosphere Research Products, 19.21.01) inserted at 45° in the soil (2–9 cm sampling depth) to monitor porewater chemistry according to established methods (Seyfferth and Fendorf 2012; Seyfferth et al. 2016). The pH and oxidation-reduction potential (ORP; AgCl reference with +197 mV offset) of the porewater was measured using electrodes, Fe2+ and H4SiO4 concentrations were determined colorimetrically (Stookey 1970; Kraska and Breitenbeck 2010), then the porewater was acidified to 2% HNO3 for elemental analysis (Thermo Scientific™ iCAP™ TQ ICP-MS) alongside check standards (Table S2). At physiological maturity (~140 days post-transplant) we separated the aboveground tissue into panicles and straw, and removed and thoroughly washed the roots, excluding any tightly pot-bound roots that occupied the interface between the soil and the pot. Because roots were much more prone to break during harvesting in the clayey soils compared to the silty soils, we do not have an accurate measurement of root biomass; instead, we estimated total root biomass as 29.5% the amount of aboveground biomass based on previous studies where entire root systems were isolated (Hu et al. 2005; Wu et al. 2015; Teasley et al. 2017). Roots and panicles were air dried while straw was oven dried at 40 °C.
Vinyl films
We used flexible (0.25 mm thickness), white vinyl films to non-destructively monitor FeOx deposition by roots over the course of the pot study, as previously described (Limmer et al. 2021, 2022). Briefly, 10 cm wide vinyl films were inserted between the soil and pot, and every week a film was removed with a new one inserted behind it. Removed films were gently washed and blotted dry before being scanned on a flatbed scanner at 200 dpi; films with dark sulfide-rich regions were re-scanned several days later to calculate sulfide deposition. Matlab (Mathworks) code was used to analyze the total area of FeOx deposited on each film using a thresholding technique and summing all Fe pixels. Film scans for each pot were then aligned and animated into videos to visually show root development patterns over time for representative plants in each soil (Fig. S1). This is a useful method to measure “root activity” as a combination of root growth, branching, and radial oxygen loss averaged over space.
Plaque and plant analysis
We processed the roots to determine the Fe plaque mineralogy and chemical composition similarly to previous methods (Amaral et al. 2017). We performed sequential acid ammonium oxalate (AAO; 0.3 M, pH 3.25, 4 hours in the dark) and dithionite-citrate-bicarbonate (DCB; 0.33 M dithionite, 0.27 M citrate, 0.11 M bicarbonate, 3 hours in the dark) dissolutions on ~1 g of roots from each plant to separate the “amorphous” and “crystalline” oxide minerals (Schwertmann 1991). These extracts were analyzed via ICP-MS and will be referred to as plaque selective dissolutions. Brown rice (unpolished grain), husk, straw, and roots (after plaque dissolution) were ground and microwave-digested in HNO3 following previous procedures (Seyfferth et al. 2016). The digestates were diluted to 2% HNO3 and analyzed via ICP-MS. Insoluble Si gel was washed 3x with diH2O, dissolved in 2 M NaOH, and analyzed colorimetrically. Standard reference materials digested with samples showed good recoveries for Si (76 ± 2%), P (100 ± 6%), As (104 ± 15%), and Se (104 ± 23%; Table S2).
A separate mass of roots was sonicated to remove Fe plaque which was then collected on nitrocellulose filters for Fe plaque mineral composition according to established methods (Amaral et al. 2017). These filters were analyzed via Fe EXAFS at beamline 7–2 at the Stanford synchrotron radiation lightsource (SSRL). The beam was calibrated to Fe foil at 7112 eV and the samples were analyzed in transmission mode from 6909 to 7682 eV using a 1 mm beam. Data analysis was done using Athena (Ravel and Newville 2005). Two replicate scans were averaged for each sample, and linear combination fitting (LCF) was used to fit the k3 weighted samples from 2 to 12 Å. PCA suggested 4 standards were ideal to fit the data, and LCF produced the best results using FHY, LEP, GOE, and siderite (SID) rather than magnetite or hematite.
Statistics
Statistical analyses were performed in JMP Pro 16 (SAS Institute). Multiple linear regression analysis was performed to determine significant factors impacting plaque mineralogy, plaque chemistry, and plant oxyanion concentration. Two-way ANOVA analysis was used to test Soil, Husk, and Soil*Husk effects on porewater, plaque, and plant chemistry; because Husk and Soil*Husk terms were never significant, Husk effects are not reported separately in this study. For root Fe plaque dissolution data, two-way ANOVA with Tukey post-hoc HSD tests were done with Soil, Fraction (e.g. AAO vs. DCB), and Soil*Fraction interaction terms. Differences between anion fractions in soil, porewater, plaque, and plant were also tested with Soil*Fraction interaction terms. Oxyanion concentrations were usually analyzed as molar ratios with Fe to control for differences in redox potential of soils. P < 0.05 was generally considered significant.
Results
Soil characterization
The soils used in this study had pH (1:10 w:v H2O) from 5.4–7.1 and SOM from 1.7–3.2% (Table 1; Table S3). CEC ranged from 9.6–41.4 cmol(+)/kg with CEC increasing with clay content. Amorphous and crystalline oxide soil Fe decreased with decreasing clay content but was much higher in the AC soil, likely due to greater weathering extent in that soil. In addition, the two vertisols differed in their soil Fe chemistry, with VR1 higher in crystalline Fe oxides and VR2 relatively higher in amorphous Fe oxides.
Comparing the sum of the sequential extractions to the EPA 3051 microwave digest showed extraction of 122–790% for Si, 101–131% for As, 80–111% for Se, and 82–103% for Fe, with no obvious differences between soils. These numbers would likely be more similar for Si if an HF digestion had been used rather than the HNO3 digestion. Most of the soil Si was found in the crystalline oxide F4 fraction (47–61%), most As was found in the amorphous F3 (24–50%) and crystalline oxide F4 fractions (17–49%), most Se was in the residual F5 fraction (60–74%), and most Fe was in the residual F5 (41–69%) and crystalline oxide F4 fractions (17–51%), though these values varied by soil (Fig. 1). All Si and Se fractions scaled with the sum of soil Si and Se fractions, but PL had significantly higher F1 As, despite having the lowest total As (Fig. 1). Complete soil descriptions are given in Table S3.
Fe, Si, As, and Se extracted in each step of the As sequential extraction; note different units used for Fe/Si and As/Se. F1 = 0.05 M (NH4)2SO4 (non-specifically adsorbed), F2 = 0.05 M (NH4)H2PO4 (specifically adsorbed), F3 = 0.2 M NH4-oxalate at pH 3.25 (amorphous oxide-bound), F4 = 0.2 M NH4-oxalate +0.1 M ascorbic acid at pH 3.25 (crystalline oxide-bound), F5 = HNO3 microwave digestion (residual). Black letters represent Tukey HSD groupings for differences between soils within each fraction, while colored letters represent Tukey HSD groupings for totals. Error bars represent standard deviation (n = 3, α = 0.05)
Fe plaque formation and mineralogy
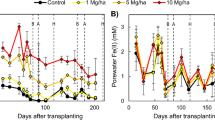
There were significant differences in porewater Fe concentrations (p < 0.001) and cumulative plaque deposition (p < 0.001) among the soils studied (Fig. 2a, b). Because the vinyl films recorded Fe oxidation spatially, they provided a good estimate of spatially-averaged root activity (i.e. growth and radial oxygen loss) over time. In general, the silty soils (PL and AC) had much higher porewater Fe concentrations and lower ORP than the clayey soils (VR1 and VR2), with the silty clay soil (ST) in between (Fig. 2a, c). The trends in Fe plaque deposition were similar except for the PL and VR1 soils. PL had the lowest plaque deposition (Fig. 2b) even though it had the highest porewater Fe (Fig. 2a), and VR1 had higher plaque deposition than VR2, despite them having very similar porewater Fe. Examination of Fe plaque deposition patterns over time suggests that individual roots were active for much longer time scales (up to 5 weeks) in the clayey VR1, VR2, and ST soils, while no roots seemed to form plaque for more than 3 weeks in the silty AC or PL soils (Fig. S1). Because of the difficulty in placing new films in the exact location of previous films, these roots are seen to shift a few mm between weeks. Precipitation of sulfide minerals on the films occurred late in the growth season and was higher for VR1 and AC compared to the other soils (Fig. S2). Maximum plaque formation occurred around booting (70–90 days after transplant; Limmer et al. 2022).
Porewater Fe concentrations throughout the pot study (a) and cumulative coverage area of Fe plaque on vinyl films placed between the soil and pot (b), porewater ORP (c), Fe plaque concentration and mineralogy on roots (d), and correlations between EXAFS fits of FHY and plaque AAO dissolution (e) and EXAFS LEP + GOE + SID and plaque DCB dissolution (f). Uppercase letters correspond to Tukey HSD groupings for differences between soils for the average of points after day 40 for porewater and cumulative total for plaque deposition. Colored letters in (d) denote differences in total plaque concentration on roots while black letters denote differences in % mineral composition. Error bars denote standard deviation (α = 0.05, n = 8)
The Fe root plaque mineralogy and total amount of Fe plaque varied between rice grown in the different soils (Fig. 2d). Total Fe root plaque (from AAO + DCB dissolution) followed a trend more similar to porewater Fe (R2 = 0.18, p = 0.004) than vinyl film plaque deposition, with the siltier soils having greater amounts of plaque compared to the clayey soils. In terms of percent composition, FHY was the dominant Fe mineral in plaque and made up 36–78% of the Fe minerals, with the highest percentage in PL and ST. LEP was the second most common mineral (10–40%) and was most dominant in AC. GOE made up 0–31% of the Fe minerals and was highest in VR1. SID was the final component found via Fe EXAFS, accounting for 7–14% of the Fe minerals and not differing significantly between soils. Example spectra and fitting results are shown in Fig. S3 and Table S4. Multiple linear regression analysis suggested that higher porewater Si favored FHY over GOE and higher porewater ORP favored LEP over FHY.
Comparing AAO and DCB dissolution data to the Fe EXAFS fits allows for determination of chemical dissolution accuracy for predicting Fe plaque mineralogy (Fig. 2e, f). Our data points are centered on the 1:1 lines comparing percent of plaque Fe extracted using AAO and percent of FHY determined via EXAFS and percent of plaque extracted using DCB and Fe EXAFS LEP + GOE + SID. The trendlines are flattened, however, with slopes of 0.48 and intercepts of 0.30 and 0.22. Color-coding the points using the %LEP from the EXAFS fit shows that high plaque LEP content causes overestimation and low plaque LEP causes underestimation of amorphous minerals (FHY) using AAO. The reverse is true for DCB estimation of crystalline minerals (LEP + GOE + SID). This data also suggests SID is extracted by DCB rather than AAO.
Porewater and plaque oxyanions
Because plaque is formed from the elements encountered by the roots in soil porewater, normalizing by Fe concentration shows whether an element is more, less, or equally likely to be retained in the plaque compared to Fe. Porewater concentrations of Si, P, As, and Se differed significantly (p < 0.001) between the soils studied (Fig. 3a-d). The general trend showed that the silty soils had higher concentrations of oxyanions in the order PL > ST > VR2 > VR1. The highly weathered AC soil showed nutrient depletion, with very low Si and P but higher As and Se. The general order of magnitude difference between molar porewater oxyanion concentrations was 10,000:1000:10:1 for Si:P:As:Se, but this varied substantially between soils. For all the oxyanion:Fe plaque ratios, the Soil*Fraction interaction terms were significant and the data showed that Si and Se are generally concentrated in the DCB fraction, though Se readsorption to GOE during AAO dissolution is likely (Gruebel et al. 1988; Jackson and Miller 2000). Conversely, P is concentrated in the AAO fraction in siltier soils while As is concentrated in the AAO fraction in one of the clayey soils.
Porewater concentrations of Si (a), P (b), As (c), and Se (d) over time. Plaque (AAO and DCB) molar ratios of Si:Fe (e), P:Fe (f), As:Fe (g), and Se:Fe (h). Colored uppercase letters represent Tukey HSD groupings for average porewater concentrations after day 40, and lowercase black letters represent groupings for Soil*Fraction interaction term; error bars denote standard deviation (α = 0.05, n = 8)
Multiple linear regression analysis of oxyanion:Fe ratio in plaque showed that only plaque As and P were positively correlated to their porewater concentrations (Table S5). Plaque mineralogy was also important for oxyanion:Fe ratios in plaque, with As:Fe negatively correlated with plaque LEP, and Si:Fe and P:Fe positively correlated with plaque FHY. The low P, high root activity clayey (VR1) and silty (AC) soils had high amounts of GOE and LEP, respectively, in their plaque at the expense of FHY (Fig. 3d).
Comparing the oxyanion:Fe ratios moving from soil to porewater to plaque to plant provides a picture of how the oxyanions are retained on plaque or excluded from the plant relative to the abundant Fe in the flooded soils. The Si:Fe and Se:Fe ratios are much higher in porewater than in plaque (Fig. 4a, d) which suggests that they are not enriched in Fe plaque. The P:Fe and As:Fe ratios are similar in porewater and plaque (Fig. 4b, c) which suggests plaque formation retains P and As to a similar degree as Fe. The plaque As:Fe ratio was higher than the porewater As:Fe ratio in the siltier soils (Fig. 4c). Single and multiple linear regression analyses showed that no plaque oxyanion concentrations or molar ratios to Fe were negatively correlated with each other, suggesting that Si, P, As, and Se do not limit the adsorption potential of each other on Fe plaque.
Oxyanion:Fe molar ratios for Si (a), P (b), As (c), and Se (d) in soil, porewater, Fe plaque, and plant tissue. Soil = HNO3 digest (EPA3051). PW = porewater time weight averaged after day 40. Plaque = root AAO + DCB dissolutions. Plant = mass balance for roots+straw+husk+grain. Note use of logarithmic scale. Letters represent Tukey HSD groupings for log-transformed data comparing fractions (i.e., Soil, PW, Plaque, and Plant) across soils, and error bars denote standard deviation (α = 0.05, n = 8)
Plant oxyanions
Concentrations of Si, P, As, and Se in different plant tissues differed significantly between soils and are shown in Fig. S4. In general, AC seems to be Si deficient while PL accumulated very high concentrations of As. Soil F1 As was the only fraction positively correlated with porewater, grain, and straw As (R2 = 0.89–0.92). This contrasts with previous work which showed F2 was best correlated with rice uptake in a single soil type with different water managements (Seyfferth et al. 2019b). F1 and F3 Si had the strongest positive correlations with porewater (R2 = 0.12–0.18) and straw Si (R2 = 0.71–0.73), and F3 and F4 Se were positively correlated with grain Se (R2 = 0.49–0.57) and straw Se (R2 = 0.20–0.23). The oxyanion:Fe ratios in plant biomass were higher than their ratios in soil, porewater, and plaque; this suggests that all oxyanions are taken up more effectively than Fe. The magnitude of difference between soil/porewater/plaque and plant oxyanions is evident in the logarithmic scale of Fig. 4. The plant Si:Fe ratio is 1200-2300x the plaque Si:Fe ratio (Fig. 4a), showing that plant uptake is highly efficient and plaque retention is low. Similar calculations show this value to be 14-112x for P (Fig. 4b), 2-7x for As (Fig. 4c), and 18-33x for Se (Fig. 4d). Yield of panicles and straw was significantly higher for PL compared to the other soils (Fig. S6), and only Se concentrations are consistently lower in plants grown in this soil which suggests that Se alone may be affected by growth dilution (Figs. S4 and S5).
Viewing the total elemental uptake (on mass basis) of Si, P, As, and Se into different plant tissues shows that each element displays a different distribution pattern (Fig. 5): Si is concentrated in the husk and straw, which implies efficient root uptake and translocation; plant P is concentrated more in the plaque in high P soils (ST and PL), but is taken up and translocated into grain very efficiently; As is mostly located in the plaque and then roots (51–79% and 17–40% of plant As, respectively), which suggests both plaque retention of As and sequestration in roots; Se is distributed evenly throughout the plaque, roots, straw, and grain. These data suggest that both P and As are retained in the plaque alongside Fe, but that As is excluded while the plant maintains high tissue P concentrations; this difference is likely due to P being diffusion limited while As moves more via mass flow in flooded soil.
Discussion
Plaque formation
Multiple sampling techniques help us overcome some biases inherent in root studies. Collecting and analyzing root plaque from soil causes overrepresentation of large roots at the expense of fine roots and also normalizes plaque Fe by root biomass, while the vinyl film method measures Fe plaque formation over an area, capturing differences in root growth. Figure 2a, b shows that the two clays (VR1 and VR2) have similar porewater Fe and plaque Fe, but VR2 has much less FeOx precipitation on vinyl films. The same is true of the silty soils, where AC and PL have similar porewater and plaque Fe, but PL has much lower FeOx on vinyl films. The best explanation for this difference is greater root growth and branching due to differences in soil P availability (Eshel and Beeckman 2013; Fu et al. 2014; Martinengo et al. 2023a, b). In the clayey soils, VR2 had 3x higher Mehlich-III and porewater P than VR1, and in the silty soils PL had 2x higher Mehlich-III and 60x higher porewater P than AC (Fig. 3, Table S3). The low P clayey and silty soils (VR1 and AC) had much higher rates of FeOx precipitation on the vinyl films than the high P clayey and silty soils (VR2 and PL). Greater root growth and activity leads to higher FeOx coverage of the vinyl films (Fig. 2b) but does not impact the Fe plaque concentration (in Fe/kg root) nearly as much as porewater Fe does (Fig. 2d). Our estimates of root biomass are based on studies of rice grown in three different soils with varying concentrations of Si and As (Hu et al. 2005; Wu et al. 2015; Teasley et al. 2017), but there are likely differences in root mass and average root size (and therefore root surface area) between our soil textures due to the varied nutrient availabilities; this requires further study. The P:Fe ratios in the plaque also follow the same trend as the porewater P (Fig. 3b, f). Based on this evidence, vinyl films provide insight into spatially-averaged root activity (growth and radial oxygen loss) while collecting roots from soil mainly gives insight into Fe plaque mineralogy and chemistry.
The differences seen in plaque mineralogy between soils were likely also due to a combination of porewater chemistry and root growth differences. Higher soil and plaque Si and P in the VR2 and PL soils (Fig. 3) may have inhibited crystallization of FHY to LEP or GOE (Fuller et al. 1993; Manceau 1995; Waychunas et al. 1995; Gálvez 1999; Chen et al. 2015; Seyfferth 2015; Teasley et al. 2017; Thomasarrigo et al. 2017, 2018; Limmer et al. 2018; Schulz et al. 2022). Electrical conductivity tests (Table 1) and porewater concentrations of ions suggest AC has a higher (~2x) ionic strength than the other soils, which along with the lower pH could be favoring LEP instead of GOE (Grigg et al. 2022). It is unclear, however, if VR2 and PL have less FHY due to less Si and P stabilizing it, or if there is less Si and P adsorption to the plaque because there is less high surface area FHY (Seyfferth et al. 2019b). We did not observe any magnetite, suggesting Fe2+ levels in the plaque are never high enough to catalyze its formation (Cornell and Schwertmann 2003a; Aeppli et al. 2019a, b; Thomasarrigo et al. 2019).
Oxyanion comparison
Porewater Si was primarily (86%) silicic acid, which has a high first pKa (~9.5) and is thus neutral in most soils, making it less likely to form strong bonds with FeOx in plaque. Adsorption of monomeric silicic acid rather than polymerization of silica is expected here due to the low Si:Fe ratios observed (0.01–0.04; Fig. 3a) (Hiemstra 2018). Finally, Si is a very important nutrient for rice (Ma 2004; Ma and Yamaji 2006) and it has highly efficient uptake and translocation systems (Mitani and Ma 2005; Ma et al. 2006, 2007), which resulted in most plant Si accumulating in the husk and straw (Figs. 5 and S4).
Selenium was present in porewater and plaque in much lower concentrations than the other oxyanions (Fig. 3). The low plaque Se:Fe ratio and distribution of Se throughout plant tissues (Figs. 5, S4 and S5) imply that plaque is not limiting plant availability. This may be due to oxidation of Se4+ to Se6+ in the rhizosphere, which would increase its mobility and plant uptake, but this has not been confirmed in the rhizosphere. Some studies using Se4+ in hydroponics suggest plaque could limit Se availability, but that root exudates can mobilize sufficient Se from Fe plaque (Zhou and Shi 2007; Zhou et al. 2007).
Phosphorus was one of the most abundant elements in Fe plaque at an average of 0.1 P:Fe molar ratio (Fig. 3b); this value exceeds the P adsorption capacities of FeOx (Liu et al. 2001; Sabur et al. 2022), and suggest that precipitation reactions or P sequestration in internal pores during FHY to GOE transformation is occurring (Gálvez 1999). The soils where plaque P:Fe ratio was higher than porewater P:Fe ratio (ST and AC; Fig. 4b) were also the soils showing the highest plaque deposition rates on vinyl films (Fig. 2b), implying that they had more actively growing and branching root systems. This extra growth likely meant more root interception of P at root tips or branches, which have less developed FeOx barriers (Seyfferth et al. 2010). Increased root interception and ligand exchange with P on soil particles would explain how the plants acquired high grain P (Fig. 5) while porewater P was retained in plaque (Fig. 4). Though porewater P is retained in plaque similarly to As, P accumulated in aboveground tissues much more efficiently than As (Figs. 5 and S7), which suggests that the protective role of plaque on As uptake does not necessarily harm plant P nutrition. Another reason for this difference is likely due to soil and porewater P concentrations being ~400x and ~ 100x higher than soil and porewater As concentrations, along with the addition of P fertilizer in two of the soils. Finally, the P uptake and translocation system is much more efficient than As uptake and translocation by rice, and resulted in straw, husk, and grain P concentrations that were ~ 2000x, ~5000x, and ~ 50,000x the As concentrations.
Arsenic adsorption on Fe plaque seems to decrease its plant availability. Most plant As is in the plaque (Fig. 5), and the plant As:Fe ratio is much closer in magnitude to the soil, porewater, and plaque As:Fe ratios than for the other oxyanions (Fig. 4). It is also evident that total soil As is not well correlated to plant As uptake, but non-specifically adsorbed (F1; (NH4)2SO4 extraction) As is a much better predictor (Figs. 1, 5 and S4). Compared to Si and Se, As is more closely associated with the amorphous (F3) and crystalline (F4) oxide fractions of the soil (Fig. 1). Plaque As:Fe ratios are elevated in soils with higher porewater As. Some authors interpret the positive correlation between plant As and plaque As to mean that plaque is a source of As to the plant (Fang et al. 2023), but this is a spurious correlation which is a result of both plant and plaque As being functions of redox potential (Seyfferth and Fendorf 2012; Seyfferth et al. 2016, 2019b; A. Farhat et al. 2021; Linam et al. 2022). Plaque/rhizosphere is concentrated in As relative to the bulk soil and porewater (Voegelin et al. 2007; Frommer et al. 2011; Mei et al. 2012), defining it as a sink and necessitating normalization of measurements by porewater or soil Fe, As, or redox potential to assess plant uptake. The main distinction separating As from the other oxyanions is that it not only adsorbs strongly to FeOx, but 60–90% of the As is oxidized to As5+ (Voegelin et al. 2007; Seyfferth et al. 2010, 2019b; Frommer et al. 2011; Yang et al. 2020), a reaction which is catalyzed by FeOx and further increases retention in the plaque (Jackson and Miller 2000; Liu 2004).
An aspect of oxyanion adsorption which we did not examine is differences in speciation. While soluble Si exists solely as H4SiO40 in most soils, P, As, and Se all have organic and inorganic forms, and As and Se undergo redox transformations. Organic P species are generally less or similarly plant available compared to inorganic orthophosphate (Amadou et al. 2022; Martinengo et al. 2023b) and can make up a significant portion of total soil P (Kunito et al. 2012; Mukai et al. 2021). It is unknown whether the organic and inorganic P species would follow similar trends going from the soil to the plant via porewater and plaque, and this study did not differentiate the two. Organic As species such as dimethylarsinic acid (DMA) accumulate in significant quantities in rice tissues but are minor components in Fe plaque and soil (Liu et al. 2006; Seyfferth et al. 2010, 2011; Dykes et al. 2021) and do not adsorb as strongly to FeOx as inorganic As (Jackson and Miller 2000; Lafferty and Loeppert 2005; Zhang et al. 2008; Situm et al. 2017). Organic selenium species have not been studied extensively in soil, likely due to the very low concentrations of Se in most soils (~7% the concentration of As). Se is expected to be oxidized to Se6+ in the rhizosphere, which would increase its mobility compared to the bulk soil (Barrow et al. 2005; Keskinen et al. 2013; Favorito et al. 2018). This would contribute to the low retention of porewater Se in plaque observed here. Conversion of Se to organic species is more likely in reduced conditions, which could increase plant uptake (Li et al. 2010); however, further reduction to elemental Se0 or selenides (Se2−) would decrease Se availability (Mayland et al. 1991; Scheinost et al. 2008). Current methods (e.g. synchrotron XANES) are inadequate to distinguish Se species in plaque and soil samples at the low concentrations in the present study. Future study examining Se and As speciation changes in the porewater using IC-ICP-MS could be beneficial to understanding how these processes occur along the redox gradient from bulk soil to rhizosphere.
A final interaction which requires further study is the effect of root exudates (and soil organic matter) on oxyanion retention in Fe plaque. The most common root exudates (citrate, oxalate, malate, tartrate) generally form weaker bonds with FeOx than the oxyanions studied here, but their effectiveness increases at low pH (Mohapatra et al. 2005; Shi et al. 2009; Zhu et al. 2011). Phosphorus deficiency greatly increases exudation (Hoffland et al. 2006; Santoro et al. 2022) and studies suggest that exudates are more effective at desorbing P than As5+ from FeOx (Liu et al. 2001), especially given that P does not adsorb on the stronger 1E sites occupied by As (Arai and Sparks 2001; Rahnemaie et al. 2007; Abdala et al. 2015). This could help explain how rice plants remain P sufficient even with adsorption in the plaque. Total Si, P, As, and Se adsorption to plaque in this study totaled 0.05–0.27 mol/mol Fe which is close to the anion adsorption site density of FHY (~0.15–0.22 mol/mol Fe; Dzombak and Morel 1990; Hiemstra and Van Riemsdijk 2009), with P over (>144%) its adsorption maximum while As is well below (<8%) its adsorption maximum (Liu et al. 2001). This would favor root exudation as an effective way for rice to acquire P from plaque without mobilizing As.
Conclusions
The formation of Fe plaque on rice roots is a complex and challenging process to observe. This leads to many simplifications in experimental design, and thus much of our current understanding is based on experiments in soil-less media and/or with seedlings. We believe our experimental design of a pot study using native soils with background levels of oxyanions and multiple Fe plaque monitoring methods allowed us to test theories about Fe plaque’s role in retention of oxyanions using natural variations in soil chemistry.
Our data partially support Hypothesis 1, because the clayey soils tend to have lower porewater Fe which results in lower Fe plaque concentration on roots, but the total amount of Fe plaque deposition by the root systems does not follow a trend with texture (Fig. 2). Phosphorus availability and acquisition seem to play a more important role in root activity and Fe plaque deposition (Figs. 2 and 3; Table S3). Hypothesis 2 is also partially supported, because the plaque concentrations of Si, P, As, and Se are directly correlated with their porewater concentrations but not with plaque mineralogy/crystallinity (Figs. 2 and 3). Our data support Hypothesis 3 because Si and Se are not retained by Fe plaque (Figs. 4 and S7), and Si is taken up very efficiently by rice (Figs. 5 and S4). Porewater P and As are retained in plaque at similar levels as Fe (Figs. 4 and S4), but most plant P is located in the grain while As is located in plaque (Fig. 5)—this suggests plaque limits As uptake but not P. The plants are likely accessing diffusion limited P which is not measured in porewater samples. We reject Hypothesis 4, because concentrations and porewater-normalized concentration factors for plaque Si, P, As, and Se are not negatively correlated to each other, suggesting competition for adsorption sites on Fe plaque is not determining their retention. Finally, we reject Hypothesis 5, because returning rice husk residues to the soils did not significantly affect porewater, plaque, or plant Si concentration (Limmer et al. 2023). In conclusion, Fe plaque seems to protect rice plants from toxic As without significantly interfering with Si, P, or Se uptake in a wide range of paddy soils.
Data availability
Data is available from the USDA Ag Data Commons at: https://data.nal.usda.gov/dataset/data-rice-root-iron-plaque-retains-arsenic-and-phosphorus-doesn%E2%80%99t-interfere-silicon-and-selenium-uptake
Abbreviations
- FHY:
-
Ferrihydrite
- LEP:
-
Lepidocrocite
- GOE:
-
Goethite
- SID:
-
Siderite
- FeOx:
-
Iron oxyhydroxides
- AAO:
-
Acid ammonium oxalate
- DCB:
-
Dithionite-citrate-bicarbonate
- VR:
-
Vertisol
- ST:
-
Stagnosol
- AC:
-
Acrisol
- PL:
-
Planosol
References
Abdala DB, Northrup PA, Arai Y, Sparks DL (2015) Surface loading effects on orthophosphate surface complexation at the goethite/water interface as examined by extended X-ray absorption fine structure (EXAFS) spectroscopy. J Colloid Interface Sci 437:297–303. https://doi.org/10.1016/j.jcis.2014.09.057
Abedin J, Cresser MS, Meharg AA et al (2002) Arsenic accumulation and metabolism in rice (Oryza sativa L.). Environ Sci Technol 36:962–968. https://doi.org/10.1021/es0101678
Aeppli M, Kaegi R, Kretzschmar R et al (2019a) Electrochemical analysis of changes in iron oxide reducibility during abiotic Ferrihydrite transformation into goethite and magnetite. Environ Sci Technol 53:3568–3578. https://doi.org/10.1021/acs.est.8b07190
Aeppli M, Vranic S, Kaegi R et al (2019b) Decreases in iron oxide reducibility during microbial reductive dissolution and transformation of Ferrihydrite. Environ Sci Technol 53:8736–8746. https://doi.org/10.1021/acs.est.9b01299
Alloway BJ (2008) Micronutrient deficiencies in global crop production. Springer, Dordrecht
Amadou I, Faucon M-P, Houben D (2022) Role of soil minerals on organic phosphorus availability and phosphorus uptake by plants. Geoderma 428:116125. https://doi.org/10.1016/j.geoderma.2022.116125
Amaral DC, Lopes G, Guilherme LRG, Seyfferth AL (2017) A new approach to sampling intact Fe plaque reveals Si-induced changes in Fe mineral composition and shoot as in Rice. Environ Sci Technol 51:38–45. https://doi.org/10.1021/acs.est.6b03558
Arai Y, Sparks DL (2001) ATR-FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrite-water interface. J Colloid Interface Sci 241:317–326. https://doi.org/10.1006/jcis.2001.7773
Armstrong W, Cousins D, Armstrong J et al (2000) Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Ann Bot 86:687–703. https://doi.org/10.1006/anbo.2000.1236
Barrow NJ, Cartes P, Mora ML (2005) Modifications to the Freundlich equation to describe anion sorption over a large range and to describe competition between pairs of ions. Eur J Soil Science 56:601–606. https://doi.org/10.1111/j.1365-2389.2005.00700.x
Chen Z, Zhu YG, Liu WJ, Meharg AA (2005) Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytol 165:91–97. https://doi.org/10.1111/j.1469-8137.2004.01241.x
Chen C, Kukkadapu R, Sparks DL (2015) Influence of Coprecipitated organic matter on Fe2+(aq)-catalyzed transformation of Ferrihydrite: implications for carbon dynamics. Environ Sci Technol 49:10927–10936. https://doi.org/10.1021/acs.est.5b02448
Christl I, Brechbühl Y, Graf M, Kretzschmar R (2012) Polymerization of silicate on hematite surfaces and its influence on arsenic sorption. Environ Sci Technol 46:13235–13243. https://doi.org/10.1021/es303297m
Colmer TD (2003) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann Bot 91:301–309. https://doi.org/10.1093/aob/mcf114
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681. https://doi.org/10.1071/FP09144
Cornell RM, Schwertmann U (2003a) Formation. In: the iron oxides: structure, properties, reactions, occurrences and uses 1st edn. Wiley, New York, pp 345–364
Cornell RM, Schwertmann U (2003b) Adsorption of ions and molecules. In: the iron oxides: structure, properties, reactions, occurrences and uses, 1st edn. Wiley, New York, pp 253–296
Couture R-M, Rose J, Kumar N et al (2013) Sorption of arsenite, arsenate, and thioarsenates to iron oxides and iron sulfides: a kinetic and spectroscopic investigation. Environ Sci Technol 47:5652–5659. https://doi.org/10.1021/es3049724
Das S, Jim Hendry M, Essilfie-Dughan J (2013) Adsorption of selenate onto ferrihydrite, goethite, and lepidocrocite under neutral pH conditions. Appl Geochem 28:185–193. https://doi.org/10.1016/j.apgeochem.2012.10.026
Deng D, Wu SC, Wu FY et al (2010) Effects of root anatomy and Fe plaque on arsenic uptake by rice seedlings grown in solution culture. Environ Pollut 158:2589–2595. https://doi.org/10.1016/j.envpol.2010.05.015
Dorau K, Pohl L, Just C et al (2019) Soil organic matter and phosphate sorption on natural and synthetic Fe oxides under in situ conditions. Environ Sci Technol 53:13081–13087. https://doi.org/10.1021/acs.est.9b03260
Dykes GE, Limmer MA, Seyfferth AL (2021) Silicon-rich soil amendments impact microbial community composition and the composition of arsM bearing microbes. Plant Soil 468:147–164. https://doi.org/10.1007/s11104-021-05103-8
Dzombak DA, Morel FMM (1990) Surface complexation modeling: hydrous ferric oxide. Wiley, New York
Eich-Greatorex S, Krogstad T, Sogn TA (2010) Effect of phosphorus status of the soil on selenium availability. Z Pflanzenernähr Bodenk 173:337–344. https://doi.org/10.1002/jpln.200900004
Eick MJ, Luxton TP, Welsh HA (2009) Effect of silica polymerization on the oxalate-promoted dissolution of goethite. Clay Clay Miner 57:578–585. https://doi.org/10.1346/CCMN.2009.0570506
Eshel A, Beeckman T (eds) (2013) Plant roots: the hidden half, 4th edn. CRC Press, Boca Raton
Evans DE (2004) Aerenchyma formation. New Phytol 161:35–49. https://doi.org/10.1046/j.1469-8137.2003.00907.x
Fang X, Christl I, Colina Blanco AE et al (2023) Decreasing arsenic in rice: interactions of soil sulfate amendment and water management. Environ Pollut 322:121152. https://doi.org/10.1016/j.envpol.2023.121152
Farhat YA, Kim S-H, Seyfferth AL et al (2021) Altered arsenic availability, uptake, and allocation in rice under elevated temperature. Sci Total Environ 763. https://doi.org/10.1016/j.scitotenv.2020.143049
Favorito JE, Eick MJ, Grossl PR (2018) Adsorption of selenite and Selenate on Ferrihydrite in the presence and absence of dissolved organic carbon. J Environ Qual 47:147–155. https://doi.org/10.2134/jeq2017.09.0352
Frommer J, Voegelin A, Dittmar J et al (2011) Biogeochemical processes and arsenic enrichment around rice roots in paddy soil: results from micro-focused X-ray spectroscopy. Eur J Soil Sci 62:305–317. https://doi.org/10.1111/j.1365-2389.2010.01328.x
Fu YQ, Yang XJ, Shen H (2014) The physiological mechanism of enhanced oxidizing capacity of rice (Oryza sativa L.) roots induced by phosphorus deficiency. Acta Physiol Plant 36:179–190. https://doi.org/10.1007/s11738-013-1398-3
Fuller CC, Davis JA, Waychunas GA (1993) Surface chemistry of Ferrihydrite: part 2. Kinetics of arsenate adsorption and coprecipitation. Geochim Cosmochim Acta 57:2271–2282. https://doi.org/10.3998/mpub.168625
Gálvez N (1999) Effect of phosphate on the crystallization of hematite, goethite, and lepidocrocite from Ferrihydrite. Clay Clay Miner 47:304–311. https://doi.org/10.1346/CCMN.1999.0470306
Gérard F (2016) Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils - a myth revisited. Geoderma 262:213–226. https://doi.org/10.1016/j.geoderma.2015.08.036
Gilmour JT, Wells BR (1980) Residual effects of MSMA on sterility in rice cultivars. Agron J 72:1066. https://doi.org/10.2134/agronj1980.00021962007200060049x
Girling CA (1984) Selenium in AGRICULTURE and the environment. Agric Ecosyst Environ 11:37–65
Goh K-H, Lim T-T (2004) Geochemistry of inorganic arsenic and selenium in a tropical soil: effect of reaction time, pH, and competitive anions on arsenic and selenium adsorption. Chemosphere 55:849–859. https://doi.org/10.1016/j.chemosphere.2003.11.041
Grigg ARC, Thomasarrigo LK, Schulz K et al (2022) Ferrihydrite transformations in flooded paddy soils: rates, pathways, and product spatial distributions. Environ Sci Process Impacts. https://doi.org/10.1039/d2em00290f
Gruebel KA, Davis JA, Leckie JO (1988) The feasibility of using sequential extraction techniques for arsenic and selenium in soils and sediments. Soil Sci Soc Am J 52:390–397. https://doi.org/10.2136/sssaj1988.03615995005200020016x
Guo W, Zhu YG, Liu WJ et al (2007) Is the effect of silicon on rice uptake of arsenate (AsV) related to internal silicon concentrations, iron plaque and phosphate nutrition? Environ Pollut 148:251–257. https://doi.org/10.1016/j.envpol.2006.10.021
Hansel CM, Fendorf S, Sutton S, Newville M (2001) Characterization of Fe plaque and associated metals on the roots of mine-waste impacted aquatic plants. Environ Sci Technol 35:3863–3868. https://doi.org/10.1021/es0105459
Hansel CM, La Force MJ, Fendorf S, Sutton S (2002) Spatial and temporal association of as and Fe species on aquatic plant roots. Environ Sci Technol 36:1988–1994. https://doi.org/10.1021/es015647d
Hansel CM, Benner SG, Fendorf S (2005) Competing Fe(II)-induced mineralization pathways of Ferrihydrite. Environ Sci Technol 39:7147–7153
Hardke JT (ed) (2021) Arkansas Rice production handbook. University of Arkansas Division of Agriculture
Hayes KF, Roe AL, Brown GE et al (1987) In situ x-ray absorption study of surface complexes: selenium oxyanions on alpha-FeOOH. Science 238:783–786. https://doi.org/10.1126/science.238.4828.783
Hiemstra T (2018) Ferrihydrite interaction with silicate and competing oxyanions: geometry and hydrogen bonding of surface species. Geochim Cosmochim Acta 238:453–476. https://doi.org/10.1016/j.gca.2018.07.017
Hiemstra T, Van Riemsdijk WH (2009) A surface structural model for Ferrihydrite I: sites related to primary charge, molar mass, and mass density. Geochim Cosmochim Acta 73:4423–4436. https://doi.org/10.1016/j.gca.2009.04.032
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hoffland E, Wei C, Wissuwa M (2006) Organic anion exudation by lowland rice (Oryza sativa L.) at zinc and phosphorus deficiency. Plant Soil 283:155–162. https://doi.org/10.1007/s11104-005-3937-1
Hu Y, Li JH, Zhu YG et al (2005) Sequestration of as by iron plaque on the roots of three rice (Oryza sativa L.) cultivars in a low-P soil with or without P fertilizer. Environ Geochem Health 27:169–176. https://doi.org/10.1007/s10653-005-0132-5
Inskeep WP, McDermott TR, Fendorf S (2002) Arsenic (V)/(III) cycling in soils and natural waters: chemical and microbiological processes. In: Frankenberger WT Jr (ed) Environmental chemistry of arsenic. Marcel Dekker, New York, pp 183–215
Jackson BP, Miller WP (2000) Effectiveness of phosphate and hydroxide for desorption of arsenic and selenium species from iron oxides. Soil Sci Soc Am J 64:1616–1622. https://doi.org/10.2136/sssaj2000.6451616x
Jacobson AT, Fan M (2019) Evaluation of natural goethite on the removal of arsenate and selenite from water. J Environ Sci 76:133–141. https://doi.org/10.1016/j.jes.2018.04.016
Jain A, Loeppert RH (2000) Effect of competing anions on the adsorption of arsenate and arsenite by Ferrihydrite. J Environ Qual 29:1422–1430. https://doi.org/10.2134/jeq2000.00472425002900050008x
Jessen S, Postma D, Larsen F et al (2012) Surface complexation modeling of groundwater arsenic mobility: results of a forced gradient experiment in a Red River flood plain aquifer, Vietnam. Geochim Cosmochim Acta 98:186–201. https://doi.org/10.1016/j.gca.2012.07.014
Kanematsu M, Waychunas GA, Boily JF (2018) Silicate binding and precipitation on iron oxyhydroxides. Environ Sci Technol 52:1827–1833. https://doi.org/10.1021/acs.est.7b04098
Keskinen R, Yli-Halla M, Hartikainen H (2013) Retention and uptake by plants of added selenium in peat soils. Commun Soil Sci Plant Anal 44:3465–3482. https://doi.org/10.1080/00103624.2013.847955
Koch M, Guppy C, Amelung W et al (2019) Insights into 33phosphorus utilisation from Fe-and Al-hydroxides in Luvisol and Ferralsol subsoils. Soil Research 57:447–458. https://doi.org/10.1071/SR18223
Kotula L, Steudle E (2009) Measurements of oxygen permeability coefficients of rice (Oryza sativa L.) roots using a new perfusion technique. J Exp Bot 60:567–580. https://doi.org/10.1093/jxb/ern300
Kraska JE, Breitenbeck GA (2010) Simple, robust method for quantifying silicon in plant tissue. Commun Soil Sci Plant Anal 41:2075–2085. https://doi.org/10.1080/00103624.2010.498537
Kunito T, Tsunekawa M, Yoshida S et al (2012) Soil properties affecting phosphorus forms and phosphatase activities in Japanese Forest soils: soil microorganisms may be limited by phosphorus. Soil Sci 177:39–46. https://doi.org/10.1097/SS.0b013e3182378153
Lafferty BJ, Loeppert RH (2005) Methyl arsenic adsorption and desorption behavior on iron oxides. Environ Sci Technol 39:2120–2127
Lee CH, Hsieh YC, Lin TH, Lee DY (2013) Iron plaque formation and its effect on arsenic uptake by different genotypes of paddy rice. Plant Soil 363:231–241. https://doi.org/10.1007/s11104-012-1308-2
Li HF, Lombi E, Stroud JL et al (2010) Selenium speciation in soil and rice: influence of water management and se fertilization. J Agric Food Chem 58:11837–11843. https://doi.org/10.1021/jf1026185
Limmer MA, Mann J, Amaral DC et al (2018) Silicon-rich amendments in rice paddies: effects on arsenic uptake and biogeochemistry. Sci Total Environ 624:1360–1368. https://doi.org/10.1016/j.scitotenv.2017.12.207
Limmer MA, Evans AE, Seyfferth AL (2021) A new method to capture the spatial and temporal heterogeneity of aquatic plant iron root plaque in situ. Environ Sci Technol 55:912–918. https://doi.org/10.1021/acs.est.0c02949
Limmer MA, Thomas J, Seyfferth AL (2022) The effect of silicon on the kinetics of rice root iron plaque formation. Plant Soil 477:171–181. https://doi.org/10.1007/s11104-022-05414-4
Limmer MA, Linam FA, Seyfferth AL (2023) The effect of rice residue management on rice paddy Si, Fe, as, and methane biogeochemistry. Sci Total Environ 903:166496. https://doi.org/10.1016/j.scitotenv.2023.166496
Linam F, McCoach K, Limmer MA, Seyfferth AL (2021) Contrasting effects of rice husk pyrolysis temperature on silicon dissolution and retention of cadmium (cd) and dimethylarsinic acid (DMA). Sci Total Environ 765. https://doi.org/10.1016/j.scitotenv.2020.144428
Linam F, Limmer MA, Tappero R, Seyfferth AL (2022) Rice husk and charred husk amendments increase porewater and plant Si but water management determines grain as and cd concentration. Plant Soil 477:135–152. https://doi.org/10.1007/s11104-022-05350-3
Liu W-J (2004) Do iron plaque and genotypes affect arsenate uptake and translocation by rice seedlings (Oryza sativa L.) grown in solution culture? J Exp Bot 55:1707–1713. https://doi.org/10.1093/jxb/erh205
Liu F, De Cristofaro A, Violante A (2001) Effect of pH, phosphate and oxalate on the adsorption/desorption of arsenate on/from goethite. Soil Sci 166:197–208. https://doi.org/10.1097/00010694-200103000-00005
Liu Q, Wang DJ, Jiang XJ, Cao ZH (2004) Effects of the interactions between selenium and phosphorus on the growth and selenium accumulation in rice (Oryza Sativa). Environ Geochem Health 26:325–330. https://doi.org/10.1023/B:EGAH.0000039597.75201.57
Liu WJ, Zhu YG, Hu Y et al (2006) Arsenic sequestration in iron plaque, its accumulation and speciation in mature rice plants (Oryza Sativa L.). Environ Sci Technol 40:5730–5736. https://doi.org/10.1021/es060800v
Luxton TP, Tadanier CJ, Eick MJ (2006) Mobilization of arsenite by competitive interaction with silicic acid. Soil Sci Soc Am J 70:204–214. https://doi.org/10.2136/sssaj2005.0101
Luxton TP, Eick MJ, Rimstidt DJ (2008) The role of silicate in the adsorption/desorption of arsenite on goethite. Chem Geol 252:125–135. https://doi.org/10.1016/j.chemgeo.2008.01.022
Ma JF (2004) Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Science and Plant Nutrition 50:11–18. https://doi.org/10.1080/00380768.2004.10408447
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397. https://doi.org/10.1016/j.tplants.2006.06.007
Ma JF, Tamai K, Yamaji N et al (2006) A silicon transporter in rice. Nature 440:688–691. https://doi.org/10.1038/nature04590
Ma JF, Yamaji N, Mitani N et al (2007) An efflux transporter of silicon in rice. Nature 448:209–212. https://doi.org/10.1038/nature05964
Ma JF, Yamaji N, Mitani N et al (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci 105:9931–9935. https://doi.org/10.1073/pnas.0802361105
Manceau A (1995) The mechanism of anion adsorption on iron oxides: evidence for the bonding of arsenate tetrahedra on free Fe(O, OH)6 edges. Geochim Cosmochim Acta 59:3647–3653. https://doi.org/10.1016/0016-7037(95)00275-5
Manceau A, Charlet L (1994) The mechanism of Selenate adsorption on goethite and hydrous ferric oxide. J Colloid Interface Sci 168:87–93
Manning BA, Fendorf SE, Goldberg S (1998) Surface structures and stability of arsenic(III) on goethite: spectroscopic evidence for inner-sphere complexes. Environ Sci Technol 32:2383–2388. https://doi.org/10.1021/es9802201
Martinengo S, Santoro V, Schiavon M et al (2023a) The influence of phosphorus availability on rice root traits driving iron plaque formation and dissolution, and implications for phosphorus uptake. Plant Soil. https://doi.org/10.1007/s11104-023-06306-x
Martinengo S, Schiavon M, Santoro V et al (2023b) Assessing phosphorus availability in paddy soils: the importance of integrating soil tests and plant responses. Biol Fertil Soils 59:391–405. https://doi.org/10.1007/s00374-023-01714-8
Mayland HF, Gough LP, Stewart KC (1991) Selenium mobility in soils and its absorption, translocation, and metabolism in plants. US Geological Survey Circular 1064:57–64
McLaughlin MJ, Parker DR, Clarke JM (1999) Metals and micronutrients - food safety issues. Field Crop Res 60:143–163. https://doi.org/10.1016/S0378-4290(98)00137-3
Meharg AA, Rahman M (2003) Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption. Environ Sci Technol 37:229–234. https://doi.org/10.1021/es0259842
Mei XQ, Wong MH, Yang Y et al (2012) The effects of radial oxygen loss on arsenic tolerance and uptake in rice and on its rhizosphere. Environ Pollut 165:109–117. https://doi.org/10.1016/j.envpol.2012.02.018
Mei K, Liu J, Xue L et al (2023) Stimulation of oxalate root exudate in arsenic speciation and fluctuation with phosphate and iron in anoxic mangrove sediment. Mar Pollut Bull 189:114823. https://doi.org/10.1016/j.marpolbul.2023.114823
Mitani N, Ma JF (2005) Uptake system of silicon in different plant species. J Exp Bot 56:1255–1261. https://doi.org/10.1093/jxb/eri121
Mohapatra D, Singh P, Zhang W, Pullammanappallil P (2005) The effect of citrate, oxalate, acetate, silicate and phosphate on stability of synthetic arsenic-loaded ferrihydrite and Al-ferrihydrite. J Hazard Mater 124:95–100. https://doi.org/10.1016/j.jhazmat.2005.04.025
Mongon J, Konnerup D, Colmer TD, Rerkasem B (2014) Responses of rice to Fe2+ in aerated and stagnant conditions: growth, root porosity and radial oxygen loss barrier. Funct Plant Biol 41:922–929. https://doi.org/10.1071/FP13359
Mukai M, Aiba S, Kitayama K (2021) Effects of tree-root exudates on the solubilization of phosphorus adsorbed to non-crystalline minerals in the rhizosphere volcanic soils on Yakushima Island, Japan. Trees 35:2031–2041. https://doi.org/10.1007/s00468-021-02170-3
Øgaard AF, Sogn TA, Eich-Greatorex S (2006) Effect of cattle manure on selenate and selenite retention in soil. Nutr Cycl Agroecosyst 76:39–48. https://doi.org/10.1007/s10705-006-9039-5
Ona-Nguema G, Morin G, Juillot F et al (2005) EXAFS analysis of arsenite adsorption onto two-line ferrihydrite, hematite, goethite, and lepidocrocite. Environ Sci Technol 39:9147–9155. https://doi.org/10.1021/es050889p
Prietzel J, Harrington G, Häusler W et al (2016a) Reference spectra of important adsorbed organic and inorganic phosphate binding forms for soil P speciation using synchrotron-based K-edge XANES spectroscopy. J Synchrotron Radiat 23:532–544. https://doi.org/10.1107/S1600577515023085
Prietzel J, Harrington G, Häusler W et al (2016b) Reference spectra of important adsorbed organic and inorganic phosphate binding forms for soil P speciation using synchrotron-based K-edge XANES spectroscopy. J Synchrotron Radiat 23:532–544. https://doi.org/10.1107/S1600577515023085
Rahman MA, Hasegawa H, Rahman MM et al (2008) Straighthead disease of rice (Oryza sativa L.) induced by arsenic toxicity. Environ Exp Bot 62:54–59. https://doi.org/10.1016/j.envexpbot.2007.07.016
Rahnemaie R, Hiemstra T, Van Riemsdijk WH (2007) Geometry, charge distribution, and surface speciation of phosphate on goethite. Langmuir 23:3680–3689. https://doi.org/10.1021/la062965n
Ravel B, Newville M (2005) ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12:537–541. https://doi.org/10.1107/S0909049505012719
Sabur MA, Parsons CT, Maavara T, Van Cappellen P (2022) Effects of pH and dissolved silicate on phosphate mineral-water partitioning with goethite. ACS Earth Space Chem 6:34–43. https://doi.org/10.1021/acsearthspacechem.1c00197
Santoro V, Schiavon M, Visentin I et al (2022) Tomato plant responses induced by sparingly available inorganic and organic phosphorus forms are modulated by strigolactones. Plant Soil 474:355–372. https://doi.org/10.1007/s11104-022-05337-0
Savant NK, Snyder GH, Datnoff LE (1997) Silicon management and sustainable rice production. Adv Agron 58:151–199
Scheinost AC, Kirsch R, Banerjee D et al (2008) X-ray absorption and photoelectron spectroscopy investigation of selenite reduction by FeII-bearing minerals. J Contam Hydrol 102:228–245. https://doi.org/10.1016/j.jconhyd.2008.09.018
Schulz K, ThomasArrigo LK, Kaegi R, Kretzschmar R (2022) Stabilization of Ferrihydrite and Lepidocrocite by silicate during Fe(II)-catalyzed mineral transformation: impact on particle morphology and silicate distribution. Environ Sci Technol. https://doi.org/10.1021/acs.est.1c08789
Schwertmann U (1991) Solubility and dissolution of iron oxides. Plant Soil 130:1–25
Schwertmann U, Friedl J, Stanjek H (1999) From Fe(III) ions to ferrihydrite and then to hematite. J Colloid Interface Sci 209:215–223. https://doi.org/10.1006/jcis.1998.5899
Seyfferth AL (2015) Abiotic effects of dissolved oxyanions on iron plaque quantity and mineral composition in a simulated rhizosphere. Plant Soil 397:43–61. https://doi.org/10.1007/s11104-015-2597-z
Seyfferth AL, Fendorf S (2012) Silicate mineral impacts on the uptake and storage of arsenic and plant nutrients in rice (Oryza sativa L.). Environ Sci Technol 46:13176–13183. https://doi.org/10.1021/es3025337
Seyfferth AL, Webb SM, Andrews JC, Fendorf S (2010) Arsenic localization, speciation, and co-occurrence with iron on rice (Oryza sativa L) roots having variable Fe coatings. Environ Sci Technol 44:8108–8113. https://doi.org/10.1021/es101139z
Seyfferth AL, Webb SM, Andrews JC, Fendorf S (2011) Defining the distribution of arsenic species and plant nutrients in rice (Oryza sativa L.) from the root to the grain. Geochim Cosmochim Acta 75:6655–6671. https://doi.org/10.1016/j.gca.2011.06.029
Seyfferth AL, Kocar BD, Lee JA, Fendorf S (2013) Seasonal dynamics of dissolved silicon in a rice cropping system after straw incorporation. Geochim Cosmochim Acta 123:120–133. https://doi.org/10.1016/j.gca.2013.09.015
Seyfferth AL, Morris AH, Gill R et al (2016) Soil incorporation of silica-rich Rice husk decreases inorganic arsenic in Rice grain. J Agric Food Chem 64:3760–3766. https://doi.org/10.1021/acs.jafc.6b01201
Seyfferth A, Ross J, Webb S (2017) Evidence for the root-uptake of arsenite at lateral root junctions and root apices in Rice (Oryza sativa L.). Soils 1:3. https://doi.org/10.3390/soils1010003
Seyfferth AL, Amaral D, Limmer MA, Guilherme LRG (2019a) Combined impacts of Si-rich rice residues and flooding extent on grain as and cd in rice. Environ Int 128:301–309. https://doi.org/10.1016/j.envint.2019.04.060
Seyfferth AL, Limmer M, Wu W (2019b) Si and water management drives changes in Fe and Mn pools that affect as cycling and uptake in Rice. Soil Systems 3:58. https://doi.org/10.3390/soilsystems3030058
Shi R, Jia Y, Wang C (2009) Competitive and cooperative adsorption of arsenate and citrate on goethite. J Environ Sci 21:106–112. https://doi.org/10.1016/S1001-0742(09)60019-X
Situm A, Rahman MA, Allen N et al (2017) ATR-FTIR and flow microcalorimetry studies on the initial binding kinetics of arsenicals at the organic-hematite Interface. J Phys Chem A 121:5569–5579. https://doi.org/10.1021/acs.jpca.7b03426
Smith E, Naidu R, Alston AM (2002) Chemistry of inorganic arsenic in soils: II. Effect of phosphorus, sodium, and calcium on arsenic sorption. J Environ Qual 31:557–563. https://doi.org/10.2134/jeq2002.5570
Stookey LL (1970) Ferrozine--a new spectrophotometric reagent for Iron. Anal Chem 42:779–781
Syu CH, Jiang PY, Huang HH et al (2013) Arsenic sequestration in iron plaque and its effect on as uptake by rice plants grown in paddy soils with high contents of as, iron oxides, and organic matter. Soil Science and Plant Nutrition 59:463–471. https://doi.org/10.1080/00380768.2013.784950
Teasley WA, Limmer MA, Seyfferth AL (2017) How Rice (Oryza sativa L.) responds to elevated as under different Si-rich soil amendments. Environ Sci Technol 51:10335–10343. https://doi.org/10.1021/acs.est.7b01740
Thomasarrigo LK, Mikutta C, Byrne J et al (2017) Iron(II)-catalyzed iron atom exchange and mineralogical changes in iron-rich organic freshwater flocs: an iron isotope tracer study. Environ Sci Technol 51:6897–6907. https://doi.org/10.1021/acs.est.7b01495
Thomasarrigo LK, Byrne JM, Kappler A, Kretzschmar R (2018) Impact of organic matter on Iron(II)-catalyzed mineral transformations in Ferrihydrite-organic matter Coprecipitates. Environ Sci Technol 52:12316–12326. https://doi.org/10.1021/acs.est.8b03206
Thomasarrigo LK, Kaegi R, Kretzschmar R (2019) Ferrihydrite growth and transformation in the presence of ferrous iron and model organic ligands. Environ Sci Technol 53:13636–13647. https://doi.org/10.1021/acs.est.9b03952
Voegelin A, Weber FA, Kretzschmar R (2007) Distribution and speciation of arsenic around roots in a contaminated riparian floodplain soil: Micro-XRF element mapping and EXAFS spectroscopy. Geochim Cosmochim Acta 71:5804–5820. https://doi.org/10.1016/j.gca.2007.05.030
Wang K, Wang Y, Zhang C et al (2022) Selenite and selenate showed contrasting impacts on the fate of arsenic in rice (Oryza sativa L.) regardless of the formation of iron plaque. Environ Pollut 312:120039. https://doi.org/10.1016/j.envpol.2022.120039
Waychunas GA, Davis JA, Fuller CC (1995) Geometry of sorbed arsenate on ferrihydrite and crystalline FeOOH: re-evaluation of EXAFS results and topological factors in predicting sorbate geometry, and evidence for monodentate complexes. Geochim Cosmochim Acta 59:3655–3661. https://doi.org/10.1016/0016-7037(95)00276-6
Wenzel WW, Kirchbaumer N, Prohaska T et al (2001) Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta 436:309–323. https://doi.org/10.1016/S0003-2670(01)00924-2
Williams PN, Islam MR, Adomako EE et al (2006) Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters. Environ Sci Technol 40:4903–4908. https://doi.org/10.1021/es060222i
Wu C, Zou Q, Xue S et al (2015) Effects of silicon (Si) on arsenic (As) accumulation and speciation in rice (Oryza sativa L.) genotypes with different radial oxygen loss (ROL). Chemosphere 138:447–453. https://doi.org/10.1016/j.chemosphere.2015.06.081
Yamaguchi N, Ohkura T, Takahashi Y et al (2014) Arsenic distribution and speciation near rice roots influenced by iron plaques and redox conditions of the soil matrix. Environ Sci Technol 48:1549–1556. https://doi.org/10.1021/es402739a
Yang Y, Hu H, Fu Q et al (2020) Phosphorus regulates as uptake by rice via releasing as into soil porewater and sequestrating it on Fe plaque. Sci Total Environ 738. https://doi.org/10.1016/j.scitotenv.2020.139869
Zhang JS, Stanforth R, Pehkonen SO (2008) Irreversible adsorption of methyl arsenic, arsenate, and phosphate onto goethite in arsenic and phosphate binary systems. J Colloid Interface Sci 317:35–43. https://doi.org/10.1016/j.jcis.2007.09.024
Zhang X, Yao H, Lei X et al (2021) A comparative study for phosphate adsorption on amorphous FeOOH and goethite (α-FeOOH): an investigation of relationship between the surface chemistry and structure. Environ Res 199:111223. https://doi.org/10.1016/j.envres.2021.111223
Zhou XB, Shi WM (2007) Effect of root surface iron plaque on se translocation and uptake by Fe-deficient rice. Pedosphere 17:580–587. https://doi.org/10.1016/S1002-0160(07)60068-X
Zhou XB, Shi WM, Zhang LH (2007) Iron plaque outside roots affects selenite uptake by rice seedlings (Oryza sativa L.) grown in solution culture. Plant Soil 290:17–28. https://doi.org/10.1007/s11104-006-9072-9
Zhu J, Pigna M, Cozzolino V et al (2011) Sorption of arsenite and arsenate on ferrihydrite: effect of organic and inorganic ligands. J Hazard Mater 189:564–571. https://doi.org/10.1016/j.jhazmat.2011.02.071
Acknowledgements
Michele L. Reba (USDA-ARS), Beatriz Moreno-Garcia, Benjamin R. K. Runkle (University of Arkansas), Chin Chen Kuo (University of Delaware), Sam Webb (SSRL). This work was funded by the United States Department of Agriculture National Institute of Food and Agriculture (Grant #2022-67011-36457). Parts of this research used beam line 7-2 at SSRL. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515.
Funding
This work was funded by the United States Department of Agriculture National Institute of Food and Agriculture (Grant #2022–67011-36457). Parts of this research used beam line 7–2 at SSRL. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515.
Author information
Authors and Affiliations
Contributions
All authors contributed to experimental design and manuscript editing. F. Linam performed the pot study, wrote the first manuscript draft, and did all data analysis. M. Limmer wrote the Matlab code and assisted with vinyl film and Fe EXAFS analysis. A. Seyfferth contributed to data interpretation, Fe EXAFS, and funding and synchrotron applications. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Jian Feng Ma.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Soil chemistry, vinyl film plaque deposition videos, EXAFS fits, and plant concentration data.
ESM 1
(DOCX 71076 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Linam, F.A., Limmer, M.A. & Seyfferth, A.L. Contrasting roles of rice root iron plaque in retention and plant uptake of silicon, phosphorus, arsenic, and selenium in diverse paddy soils. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06553-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06553-6