Abstract
Phosphorus (P) cycling in paddy soil is closely related to iron (Fe) redox wheel; its availability to rice has thus generally been ascribed to Fe minerals reductive dissolution. However, the literature aimed to identify the best method for predicting rice available P does not uniformly point to Fe reductants. Rice plants can indeed solubilize and absorb P through many strategies as a function of P supply, modifying the chemical environment. Therefore, this study aims to estimate P availability in paddy soils coupling the redox mechanisms driving P cycling with concurrent plant responses. Soil available P was estimated in three groups of paddy soils with low, medium, or high P content assessing easily desorbable pools (0.01 M calcium chloride, Olsen, Mehlich-III, anion exchanging resins) and Fe-bound P pools (EDTA, citrate-ascorbate, and oxalate). Rice P uptake and responses to P availability were assessed by a mesocosm cultivation trial. Although P released in porewater positively correlated with dissolved Fe(II), it did not with plant P uptake, and readily desorbable P pools were better availability predictors than Fe-bound pools, mainly because of the asynchrony observed between Fe reduction and plant P demand. Moreover, in low P soils, plants showed higher Fe(II) oxidation, enhanced root growth, and up-regulation of P root transporter encoding genes, plant responses being related with changes in P pools. These results indicate the generally assumed direct link between Fe reduction and rice P nutrition in paddy soils as an oversimplification, with rice P nutrition appearing as the result of a complex trade-off between soil redox dynamics, P content, and plant responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is an essential nutrient for plants, and its availability impacts both rice quality and yields (Seck et al. 2012). The fluctuating redox conditions occurring in paddy soils affect the solubility and availability of Fe-bound P (Scalenghe et al. 2002; Marschner 2021). Under reducing conditions, P concentration in the soil solution increases as a result of the microbial reductive dissolution of poorly crystalline Fe (hydr)oxides (Willett and Higgins 1978; Wei et al. 2019). However, if flooding conditions are protracted in time or oxic conditions are re-established by mid-season field draining, dissolved P can be re-adsorbed or co-precipitated (de Mello et al. 1998; Zhang et al. 2003; Santoro et al. 2019), likely reducing P availability for crops more than supposed until now. On the other hand, the alerting depletion of phosphate rock resources expected over the next 50 years and the constantly rising prices of mineral fertilizers have caused a three-fold decrease in the use of phosphate fertilizers in the European rice-intensive agroecosystems over the last years (Van Vuuren et al. 2010), potentially compromising rice productivity especially in P-poor soils. Thus, a correct assessment of the P-supplying capacity of paddy soils is essential to optimize fertilization management and increase P-use efficiency without negatively affecting crop yields.
A number of methods are being used for estimating P availability to paddy rice in different pedoclimatic environments (Pierzynski 2000), and previous studies have been devoted to identify the best methods, achieving different results. The acknowledged relationship between the reductive dissolution of Fe (hydr)oxides and P mobilization would well explain the results of those papers that identified extractants targeting Fe pools (e.g., citrate-ascorbate, (de Mello et al. 1998); oxalate, (Rabeharisoa et al. 2012); citric acid, (Hernández et al. 2013); EDTA, (Simonete et al. 2015)) as good predictors of P availability to rice, while a number of other works point to methods related to P desorption (e.g., Olsen, (Olsen et al. 1954; Maftoun et al. 2003); anion extracting resins, (Teo et al. 1995); diffusive gradients in thin films (DGT), (Six et al. 2013)). However, most of these studies focused mainly on tropical soils; thus, their results are poorly adaptable to temperate paddy systems because of the differences in soil parent materials and soil-forming processes that affect P release for plant uptake (Kögel-Knabner et al. 2010; Moody et al. 2013). In subtropical and tropical regions where soils are highly weathered, the porewater P concentration could be very low compared to the plant P demand, due to the high content of stable Fe and aluminum oxides (Hinsinger 2001) and to their lower reducibility. As P availability in paddy soils is known to be associated with the dissolution of the most reactive Fe (hydr)oxide forms (de Mello et al. 1998; Wang et al. 2022a, b), redox-driven changes in Fe mineralogy could strongly impact P availability for rice plants. This is the reason why acid oxalate-extractable P, i.e., P associated with poorly crystalline oxides, is reported to correlate with P availability (Shahandeh et al. 1995), although there is evidence that this pool includes some P forms associated with crystalline Fe phases, potentially overestimating the amount of plant available P. Reyes and Torrent (1997) thus proposed the citrate-ascorbate extraction as a more specific method to extract poorly crystalline Fe (hydr)oxides. Similarly, the extraction with EDTA at neutral pH was also proposed to release P from less labile pools by promoting the chelation of cations including Fe (McDowell et al. 2008).
Moreover, the abovementioned methods do not consider the potentially important contribution of soil organic P (Po) to available pools. Although plants take up almost exclusively inorganic P (Pi), this pool can be replenished by Po mineralization over time, further confounding the relationship between soil Pi contents and P availability for plants (McDowell et al. 2008). Plants can access Po resources through the root exudation of organic acids and/or by producing phosphatase and phytase enzymes, but the hydrolysis of Po is not the only strategy adopted by plants to increase soil P availability (McDowell et al. 2008; Rose et al. 2013; Wu et al. 2021). Because of the low mobility of P in soils, rice plants could respond to P-limiting conditions by increasing soil exploration with roots, uptake efficiency through up-regulation of the expression of P-transporter encoding genes, and promoting radial oxygen loss (Kirk and Van Du 1997; Rose et al. 2013; Fu et al. 2014). In particular, rice phosphate root transporters actively take up inorganic phosphate (Pi) from the soil solution and translocate it within the plant (Jia et al. 2011). Under flooded soil conditions, because of the reduced tortuosity of diffusion paths, the uptake of P across the root membrane may become the rate-limiting step for P acquisition, rather than the speed of replenishment of the soil solution P (Rose et al. 2013). Thus, the activation of phosphate root transporters, in association with other above-mentioned strategies, may play a crucial role in regulating P uptake by rice plants.
The interaction between plant P acquisition strategies and soil redox dynamics could drive changes in the different soil P forms and their availability, thus justifying the large variability of mechanisms proposed to explain rice P nutrition in paddies and the involved P pools (Shahandeh et al. 1995; Maftoun et al. 2003; Madurapperuma and Kumaragamage 2008; Hernández et al. 2013; Six et al. 2013). In addition, the type and extent of plant-soil interactions evolve during crop growth, hence, the P supplying capacity of paddy soils may be related also with the temporal changes in P availability during rice cultivation that are driven by management-dependent fluctuations in soil redox conditions and plant development. However, the effects of these temporal dynamics on P availability for rice plants are still unclear so far and thus need a better understanding.
Based on these considerations, we hypothesized that the chemical methods used to estimate Fe forms could better represent P availability to rice than the methods commonly used in aerobic crops. In addition, the plant responses to flooding and soil P supply could profoundly change the rhizosphere dynamics regulating P release into solution, further complicating the correct assessment of available P pool. Therefore, the current study aims to assess the involvement of different P pool(s) in rice P nutrition, as a function of soil P content, while combining the changes in Fe and P dynamics with the response of rice plants to P supply. We hypothesized that P bound to reducible Fe pools could play a major role in paddy soils from temperate regions, as recently reported for tropical soils (Wang et al. 2022a, b), and that plant adaptation mechanisms could enhance P uptake under P deficiency by acquiring P from less available pools, including organic P. We tested our hypotheses by assessing soil P availability with seven different chemical methods in 12 paddy soils, selected from an initial set of 100 soils typical of temperate rice cropping systems (NW Italy) and representative of a large range of total P contents. The estimated P availability was compared to the effective P uptake by rice plants cultivated in mesocosms under continuous flooding and related to plant responses.
Materials and methods
Assessing the soil P pools most involved in plant uptake
Study sites, soil sampling, and chemical analyses
In the first phase of the current study, a set of 100 paddy topsoils was randomly collected from different sites to represent all the rice cropping areas of Lombardy (NW Italy, Fig. S1), one of the main rice-producing regions of Italy. These sampling sites were located in the plains of the river Po, characterized by the presence of bumps of Holocene fluvial dynamics, modified by anthropogenic soil levelling due to the more recent agricultural processes. Most soils fell in the orders of Alfisols and Inceptisols, according to USDA classification (Soil Survey Staff 2022). The climate is temperate, characterized by hot summers and two main rainy periods in spring and autumn. The mean annual precipitation for the last 20 years was 704 mm, while the mean annual temperature was 17.7 °C (Miniotti et al. 2016).
The soils were sampled at 0–20 cm, air-dried, and sieved < 2 mm. The pH was determined potentiometrically in a 1:2.5 soil:H2O suspension. Total carbon (C) and nitrogen (N) contents were determined by dry combustion (UNICUBE, Elementar Analyses System GmbH, Langensbold, Germany). When present, the CaCO3 content was calculated from the difference between total and organic C determined by dry combustion after fumigation with 0.1 M HCl. Total P (Ptot) was determined after sulfuric-perchloric digestion (Olsen and Sommers 1982), and the concentration of molybdate-reactive P (MRP) in the extracts was determined according to Ohno and Zibilske (1991). Bicarbonate extractable P (POlsen) was determined on air-dried soils according to Olsen et al. (1954). Iron (Fe) and aluminum (Al) were extracted with ammonium oxalate (Schwertmann 1964), and the total Fe and Al concentration in the extracts was determined by atomic absorption spectroscopy (AAS, PerkinElmer AAnalyst 400, Norwalk, CT, USA). Particle-size distribution was assessed by the pipette method after sample dispersion with Na-hexametaphosphate (Gee and Bauder 1986). The soil cation exchange capacity was determined in 10% BaCl2 (Bascomb 1964), and the concentration of exchangeable cations in the extracts was determined by AAS.
Soil P availability assessment
The soils were subsequently grouped into 12 groups based on their total P content (Ptot), pH, and particle size distribution (Table S1). One soil per group, totally 12 representative soils were further selected as the most representative and grouped based on total P content as high P, medium P, and low P. The GIS coordinates and USDA soil classification (Soil Survey Staff 2022) of the 12 representative soils are reported in Table S2. These soils were analyzed for P availability using seven extraction methods according to the target P pool, as listed in Table 1. The selected extraction methods were divided into the following: (i) methods assessing easily desorbable pools, including the extraction in CaCl2 (Soltanpour et al. 1974), NaHCO3 (Olsen et al. 1954), Mehlich III (Mehlich 1984), and anionic resins (Saggar et al. 1990), and (ii) methods targeting Fe-bound P pools, as EDTA (Bowman and Moir 1993), citrate-ascorbate (Reyes and Torrent 1997), and oxalate (Schwertmann 1964). The molybdate-reactive phosphate (MRP) in the extracts was determined colorimetrically according to Murphy and Riley (1962) using excess molybdate when necessary (Weaver 1974). Aliquots of the extracts were dried in the oven at 105 °C and digested with sulfuric-perchloric acid mixture (Martin et al. 1999). The concentration of molybdate-unreactive P (MUP) was determined as the difference between P concentration in the digested and undigested sample. As stated by Cade-Menun and Liu (2014), the MUP includes organic P along with some inorganic forms that do not react with molybdate. In relation to the scope of this study, we assumed that the MUP pool corresponded mainly to organic P forms. Therefore, we will hereafter refer to it as organic P in the extract.
Evaluation of plant responses to different soil P supply
Mesocosm experiment setup
Rice cultivation was performed in the 12 selected soils for 60 days under continuous flooding. 2.5 kg of fresh soil was used and carefully put in each mesocosm by reproducing the original bulk density. The soils were then flooded by maintaining 5 cm of water above the soil surface and the Eh measured potentiometrically and monitored to confirm the establishment of soil-reducing conditions (7 days after flooding, DAF). The experiment was carried out with four replicates. Seeds of the rice cultivar Selenio were pre-germinated for 3 days at 25 °C in the dark and transferred to the mesocosms at 5 DAF. The mesocosms were then transferred inside a climatic chamber at a constant temperature of 20 °C and 12 h light per day, with a light intensity of 600 µmol m−2 s−1. At 20 DAF, plants were fertilized with 40 kg N ha−1, considering a mesocosm volume of 0.02 m3.
In each mesocosm, one Rhizon sampler (Rhizon MOM 19.21.21F, Rhizosphere, Wageningen, The Netherlands) was installed vertically in the proximity of the root system, and the soil solution was collected weekly and analyzed for MRP and MUP, Fe(II), and dissolved organic C (DOC). Molybdate unreactive P and MRP in the soil solution were quantified as previously described for soil extracts. Dissolved Fe(II) concentration was measured colorimetrically immediately after porewater sampling, using the 1,10-orthophenanthroline method (Loeppert and Inskeep 1996). Dissolved organic C was determined using Pt-catalyzed, high-temperature combustion (850 °C) followed by infrared detection of CO2 (VarioTOC, Elementar, Hanau, Germany), after removing inorganic C by acidifying to pH 2 and purging with CO2-free synthetic air.
Additionally, for each soil P level, unplanted soil mesocosms were also set up, and soil solution was weekly collected and analyzed for Fe(II) concentration. The difference in porewater Fe(II) concentration between non-planted and planted samples was used as a proxy to monitor radial O2 loss (ROL) from plant roots. Indeed, the extent of Fe mineral reduction could be used as an indicator of the ability of rice plants to control rhizospheric soil redox conditions under flooding (Doran et al. 2006).
Plant sampling and elemental analysis
Plant shoots and roots were harvested separately at 60 DAF, when plants had the maximum number of detectable tillers, that is at the end of the vegetative stage, and the concentration of P in plants mainly depends on plant uptake rather than internal redistribution (Wissuwa 2003). Roots were carefully washed with deionized water, and a subsample (200 mg) was rapidly frozen with liquid N2 and stored at − 80 °C for enzymatic and gene expression analyses. The remaining root and shoot material was dried at + 40 °C for determining dry biomass and elemental composition. Total C, N, and P content (Pplant) was determined according to the methods previously described for soil samples. Phosphorus-acquisition efficiency (PAE) was calculated as the ratio of P accumulated in tissues to soil total P, P-utilization efficiency (PUE) was calculated as the ratio of dry biomass to P concentration in the plant tissues (Neto et al. 2016), and P-translocation efficiency (PTE) was calculated as the ratio of P concentration in the shoot to the P concentration in the root (Santoro et al. 2021).
Phosphate transporters gene transcript quantification
Aliquots (100 mg) of the frozen roots were ground using a Qiagen TissueLyzer II (Retsch MM300, Germany). Total RNA was extracted using the Trizol Reagent (ThermoScientific) according to the manufacturing instructions and treated with DNase I (ThermoScientific) at 37 °C for 30 min to remove residual genomic DNA. First-strand cDNA was synthesized from 500 ng of purified total RNA using SuperScript® IV Reverse Transcriptase (ThermoScientific) according to the manufacturer’s instructions. For transcript quantification of target genes, the quantitative reverse-transcriptase PCR (qRT-PCR) reactions were carried out by Chromo4™ Real-Time PCR Detection System (Bio-Rad Laboratories) using the SYBR Green (Applied Biosystems) methods. Thermal conditions were 95 °C for 3 min as the first denaturing step, followed by 95 °C for 10 s and 55 °C for 30 s for 39 cycles. The CT values were analyzed with the Q-gene software by averaging three independently calculated normalized expression values for each sample. Expression values are given as the mean of the normalized expression values of the triplicates, calculated according to Eq. 2 of the Q-gene software (Muller et al. 2002). Three independent biological replicates (= 3 plants) were analyzed per soil, and each qRT-PCR reaction was run in technical duplicates. A list of primers used in this study is shown in Table S3.
Enzyme activity in root tissues
Root enzymatic activity was assayed following the method of Hayes et al. (1999). Briefly, the root material (0.5 g) was ground in 15 mM 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 5.5) containing 0.5 mM CaCl2 and 1 mM EDTA. After centrifugation, the extract was subjected to gel filtration on Sephadex G-25 columns. The activity of acid phosphatase and phytase enzymes was measured on the same root extracts using the substrates 10 mM p-nitrophenyl phosphate (pNPP) and 2 mM potassium myo-inositol hexaphosphate (myoInsP6), respectively. Assays were conducted for 60 min, and the reactions were stopped by the addition of ice-cold 10% trichloroacetic acid (TCA). Solutions were subsequently centrifuged (2000 × g) to remove precipitated material, and p-nitrophenol and phosphate concentrations were determined by measuring the absorbance against standard solutions, at 412 nm and 600 nm, respectively (Helios Gamma Spectrophotometer, Thermo Electron, Waltham, MA).
Statistical analyses
The statistical analyses were performed using the R version 4.1.1. Normality and data homoscedasticity were checked with the Shapiro–Wilk and Levene tests, respectively. When necessary, data were transformed according to the data distribution. All the variables were tested for the analysis of variance (two-way ANOVA), followed by pair-wise post hoc analyses (the Student–Newman–Keuls test) to determine the differences among the mean value at p < 0.05. Correlation analyses were used to relate P content in the plant tissues (Pplant) to the amount of P extracted by the different extractants in order to determine the best method to estimate P availability in flooded paddy soils. The R package corrplot was used to compute the correlation matrices, and the native “R cor.test function” was implemented to test the significance of correlation. The ggplot2 package was used to plot all the figures.
Results
Potential availability of soil P pools
Soil chemical and physical properties
The majority of the 100 analyzed soils had a sandy loam texture, and the pH was close to neutrality on average. The total P content ranged from 200 to 1000 mg P kg−1, and POlsen from 5 to 65 mg P kg−1 (mean value 31 mg P kg−1). Over 70% of the samples had POlsen values higher than 20 mg P kg−1, thus indicative of a relatively high P availability. Fig. S2 reports the average characteristics of the 100 collected soils, while Table 2 details the properties of the 12 representative soils selected for the mesocosm experiment. In the representative 12 soils, total P values were over 800 mg kg−1 in high P soils, around 500–550 mg kg−1 in medium P soils, and below 420 mg kg−1 in low P soils (Table S1). Conversely, the 12 representative soils showed a low and comparable value of total organic C, being on average 11.3 ± 0.98 g kg−1. The average value of Feox was 3.04 ± 0.58 g kg−1, with the lower values associated with higher sand content.
Assessment of soil available P pools
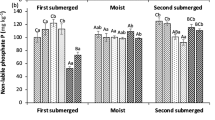
Soil extractable MRP (Pi) and MUP (assumed to be mainly Po) obtained with the different chemical methods were grouped and averaged based on the three total P levels (Fig. 1a). As expected, the amount of MRP and MUP extracted by the different methods was related to the total soil P, although differences between high and medium P soils were much higher than between medium and low P soils and largely varied according to the extraction strength of each chemical method. On average, CaCl2 (0.01 M) extracted the lowest amount (1.81 ± 2.56 mg kg−1), followed by Olsen (29.5 ± 19.2 mg kg−1), Mehlich-III (44.8 ± 31.6 mg kg−1, data not shown), and anion exchanging resins (45.3 ± 38.2 mg kg−1). The methods commonly used to determine Fe-associated P forms extracted the highest amounts of MRP: EDTA (202 ± 150 mg kg−1), citrate-ascorbate (222 ± 132 mg kg−1), and oxalate (331 ± 167 mg kg−1). The extracted MUP displayed a similar trend, despite lower values for all extractants: CaCl2 (0.26 ± 0.25 mg kg−1), Olsen (2.14 ± 1.88 mg kg−1), anion exchanging resins (7.39 ± 9.60 mg kg−1), EDTA (43.6 ± 52.2 mg kg−1), citrate-ascorbate (73.4 ± 61.9 mg kg−1), and oxalate (34.9 ± 18.9 mg kg−1). The amounts of MRP extracted in CaCl2 (0.01 M) and Olsen were not significantly different between soil groups with medium and low P levels, while the amounts of P extracted in EDTA, citrate-ascorbate, and oxalate (p < 0.05) well discriminated among the three groups. Across all methods, MRP represented the greatest proportion of extractable P (on average 83.1 ± 16.8%; Fig. 1b), while the proportion of MUP appeared to be higher in low P than in medium and high P soils, despite the differences were not statically significant (Fig. 1b). Such effect was most pronounced in 0.01 M CaCl2 extracts, where MUP was negligible in high P soils, while in low P soils, it represented over 50%.
Average concentration of inorganic P (MRP) and organic P (MUP) extracted with calcium chloride, Olsen, anion exchanging resins, EDTA, citrate-ascorbate, and acid NH4 oxalate for each of the three soil P levels (a). Percentage of MRP and MUP obtained in the same extracts (b). Different letters above bars represent significant differences among the three soil P levels within each chemical method (p < 0.05). Note the different values on the y-axis for each extractant. The data obtained using Mehlich III are not shown. For the sake of graph readability, SEs are not reported in Fig. 1b
Plant responses to soil P supply
Temporal variation of Fe(II), MRP, MUP, and DOC in porewater during plant growth
In the soil-rice mesocosms, after the beginning of the submersion, soil redox potential passed from values well above + 300 mV to nearly zero mV within 1 week, indicating the instauration of reducing conditions. The temporal trends in porewater Fe(II), DOC, and MRP concentrations were similar, but at different extents, for the three soil P levels over the plant growing period (Fig. 2) and significantly correlated (Fig. S3). Three major phases could be identified: (i) a Fe(II) and DOC increase to the maximum values during the first 14 days, (ii) a decreasing phase within the subsequent 10 days, and (iii) a last time span characterized by a steady increase of both parameters. The porewater MRP concentration during rice growth was first related to soil total P with higher mean values in high P soils with respect to medium and low P soils. Although the pattern during plant growth reflected those of Fe(II) and DOC, the MRP fluctuations were temporarily asynchronous with an initial peak anticipated at 10 DAS (high P soils 1.34 ± 1.27 mg L−1, medium P soils 0.47 ± 0.34 mg L−1, and low P soils 0.13 ± 0.06 mg L−1), a minimum concentration reached in the subsequent 20 days (high P soils 0.94 ± 1.26 mg L−1, medium P soils 0.38 ± 0.45 mg L−1, and low P soils 0.10 ± 0.08 mg L−1), followed by a slight increase until a final concentration of 1.75 ± 0.77 mg L−1 in high P, 0.40 ± 0.39 mg L−1 in medium P and 0.12 ± 0.08 mg L−1 in low P soils. Conversely, the MUP porewater concentrations started from relatively high values and differed according to soil P levels, with the highest values in high P soils than medium and low P soils and then progressively decreased reaching the lowest values after only 15 DAF.
Average concentration of Fe(II) (a), dissolved organic carbon (DOC) (b), molybdate unreactive phosphate (MUP) (c), and molybdate reactive phosphate (MRP) (d) in the porewater of the twelve soils in planted mesocosms. For the sake of graph readability, SEs are not reported. Each value represents the average of four independent replicates
When plant had reached a significant root development (20 DAF), the release of Fe(II) in the soil solution was different in mesocosms with plants compared to those without plants (Fig. 3). In all cases, the Fe(II) porewater concentrations were lower in planted mesocosms. Although in high-P soils, the differences between planted and unplanted mesocosms were not significant, and in medium and low P soil, the Fe(II) porewater concentration was significantly higher in mesocosms without rice plants (p < 0.05). Particularly, in medium and low P soils, the Fe(II) porewater concentration was two and three times higher in unplanted than in planted mesocosms, respectively.
Plant biomass and elemental composition
Notwithstanding the similar total (shoot plus root) dry biomass of rice plants grown in soils with different P availability, significantly larger root biomass was observed in plants grown in low P soils (p < 0.01). All plants showed no significant differences in total C and N concentrations, whereas P concentrations in total biomass decreased in the order high P > medium P > low P (Table 3), with a stronger effect in the roots than in the shoots. Plants grown in high P soils accumulated indeed two times more P than those grown in low P soils. However, plant P use efficiency (PUE) was twofold higher for plants grown in low P than in high P soils. Similarly, P acquisition efficiency (PAE) and translocation efficiency (PTE) were greater in low P plants than in medium and high P plants (Fig. 4). For all parameters, no significant differences were evidenced between medium and high P soils.
Phosphate transporter gene expression and activity of P-enzymes in roots
The genes encoding the root high affinity P transporters OsPt1 and OsPt8 were more expressed in plants grown in low than high P soils. The transcript abundance of the low-affinity P transporters OsPt2 and OsPt6 was generally lower compared to OsPt1 and OsPt8, but an increase in gene expression of OsPt2 was observed in roots of plants grown in low P soils (Fig. 5). Within each class of affinity for phosphate (high and low), the transcripts positively correlated. In addition, OsPT8 and OsPT2 showed a positive correlation (Fig. 7).
The activity of acid phosphatase was generally higher than that of phytase (Fig. S4), and, in the former, no significant differences were observed among plants grown at different soil P levels.
Correlations between soil P forms and plant responses
All inorganic P forms obtained with the tested extraction methods positively correlated with Pplant (Fig. 6a); however, the best performances were obtained with anion exchanging resins (r = 0.915, p < 0.001), CaCl2 (r = 0.861, p < 0.01), and Olsen (r = 0.848, p < 0.001). Only the correlation between Mehlich-III and Pplant was very weak and not significant (r = 0.197, p > 0.05). Conversely, the organic P fraction in the extracts was only weakly correlated to plant uptake, with only MUP extracted in EDTA, citrate, oxalate, and anion exchanging resins being positively correlated with Pplant (Fig. 6b). When discriminating between the three groups of soils, the correlation among Pplant and the MRP extracted by the different methods were different in low P soil compared to high and medium P. In the latter, the correlation patterns were similar to the general correlation reported in Fig. 6 a and b, while in the former, the correlations were weaker, except for Olsen P (Fig. 6c), whereas all methods showed a positive, though weak, correlation between MUP and Pplant in low P soils (Fig. 6d). Moreover, OsPT1, OsPT2, and OsPT8 positively correlated with the root biomass, PUE, PAE, and PTE, while OsPT6 showed positive correlation only with PUE. Other positive correlations were found between the root biomass and PUE, whereas negative correlations existed between root biomass and Pplant and PResins (Fig. 7). Low P plants showed a slight increase in phytase activity, and a positive correlation was observed between phytase and Pplant (Fig. 7).
Correlation heatmap of MRP (a) and MUP (b) extracted by the chemical methods used to assess soil P availability and P concentration in plant tissues (Pplant) for the three soil P levels; MRP (c) and MUP (d) extracted by the chemical methods used to assess soil P availability and P concentration in plant tissues (Pplant) grown in low P soils. Stars represent significant correlation at p-value < 0.05 (*), < 0.01 (**), and < 0.001 (***)
Correlation heatmap of the plant strategies to overcome P starvation represented by OsPT1, OsPT2, OsPT6, and OsPT8 expression, enzymatic activity (phytase and phosphatase), root biomass development (Root biomass), P concentration in total plant tissues (Pplant), P extracted by anion exchanging resins (Pre), and P use (PUE), acquisition (PAE) and translocation (PTE) efficiency indexes
Discussion
Desorbable P is the best-related pool with rice uptake
Despite the effect of reductive dissolution on the release of the Fe-bound P into paddy soil porewaters is widely acknowledged (Ponnamperuma 1972; Shahandeh et al. 1995; Ajmone-Marsan et al. 2006), the relationship between redox transformations of Fe (hydr)oxides and P availability for rice plants is still debated. Extraction methods involving the release of P from Fe minerals with EDTA, citrate-ascorbate, or oxalate have been proposed as alternative and more appropriate approaches for estimating the P supply capacity of paddy soils than 0.01 M CaCl2, Olsen, and anion exchanging resins. The reason is because the former methods mimic, to a different extent, the effect of reductive dissolution of Fe mineral pools on P release in submerged paddies, particularly in highly weathered environments (Shahandeh et al. 1995; de Mello et al. 1998; McDowell et al. 2008), whereas the latter methods estimate P release into soil solution based exclusively on adsorption–desorption mechanisms (Moody et al. 2013). In our study, desorption methods solubilized lower amounts of P, as expected, but the differentiation among the three soil P groups was poorly underlined (Fig. 1a). In contrast, the methods based on P release from Fe minerals extracted higher amounts of P with a clear differentiation between the three soil P groups. This was further confirmed by the positive correlation between P and Fe(II) concentrations in the soil porewaters during the rice growth experiment under continuous soil flooding, which, however, did not correspond to a parallel P uptake by plants (Fig. 6a). This was attributed to a temporal decoupling of P release during the reductive dissolution of Fe (hydr)oxides and plant P requirements. The temporal changes in porewater composition over time (Fig. 2) evidenced increasing concentrations of Fe(II), MRP, and DOC (Fig. 2) during the initial phase of plant growth (0–20 DAF) related to the rapid reductive dissolution of the Fe minerals with the onset of anoxic conditions, driven by anaerobic Fe-reducing microorganisms that use DOC as prompt source of C for their metabolism (Ponnamperuma 1972; Zhang et al. 2003; Wei et al. 2019). Consequently, inorganic phosphate was readily released increasing the available pool up to values as high as 1.4 mg P L−1 in those soils rich in oxalate-extractable Fe, organic C, and total P. However, already after 10–20 DAF, the concentration of both porewater Fe(II) and MRP decreased, possibly for the (co)precipitation and P re-adsorption onto less reducible or newly formed mineral active surfaces (Santoro et al. 2019). These processes have been related to an increase in soil P sorption capacity after soil flooding (Zhang et al. 2003; Refait et al. 2007; Heiberg et al. 2010), and the re-adsorption of the P released during the first phase would explain the decrease in plant available P during the second, higher nutrient-demanding phase. Although we have no direct evidence of the formation of these Fe–P associations, their occurrence could explain both the time course of P and Fe(II) in solution and the weak estimate of available P offered by EDTA, citrate-ascorbate, or oxalate. The increasing demand for available P by the plants after the depletion of seed reserves at about 20 DAS (Wissuwa 2003) could have indeed coincided with the decrease in porewater-P at later stages of plant development. Thus, the initial Pi release from Fe minerals under anoxic conditions could be temporally decoupled from the increasing plant P demand, suggesting that other interacting mechanisms could be involved in driving rice P nutrition in paddy soils, possibly with even greater importance. Besides the progressive slowing down of P uptake in the final stages of the plant growing cycle, the late steady-increasing phase of porewater MRP could be enhanced by the boosting effect of C compounds released in the first phase (0–20 DAF), which likely act as electron donors to Fe-reducing microorganisms (Scalenghe et al. 2002) and as competitors for the same sorption sites of P, thus replenishing the soil solution after P depletion (McDowell et al. 2008; Moody et al. 2013). This may further explain the more effective estimation of P availability offered by anion exchanging resins, which act as a P-sink.
The particular environment characterizing rice soils can affect the available organic P pool (Po) as well. In this case, Pplant better correlated with the methods targeting Fe forms than with those based on desorption; actually, the organic P desorbed in CaCl2 and, to a lesser extent, Olsen P was inversely related to plant P concentration (Fig. 6b). Organic P forms are not readily available to plants, and, after desorption, enzymatic hydrolysis is required for the release of inorganic phosphate into the solution suggesting a less immediate P supply efficiency to plants. In addition, organic P forms can be adsorbed/coprecipitated following Fe(II) oxidation at a larger extent and more rapidly compared to Pi (Celi et al. 2020; Santoro et al. 2021), limiting their extraction by methods exclusively based on desorption mechanisms. Thus, the methods targeting Fe forms can access more strongly bound Po pools, especially the citrate-ascorbate, which extracted the largest Po pools through a combined Fe reduction/complexation mechanism. Notwithstanding the larger fraction extracted by Fe dissolution methods, no discrimination among the three soil groups was observed, probably due to the large heterogeneity of organic P compounds. Indeed, the rate of Po mineralization depends on organic matter amount and quality, including its C/P ratio (Wu et al. 2021) or the relative abundance of easily hydrolyzable compounds, such as nucleic acids, that could represent an important P source in soils that experienced waterlogging (De Sena et al. 2022). The microbial soil biomass, involved in organic matter turnover, can also represent a potential sink of immobilized P (Peng et al. 2021), increasing the complexity of Po dynamics.
During rice growth, the initial high presence of Po in the porewater was followed by a progressive decrease during the first 20 DAF, to constant concentrations until 40 DAF, for slightly increasing in the last period, resulting in a temporal asynchrony between porewater Po and Pi concentration, more emphasized in the high P soils with respect to medium and low soils. The larger supply of organic compounds present in the high P soils might activate microbial community and the relative microbially driven Fe-reducing and organic-P hydrolytic processes (Peng et al. 2021; Wu et al. 2021; Wang et al. 2022b), alternating to the release and consequent hydrolysis of organic P compounds, phases of P re-adsorption on the newly formed surfaces that retain more Po than Pi (Santoro et al. 2019). This could reduce the availability of this important P reservoir, more than supposed by the contemporaneous high Fe(II) concentration levels, although further investigation should be devoted to better understand the Po forms involved.
Plant responses to soil P availability
The correlation patterns between estimated soil P availability and Pplant were different in the three soils P groups. Particularly, the correlations of both Pi and Po with Pplant were weaker at low soil P availability compared to medium and high P soils (Fig. 6 c and d), indicating that the mechanisms underpinning P release into solution and subsequent plant P uptake likely differ as a function of soil P content, since rice plants may adopt specific strategies to cope with P limitation by accessing less readily available pools. The first evident effect was the manipulation of rhizosphere redox conditions. Indeed, although no significant differences were observed among the soil P groups in terms of poorly crystalline Fe content (Feox), the low P soils showed a slightly lower Fe(II) concentration in porewater during the last phases of plant development (Fig. 2a). Furthermore, the significantly lower porewater Fe(II) concentration observed with plants than without (Fig. 3) suggests a higher degree of Fe oxidation driven by plants, particularly in low P soils and attributed to a larger radial oxygen loss (ROL). This process has been reported to be more expressed under low P availability (Kirk and Van Du 1997; Fu et al. 2014) and involves a higher oxidation potential in the rhizosphere compared to the anaerobic bulk soil (Colmer 2003; Zhang et al. 2004), likely shaping the redox gradient governing P dynamics in the root surrounding. Rhizosphere oxygenation may contribute to counteract the formation of green rust-like Fe–P associations that can limit P availability under persisting anaerobic conditions (Zhang et al. 2004). It has been demonstrated that anaerobic conditions may limit the rate of P release from green rust-P complexes because the high Fe(II)/Fe(III) ratio which characterizes these minerals retards their transformation to Fe(III) (hydr)oxides with lower P retention capacity (Huang et al. 2022). Conversely, under oxidative conditions, the evolution of green rust-P complexes to Fe(III) (hydr)oxides prompts the release of P (Fang et al. 2021; Huang et al. 2022), thus possibly offering a further explanation for the increase in Pi and Po porewater concentration occurred in the last plant development stage (40–60 DAS). The observation that P release from the Fe (hydr)oxides newly formed under oxidative conditions after the transformation of green rust-like materials is governed by anion-exchange mechanisms (Huang et al. 2022) could further corroborate the effectiveness of anion exchanging resins in predicting P availability to rice plants.
Other observed differences involved P uptake and translocation capacity. In our study, P concentration in rice shoots was comparable between medium and low P soils (Table 3), possibly due to the higher PTE of plants grown in these soils, especially in low P compared to high P soils (Fig. 4). In support of this, the gene encoding OsPT8, which has a major role in P translocation, was up-regulated in plants of low and medium P soils. Furthermore, the total biomass produced by rice plants was similar across different soils groups, but plants grown in low P soils had a higher root-to-shoot ratio as a consequence of the larger root biomass, which indicated a differential allocation of C resources to this organ to promote its growth and mining activity. The trend of PTE was the same as that of PUE; this latter was primarily dictated by the regulation of genes encoding P root transporters. We found that the lower the PCaCl2, the higher the induction of P transporters that aim to increase Pplant. More specifically, the high-affinity P transporter principally involved in the primary P uptake, OsPT1 (Rose et al. 2013), was most expressed in the roots of plants grown in low P soils. In the same plants, the transcription of OsPT2 was also increased. OsPT2 is a root low-affinity Pi transporter that mediates the translocation of the stored P in the plant, therefore playing a critical role in the initial stage of plant development for P acquisition (Rose et al. 2013; Julia et al. 2018). The parallel increase of OsPT1, OsPT2, and OsPT8 transcription suggests a coordination in the response of rice plants to P limitation in terms of acquisition, usage, and translocation. The transporter OsPT6, which is reported to have a role in P translocation as OsPT8 (Julia et al. 2018), was however substantially less expressed in plants, regardless of P available in soil. Thus, a minor role in P translocation for OsPT6 can be hypothesized in rice plants under the experimental conditions used in this study.
The positive correlation between Pplant and the Po extracted by 0.01 M CaCl2, suggests the importance of the labile form of Po to plant P uptake (Fig. 6d). Nevertheless, no significant differences were observed among soil P levels in terms of enzymatic activity. Such an effect underlined that the complex soil–plant dynamics governing the availability of the organic P forms are still to clarify.
Conclusions
The results obtained in this work indicate that combined multifaceted effects contribute to rice P nutrition besides P release from Fe minerals, which might not be the only nor the most relevant mechanism, probably because of the asynchrony between Pi and Po release into soil solution and plant requirements. Additionally, in moderately weathered soils, where the dominance of Fe minerals on soil chemical properties is not so much expressed as in highly weathered tropical environments, plant responses to P limitations can effectively enhance P uptake. In particular, ROL could boost the release of P after its temporary solubility decline. The different P utilization efficiency measured in low P compared to high P soils further confirmed the interplay between plant strategies and soil P pools. The increased root development and up-regulation of phosphate root transporters encoding genes contribute to P uptake in the complex environment of rice paddies. The relationship between plant strategies and changes of soil P pools suggests an important role of organic P in rice nutrition under conditions of P deficiency, with the labile organic pools likely contributing in replenishing available ones progressively taken up by plants. However, the mechanisms underpinning the transformations of organic P forms in rice paddies still deserve further research.
Abbreviations
- Pplant :
-
Total P content in plant tissues
- Pi :
-
Inorganic P
- Po :
-
Organic P
- MRP:
-
Molybdate reactive phosphate
- MUP:
-
Molybdate unreactive phosphate
- PCaCl 2 :
-
P extracted with 0.01 M calcium chloride
- POlsen :
-
P extracted with the Olsen method
- PMehlich :
-
P extracted with the Mehlich-III method
- Presins :
-
P extracted with anion exchanging resins
- PEDTA :
-
P extracted with 0.025 M EDTA
- Pcitrate :
-
P extracted with citrate-ascorbate
- Poxalate :
-
P extracted with oxalate
References
Ajmone-Marsan F, Côté D, Simard RR (2006) Phosphorus transformations under reduction in long-term manured soils. Plant Soil 282:239–250. https://doi.org/10.1007/s11104-005-5929-6
Bascomb CL (1964) Rapid method for the determination of cation exchange capacity of calcareous and non-calcareous soils. J Sci Food Agric 15:821–823
Bowman RA, Moir JO (1993) Basic edta as an extractant for soil organic phosphorus. Soil Sci Soc Am J 57:1516–1518. https://doi.org/10.2136/sssaj1993.03615995005700060020x
Cade-Menun B, Liu CW (2014) Solution phosphorus-31 nuclear magnetic resonance spectroscopy of soils from 2005 to 2013: a review of sample preparation and experimental parameters. Soil Sci Soc Am J 78:19–37. https://doi.org/10.2136/sssaj2013.05.0187dgs
Celi L, Prati M, Magnacca G, Santoro V, Martin M (2020) Role of crystalline iron oxides on stabilization of inositol phosphates in soil. Geoderma 374:114442. https://doi.org/10.1016/j.geoderma.2020.114442
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots: Gas transport in plants. Plant Cell Environ 26:17–36. https://doi.org/10.1046/j.1365-3040.2003.00846.x
de Mello JWV, Barrón V, Torrent J (1998) Phosphorus and iron mobilization in flooded soils from Brazil. Soil Sci 163:122–132. https://doi.org/10.1097/00010694-199802000-00006
De Sena A, Madramootoo CA, Whalen JK, von Sperber C (2022) Nucleic acids are a major pool of hydrolyzable organic phosphorus in arable organic soils of Southern Ontario, Canada. Biol Fertil Soils 58:7–16. https://doi.org/10.1007/s00374-021-01603-y
Doran G, Eberbach P, Helliwell S (2006) The impact of rice plant roots on the reducing conditions in flooded rice soils. Chemosphere 63:1892–1902. https://doi.org/10.1016/j.chemosphere.2005.10.027
Fang W, Williams PN, Zhang H, Yang Y, Yin D, Liu Z, Sun H, Luo J (2021) Combining multiple high-resolution in situ techniques to understand phosphorous availability around rice roots. Environ Sci Technol 55(19):13082–13092. https://doi.org/10.1021/acs.est.1c05358
Fu YQ, Yang XJ, Shen H (2014) The physiological mechanism of enhanced oxidizing capacity of rice (Oryza sativa L.) roots induced by phosphorus deficiency. Acta Physiol Plant 36:179–190. https://doi.org/10.1007/s11738-013-1398-3
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis, part 1, physical and mineralogical methods, 2nd edn. Agronomy monograph 9. Soil Science Society of America Inc. and American Society of Agronomy Inc., Madison, Wisconsin, pp 383–409
Hayes JE, Richardson AE, Simpson RJ (1999) Phytase and acid phosphatase activities in extracts from roots of temperate pasture grass and legume seedlings. Functional Plant Biol 26:801. https://doi.org/10.1071/PP99065
Heiberg L, Pedersen TV, Jensen HS, Kjaergaard C, Hansen HCB (2010) A comparative study of phosphate sorption in lowland soils under oxic and anoxic conditions. J Environ Qual 39:734–743. https://doi.org/10.2134/jeq2009.0222
Hernández J, Berger A, Deambrosi E, Lavecchia A (2013) Soil phosphorus tests for flooded rice grown in contrasting soils and cropping history. Commun Soil Sci Plant Anal 44:1193–1210. https://doi.org/10.1080/00103624.2012.756000
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195. https://doi.org/10.1023/A:1013351617532
Huang LZ, Zhang X, Liu R, Fang L (2022) The redox chemistry of phosphate complexed green rusts: Limited oxidative transformation and phosphate release. Chem Eng J 429:132417. https://doi.org/10.1016/j.cej.2021.132417
Jia H, Ren H, Gu M, Zhao J, Sun S, Zhang X, Chen J, Wu P, Xu G (2011) The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol 156:1164–1175. https://doi.org/10.1104/pp.111.175240
Julia CC, Rose TJ, Pariasca-Tanaka J, Jeong K, Matsuda T, Wissuwa M (2018) Phosphorus uptake commences at the earliest stages of seedling development in rice. J Exp Bot 69:5233–5240. https://doi.org/10.1093/jxb/ery267
Kirk GJD, Van Du L (1997) Changes in rice root architecture, porosity, and oxygen and proton release under phosphorus deficiency. New Phytol 135:191–200. https://doi.org/10.1046/j.1469-8137.1997.00640.x
Kögel-Knabner I, Amelung W, Cao Z, Fiedler S, Frenzel P, Jahn R, Kalbitz K, Kölbl A, Schloter M (2010) Biogeochemistry of paddy soils. Geoderma 157:1–14. https://doi.org/10.1016/j.geoderma.2010.03.009
Loeppert RH, Inskeep WP (1996) In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soluanpour PN, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis. Part 3, chemical methods. Book series no 5. Soil Science Society of America Inc. and American Society of Agronomy Inc., Madison, Wisconsin, pp 639–664
Madurapperuma WS, Kumaragamage D (2008) Evaluation of ammonium bicarbonate–diethylene triamine penta acetic acid as a multinutrient extractant for acidic lowland rice soils. Commun Soil Sci Plant Anal 39:1773–1790. https://doi.org/10.1080/00103620802073768
Maftoun M, Ardekani MAH, Karimian N, Ronaghy AM (2003) Evaluation of phosphorus availability for paddy rice using eight chemical soil tests under oxidized and reduced soil conditions. Commun Soil Sci Plant Anal 34:2115–2129. https://doi.org/10.1081/CSS-120024052
Marschner P (2021) Processes in submerged soils – linking redox potential, soil organic matter turnover and plants to nutrient cycling. Plant Soil 464:1–12. https://doi.org/10.1007/s11104-021-05040-6
Martin M, Celi L, Barberis E (1999) Determination of low concentrations of organic phosphorus in soil solution. Commun Soil Sci Plant Anal 30:1909–1917. https://doi.org/10.1080/00103629909370341
McDowell RW, Condron LM, Stewart I (2008) An examination of potential extraction methods to assess plant-available organic phosphorus in soil. Biol Fertil Soils 44:707–715. https://doi.org/10.1007/s00374-007-0253-3
Mehlich A (1984) Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 15:1409–1416. https://doi.org/10.1080/00103628409367568
Miniotti EF, Romani M, Said-Pullicino D, Facchi A, Bertora C, Peyron M, Sacco D, Bischetti GB, Lerda C, Tenni D, Gandolfi C, Celi L (2016) Agro-environmental sustainability of different water management practices in temperate rice agro-ecosystems. Agric Ecosyst Environ 222:235–248. https://doi.org/10.1016/j.agee.2016.02.010
Moody PW, Speirs SD, Scott BJ, Mason SD (2013) Soil phosphorus tests I: what soil phosphorus pools and processes do they measure? Crop Pasture Sci 64:461. https://doi.org/10.1071/CP13112
Muller PY, Janovjak H, Miserez AR, Dobbie Z (2002) Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32:1372–1374, 1376, 1378–1379
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Neto AP, Favarin JL, Hammond JP, Tezotto T, Couto HTZ (2016) Analysis of phosphorus use efficiency traits in Coffea genotypes reveals Coffea arabica and Coffea canephora have contrasting phosphorus uptake and utilization efficiencies. Front Plant Sci 7:408. https://doi.org/10.3389/fpls.2016.00408
Ohno T, Zibilske LM (1991) Determination of low concentration of phosphorus in soil extracts using malachite green. Soil Sci Soc Am J 55:892–895
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL et al (eds) Methods of Soil Analysis, part 2, 2nd Edition, Agronomy 9. ASA, Madison, WI, pp 403–430
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular 939, U.S. Government Printing Office, Washington D.C., 1–19.
Peng Y, Duan Y, Huo W, Xu M, Yang X, Wang X, Wang B, Blackwell MSA, Feng G (2021) Soil microbial biomass phosphorus can serve as an index to reflect soil phosphorus fertility. Biol Fertil Soils 57:657–669. https://doi.org/10.1007/s00374-021-01559-z
Pierzynski GM (2000) Methods of phosphorus analysis for soils, sediments, residuals, and waters. North Carolina State University, North Carolina
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96
Rabeharisoa L, Razanakoto OR, Razafimanantsoa MP, Rakotoson T, Amery F, Smolders E (2012) Larger bioavailability of soil phosphorus for irrigated rice compared with rainfed rice in Madagascar: results from a soil and plant survey. Soil Use Manag 28:448–456. https://doi.org/10.1111/j.1475-2743.2012.00444.x
Refait Ph, Reffass M, Landoulsi J, Sabot R, Jeannin M (2007) Role of phosphate species during the formation and transformation of the Fe(II–III) hydroxycarbonate green rust. Colloids Surf A Physicochem Eng Asp 299:29–37. https://doi.org/10.1016/j.colsurfa.2006.11.013
Reyes I, Torrent J (1997) Citrate-ascorbate as a highly selective extractant for poorly crystalline iron oxides. Soil Sci Soc Am J 61:1647–1654. https://doi.org/10.2136/sssaj1997.03615995006100060015x
Rose TJ, Impa SM, Rose MT, Pariasca-Tanaka J, Mori A, Heuer S, Johnson-Beebout SE, Wissuwa M (2013) Enhancing phosphorus and zinc acquisition efficiency in rice: a critical review of root traits and their potential utility in rice breeding. Ann Bot 112:331–345. https://doi.org/10.1093/aob/mcs217
Saggar S, Hedley MJ, White RE (1990) A simplified resin membrane technique for extracting phosphorus from soils. Fert Res 24:173–180. https://doi.org/10.1007/BF01073586
Santoro V, Martin M, Persson P, Lerda C, Said-Pullicino D, Magnacca G, Celi L (2019) Inorganic and organic P retention by coprecipitation during ferrous iron oxidation. Geoderma 348:168–180. https://doi.org/10.1016/j.geoderma.2019.04.004
Santoro V, Schiavon M, Visentin I, Constán-Aguilar C, Cardinale F, Celi L (2021) Strigolactones affect phosphorus acquisition strategies in tomato plants. Plant Cell Environ 44:3628–3642. https://doi.org/10.1111/pce.14169
Scalenghe R, Edwards AC, Ajmone Marsan F, Barberis E (2002) The effect of reducing conditions on the solubility of phosphorus in a diverse range of European agricultural soils: Redox conditions and P solubility. Eur J Soil Sci 53:439–447. https://doi.org/10.1046/j.1365-2389.2002.00462.x
Schwertmann U (1964) Differenzierung der Eisenoxide des Bodens durch Extraktion mit Ammoniumoxalat-Lösung. Z Pflanzenernaehr Dueng Bodenk 105:194–202. https://doi.org/10.1002/jpln.3591050303
Seck PA, Diagne A, Mohanty S, Wopereis MCS (2012) Crops that feed the world 7: rice. Food Sec 4:7–24. https://doi.org/10.1007/s12571-012-0168-1
Shahandeh H, Hossner LR, Turner FT (1995) A comparison of extraction methods for evaluating Fe and P in flooded rice soils. In: Abadía J (ed) Iron Nutrition in Soils and Plants. Springer, Netherlands, Dordrecht, pp 175–181
Simonete MA, Ernani PR, Moro L, Teixeira-Gandra CFA, Gatiboni LC (2015) Eficiência de métodos analíticos na predição da disponibilidade de fósforo para arroz irrigado em solos catarinenses. Rev Bras Ciênc Solo 39:1151–1160. https://doi.org/10.1590/01000683rbcs20140797
Six L, Smolders E, Merckx R (2013) The performance of DGT versus conventional soil phosphorus tests in tropical soils—maize and rice responses to P application. Plant Soil 366:49–66. https://doi.org/10.1007/s11104-012-1375-4
Soil Survey Staff (2022) Keys to soil taxonomy, 13th edn. USDA-Natural Resources Conservation Service, Washington DC
Soltanpour PN, Adams F, Bennett AC (1974) Soil phosphorus availability as measured by displaced soil solutions, calcium-chloride extracts, dilute-acid extracts, and labile phosphorus. Soil Sci Soc Am J 38:225–228. https://doi.org/10.2136/sssaj1974.03615995003800020010x
Teo YH, Beyrouty CA, Norman RJ, Gbur EE (1995) Nutrient uptake relationship to root characteristics of rice. Plant Soil 171:297–302. https://doi.org/10.1007/BF00010285
Van Vuuren DP, Bouwman AF, Beusen AHW (2010) Phosphorus demand for the 1970–2100 period: a scenario analysis of resource depletion. Glob Environ Change 20:428–439. https://doi.org/10.1016/j.gloenvcha.2010.04.004
Wang C, Thielemann L, Dippold MA, Guggenberger G, Kuzyakov Y, Banfield CC, Guenther Ge T, Guenther S, Bork P, Horn MA, Dorodnikov M (2022) Can the reductive dissolution of ferric iron in paddy soils compensate phosphorus limitation of rice plants and microorganisms? Soil Biol Biochem 168:108653. https://doi.org/10.1016/j.soilbio.2022.108653
Wang C, Thielemann L, Dippold MA, Guggenberger G, Kuzyakov Y, Banfield CC, Ge T, Guenther S, Bork P, Horn MA, Dorodnikov M (2022) Microbial iron reduction compensates for phosphorus limitation in paddy soils. Sci Total Environ 837:155810. https://doi.org/10.1016/j.scitotenv.2022.155810
Weaver RM (1974) A simplified determination of reductant-soluble phosphate in soil phosphate fractionation schemes. Soil Sci Soc Am J 38:153–154. https://doi.org/10.2136/sssaj1974.03615995003800010048x
Wei X, Zhu Z, Wei L, Wu J, Ge T (2019) Biogeochemical cycles of key elements in the paddy-rice rhizosphere: microbial mechanisms and coupling processes. Rhizosphere 10:100145. https://doi.org/10.1016/j.rhisph.2019.100145
Willett I, Higgins M (1978) Phosphate sorption by reduced and reoxidized rice soils. Soil Res 16:319. https://doi.org/10.1071/SR9780319
Wissuwa M (2003) How do plants achieve tolerance to phosphorus deficiency? Small causes with big effects. Plant Physiol 133:1947–1958. https://doi.org/10.1104/pp.103.029306
Wu G, Wei K, Chen Z, Jiang D, Xie H, Jiang N, Chen L (2021) Crop residue application at low rates could improve soil phosphorus cycling under long-term no-tillage management. Biol Fertil Soils 57:499–511. https://doi.org/10.1007/s00374-020-01531-3
Zhang Y, Lin X, Werner W (2003) The effect of soil flooding on the transformation of Fe oxides and the adsorption/desorption behavior of phosphate. J Plant Nutr Soil Sci 166:68–75. https://doi.org/10.1002/jpln.200390014
Zhang Y, Lin X, Werner W (2004) Effects of aerobic conditions in the rhizosphere of rice on the dynamics and availability of phosphorus in a flooded soil – a model experiment. J Plant Nutr Soil Sci 167:66–71. https://doi.org/10.1002/jpln.200320349
Acknowledgements
We would like to show our gratitude to Michele D’Amico, Bruna Marcato, Fabio Mazza, Franco Sciorati, and Massimo Zini for technical assistance during soil sampling and to Prof. Roberta Gorra and Dr. Ilaria Mania for allowing us to use the laboratory equipment for gene expression quantification.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. Research carried out in the framework of the P-RICE research project “Fosforo in risaia: equilibrio tra produttività e ambiente nell’ottica delle nuove pratiche agronomiche” selected with the call for funding of research projects in agriculture and forestry 2018 of Regione Lombardia. Project funded with d.d.s. March 28th—n. 4403.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization: Sara Martinengo, Maria Martin, and Luisella Celi. Methodology: Sara Martinengo, Maria Martin, and Luisella Celi. Formal analysis and investigation: Sara Martinengo, Michela Schiavon, and Veronica Santoro. Writing—original draft preparation: Sara Martinengo, Michela Schiavon, and Veronica Santoro. Writing—review and editing: all authors. Funding acquisition: Maria Martin, Luisella Celi, and Marco Romani. Supervision: Maria Martin. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martinengo, S., Schiavon, M., Santoro, V. et al. Assessing phosphorus availability in paddy soils: the importance of integrating soil tests and plant responses. Biol Fertil Soils 59, 391–405 (2023). https://doi.org/10.1007/s00374-023-01714-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-023-01714-8