Abstract
Background
Precipitation of Zn phosphates may limit Zn availability in cogranulated P fertilizers. We assessed whether the Zn availability of Zn could be improved by post-granulation acid treatment.
Methods
Uncoated Zn-fortified monoammonium phosphate granules were compared with sulfuric acid-coated granules in which Zn was either cogranulated or dissolved in the acid coating. Spatially resolved XRF and XANES was used to assess the distribution and speciation of Zn in the granules (before and after incubation in soil) and in the exposed soil. The amount of Zn remaining in the granule was determined after incubation in various soils. The effect of acid coating rate on corn yield was determined in a highly Zn-deficient soil in a pot trial.
Results
The speciation of Zn in the untreated granules was dominated by Zn phosphates. In the sulfuric acid treatments, sulfate species accounted for ~ 45% (if cogranulated) or ~ 80% (if coated) of the Zn. After one week incubation in soil, 10–86% of the added Zn remained in the residual granule, mostly as sparingly soluble compounds. The Zn speciation in the soil near the granule was dominated by Zn phosphates irrespective of treatment, but Zn moved further away from the application site in the acid treatments, as more Zn was released from the granule. In the pot trial, the dry matter yield increased by 70% at a coating rate of 0.75% H2SO4 compared to the uncoated control.
Conclusions
Post-granulation acid treatment of Zn-fortified P fertilizers is an effective way to enhance the phytoavailability of fertilizer Zn.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is an essential micronutrient for both plants and humans and hence Zn deficiency in soil may negatively affect crop growth and nutritional quality. Millions of hectares of agricultural land are affected by Zn deficiency and it has been estimated that over one billion people are at risk of inadequate dietary Zn intake, especially in low income countries (Alloway 2008; Wessells and Brown 2012).
One of the main strategies to tackle Zn deficiency both in crop production and human nutrition is the application of Zn fertilizers to crop plants in order to increase yield and Zn concentrations in edible produce (Cakmak 2008). A convenient way to apply Zn or other micronutrients is through granulation with, or as a coating onto, macronutrient fertilizers. This approach reduces application costs, avoids segregation problems that may occur with bulk blended fertilizers and ensures maximum spread for efficient access by plants (da Silva et al. 2018; Mortvedt 1991). However, the availability of fertilizer Zn may be affected by the macronutrients in the granule, as precipitation reactions may occur that limit Zn solubility. Mortvedt and Giordano (1969) found that the immediate plant availability of Zn in orthophosphate fertilizers was positively correlated with the concentration of water-soluble Zn in the fertilizer. Degryse and McLaughlin (2014) showed that combining Zn with P fertilizer restricted its diffusion in soil, particularly with diammonium phosphate (DAP, (NH4)2HPO4) fertilizer or in high pH soils. Solubility considerations indicated that this was due to the formation of Zn phosphate minerals in or near the granule. Hettiarachchi et al. (2008) demonstrated the formation of Zn phosphates in and around granular Zn-fortified monoammonium phosphate (MAP, NH4H2PO4) fertilizers applied to a calcareous soil, using a combination of synchrotron-based X-ray techniques. Most Zn remained inside or near the residual granule as hopeite (Zn3(PO4)2·4H2O) or scholzite (CaZn2(PO4)2·2H2O) after 4 weeks of incubation. Similarly, Milani et al. (2015) found that Zn coated on MAP as ZnO nanoparticles remained at or close to the point of application after 5 weeks of incubation in a calcareous soil, and had transformed to mainly scholzite and zinc ammonium phosphate (ZnNH4PO4).
A lower fertilizer pH in Zn-fortified P fertilizers results in higher Zn solubility and availability (Degryse et al. 2020; Mortvedt 1991). Thus, it would seem that lowering the pH of Zn-fortified MAP might be an easy way to increase plant-available Zn. However, the granulation process requires the molar N:P ratio to be close to 1, and so the operating pH window during granulation is very narrow. Hence, post-granulation acid treatment is a potential method to increase the availability of Zn in phosphate fertilizers (McLaughlin et al. 2019). It is not clear whether such an acid treatment would change the speciation of Zn in the fertilizer and if so, whether this change would occur throughout the granule or only at the granule surface.
The aim of this study was to assess if and how a post-granulation acid treatment could improve the availability of Zn in P fertilizers. For that purpose, we determined the speciation of Zn in and around granules with or without acid treatment using synchrotron X-ray imaging and spectroscopic techniques. Additionally, incubation experiments were carried out to assess how soil properties and acid coating rates affect the release of Zn from the granule. Finally, a pot trial was carried out to assess the effect of the acid coating on Zn uptake by corn plants and on crop yield.
Materials and methods
Soils and soil analysis
Five soil samples taken from the top 0–10 cm were used across the various experiments (Table 1). Four of the soils were sampled in South Australia while a fifth soil, Sultanonu, was collected from a Zn-deficient location in Central Anatolia, Turkey. The pH of each soil was determined in a 1:5 soil/water extract. Total C was measured with a dry combustion method (Matejovic 1997). The CaCO3 content was determined with a pressure calcimeter method (Martin and Reeve 1955). Particle size analysis was determined using the pipette method after oxidation of organic matter by hydrogen peroxide and destruction of carbonate by acetic acid (McKenzie et al. 2002). The cation exchange capacity (CEC) at pH 7.0 (ammonium acetate) and oxalate-extractable Al and Fe concentrations were determined according to Rayment and Higginson (1992).
Synchrotron-based analyses
Fertilizers
Three fertilizer treatments were included:
-
(1)
a Zn-fortified MAP fertilizer (1% w/w Zn) with low water-soluble Zn (25% of total Zn soluble in water at a liquid:solid ratio of 250 L kg−1).
-
(2)
the same fertilizer, coated with 2% w/w H2SO4. To avoid an uneven coverage, the coating was not sprayed, but 2 µL of a 3.5 M H2SO4 solution was pipetted onto a 35-mg granule. The granules were dried in an oven at 40 °C overnight.
-
(3)
a MAP fertilizer, coated with Zn (1% w/w) and H2SO4 (2% w:w). The coating solution was made by dissolving 2.3 g of ZnO in 10 mL of 3.5 M H2SO4, and 2 µL of this solution was pipetted onto a 35-mg granule. The granules were dried in an oven at 40° overnight.
Sample preparation
Two soils were used to assess Zn mobilization and speciation in and around fertilizer granules (Monarto and Bordertown, Table 1). The fertilizer granules were incubated at 25 °C for either 1 or 4 weeks in a Petri dish (5.5 cm diameter, 1 cm high) filled with soil moistened to field capacity. A hole was made in the center of the Petri dish, in which the granule was placed and carefully covered with soil so that the granule was about 1 mm under the soil surface. A sample without added fertilizer was included for each soil. Prior to the synchrotron measurements, the lid of the Petri dish was removed, and the soil surface was covered with Kapton® film. The whole Petri dishes were attached to the sample mount. Two days after the synchrotron measurements, the surface of the soil was rewetted with 2 mL of water and the Zn diffusion patterns were visualized using CaCO3-impregnated filter papers as a sink and dithizone as a dye (Degryse et al. 2015).
An additional set of Petri dishes was prepared in the same manner, and incubated for 4 weeks at 25 °C, after which the residual granule was carefully removed from the soil using tweezers and dried in the oven at 40 °C. These residual granules as well as original granules (not incubated) were ground, diluted tenfold using cellulose and pressed into pellets (6 mm diameter) for X-Ray Absorption Near Edge Structure (XANES) analysis (see below).
The original granules (not incubated) were also prepared by embedding them in a transparent epoxy resin, lightly grinding evenly to reveal the granule cross-sections and then dry fine polishing the sample surface.
XANES standards were prepared by diluting standard compounds with cellulose to bring the concentration of Zn in each standard to around 0.5% Zn and pressing the powders into pellets. The standard compounds included zincite (ZnO), smithsonite (ZnCO3), hydrozincite (Zn5(CO3)2(OH)6), hopeite (Zn3(PO4)2.4H2O), ZnNH4PO4, scholzite (CaZn2(PO4)2.2H2O), willemite (Zn2SiO4), zinc sulfate heptahydrate (ZnSO4.7H2O), zinc ammonium sulfate hexahydrate (Zn(NH4)2(SO4)2.6H2O) and ferrihydrite-adsorbed Zn. The XANES spectra of the standards are given in Figure S1 and Table S1.
X-ray fluorescence (XRF) and XANES measurements and analysis
All measurements were carried out at the XFM beamline of the Australian Synchrotron (Paterson et al. 2011) using the 384-element Maia detector (Howard et al. 2020). Both the cross-section of the epoxy-mounted granules and the surface of the incubated soils were analyzed using fast scanning 2D elemental mapping with 0.1 × 0.1 mm pixels and a 20 mm s−1 scan velocity with an incident X-ray energy of 9.8 keV. Zinc maps were generated from the XRF spectra using GeoPIXE (Ryan et al. 1990).
XANES measurements were carried out on all pelletized samples (standards, incubated granules and non-incubated granules) using line-XANES (a one-dimensional XANES image stack) with a step size of 1 µm and a scan velocity of 5 mm s−1, scanning 63 energies from 9609 to 9803 eV.
For the incubated soils and cross-sections of the mounted granules, 2D XANES imaging was carried out for selected regions. For the soils, a region of 6–10 mm width and 0.5–0.7 mm height was scanned with a 20 µm step size, scanning 129 energies from 9609 to 9803 eV (using 5-eV steps below 9649 eV, 0.5-eV steps from 9649 to 9700 eV, 2-eV steps from 9700 to 9723 eV and 10-eV steps above 9723 eV) using a per pixel dwell time of 4 ms. The region was selected to extend from the highly Zn-enriched soil at the granule application site to background Zn levels. For the mounted granules, a region of circa 2 mm width and 0.5 mm height was scanned from the center to the outside of the granule, using the same scan parameters.
The collected XANES spectra were analyzed using the Athena software package (Ravel and Newville 2005). For the homogenous samples (standards and homogenized granules), spectra were averaged over the entire scan area. For the mounted cross-sectioned granules, the Zn speciation was homogeneous for treatments (1) and (3). However, for treatment (2), the sample showed two regions with distinct spectral features (see results), for which separate XANES spectra were extracted. For the soils, three separate spectra were extracted from each sample, based on the Zn concentrations (see results). The averaged spectra were background-corrected and normalized. Linear combination fitting was carried out over the spectral region from 20 eV below the Zn K-edge to 80 eV above the absorption edge. Components with < 5% contribution were excluded. For the soil spectra fits, a “soil Zn” spectrum, collected from the unamended soil was included as a possible component (see results).
Petri dish experiments
Comparison of Zn release from granules in different soils
In this experiment, it was assessed if and how soil properties affected the release of Zn from the fertilizer granules (the same granule treatments as for the synchrotron measurements). Four soils were used; the two soils used for the synchrotron measurements (Monarto and Bordertown) and two additional soils (Kangaroo Island and Streaky Bay, Table 1). For each fertilizer treatment, four granules of circa 30 mg each were incubated in a Petri dish (9 cm diameter and 1 cm height) filled with soil moistened to field capacity. The granules were incubated for 1 week, and then carefully retrieved from the soil and dissolved in 10 mL of 5 M HNO3. Concentrations of Zn, Al, Ca, Fe and P in the acid solution were analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES, Perkin Elmer, Avio 200).
Effect of acid rate
In this experiment, the effect of acid coating rate was assessed, using the same Zn-deficient soil as for the pot experiment described below (Sultanonu, Table 1). The coating was applied by carefully pipetting 3 µL of water or a H2SO4 solution on a 35-mg granule (MAP co-granulated with 1% Zn), in order to obtain a coating rate of 0.25, 0.50, 0.75 or 1% (w:w) H2SO4. The granules were dried in an oven at 40 °C. For each treatment, three granules of circa 35 mg each were incubated in a Petri dish (9 cm diameter and 1 cm height) filled with soil moistened to field capacity. After 1 week of incubation, the residual granules were carefully retrieved and analyzed as described above.
Pot experiment
For the pot experiment, the acid coating was applied to granules by spraying the MAP-based fertilizer cogranulated with 1% Zn. As each pot contained 15–20 granules, possible variation in the coating rate between granules as a result of spraying was considered unproblematic compared to the single granule experiments described above where spraying was avoided. The acid coating was sprayed through a glass nebulizer at rates of 0.25, 0.5, 0.75 or 1 g of H2SO4 per 100 g fertilizer, using a 1:1 solution of concentrated sulfuric acid (95% H2SO4):demineralized water. This solution was sprayed onto a rolling bed of 100 g fertilizer granules in a rotating stainless steel drum. The pH and water-soluble Zn was determined at a liquid:solid ratio of 250 L kg−1 (Table S2). A control sample without coating was also included.
A pot trial using a highly Zn-deficient calcareous soil (Sultanonu, Table 1) was carried out at Sabanci University. The fertilizer granules were added at 2.5 mg Zn kg−1 and were mixed throughout the soil. Additionally, a Zn response curve study was included by applying powdered ZnSO4 mixed throughout the soil at 0, 0.25, 1, 2.5 or 7.5 mg Zn kg−1. Basal nutrients were added to all pots as a solution. Each treatment was replicated four times. The plant used was corn (Zea mays, cv: Shemal). Plants (four per pot) were grown in pots with 3.1 kg of soil. All pots were randomized and watered regularly to field capacity using deionized water. Plants were harvested at 44 days after planting, dried at 65 °C, weighed and analyzed for Zn by ICP-OES after microwave-assisted acid digestion.
Statistical analysis
To assess differences in Zn release from the granules, two-way ANOVA with soil and fertilizer treatment as independent variables was carried out. For each soil, statistical differences between the fertilizer treatments were tested by one-way ANOVA followed by a post-hoc Duncan test (at P ≤ 0.05). A one-way ANOVA and post-hoc Duncan test were also used to assess the effect of acid rate on Zn release from the granule and on plant yield. SPSS (Version 28.0.1.0) was used for the statistical analyses.
Results
Speciation of zinc in the fertilizer
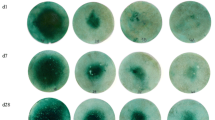
The distribution of Zn in the granules revealed by X-ray imaging was as expected from their production method (Fig. 1). In treatments (1) and (2), Zn was homogenously distributed through the granule. In treatment (3), in which both Zn and acid were coated on the granule, Zn was localized in the surface layer.
Speciation analysis was carried out both on the cross-section of the fertilizer granule and on the homogenized powder (Table 2). The XANES spectra derived from the cross-sections are shown in Fig. 2 and LCF fits are shown in Figure S2. In the control fertilizer (Treatment 1), Zn phosphates (hopeite and zinc ammonium phosphate) were the predominant species, estimated to account for about 70% of total Zn. The remaining 30% was present as gahnite (ZnAl2O4) and ZnO. Aluminium is an impurity in the fertilizer (about 0.8% by weight) explaining the formation of gahnite.
For the cross-section of the acid-coated fertilizer (Treatment 2), analysis using the GeoPIXE “energy association” module indicated that there were two distinct spectral regions. This module allows comparison of the intensity ratios between two energies at each pixel. By plotting the 9.672 keV energy vs the 9.664 keV energy and selecting the two branches using a spline, XANES spectra for the two regions were obtained (Figure S3). One region corresponded to the centre of the granule; the Zn speciation for this region indicated by XANES spectral fitting was similar to that of the control granule, with > 60% of Zn as Zn phosphate species and the remainder as ZnO and ZnAl2O4. The other region corresponded to the outer shell of the granule (with a shell thickness of 0.45–0.5 mm or circa 40% of the total granule volume). The predominant Zn species indicated by XANES fitting in this shell was zinc ammonium sulfate (Zn(NH4)2(SO4)2·6H2O), suggesting that the acid treatment resulted in conversion of the phosphate and oxide species to zinc ammonium sulfate. Based on the volume of the shell, it is estimated that 32% of the Zn was as zinc ammonium sulfate on a whole granule basis for this granule treatment method. When the acid-coated fertilizer was first ground and the homogenized powder was analysed, 55% was estimated to be as Zn phosphate species and 45% as zinc ammonium sulfate, i.e. a similar result as estimated from the cross-section of a Treatment 2 granule, but with a ~ 10% higher contribution of zinc ammonium sulfate. This discrepancy may arise from uncertainties, both in the estimation of the volume of the outer shell affected by the acid coating and in the XANES fitting speciation process. Uncertainty in the proportion of an individual compound is likely ~ 10% (Isaure et al. 2002). This also explains why the oxides (ZnO and ZnAl2O4) were not detected for the homogenized sample as the contribution for the whole fertilizer determined in the XANES imaging analysis of the granule cross section was only ~ 10% or less.
For the fertilizer coated with both ZnO and acid (Treatment 3), analysis of the cross-section indicated that Zn was mostly present as zinc sulfate. Dissolution of ZnO in the sulfuric acid would have resulted in full conversion to zinc sulfate (as there was excess acid), and these results indicated that it remained mainly as zinc sulfate after coating on the granule, although some conversion (circa 20%) to hopeite took place according to the XANES fitting analysis. Interestingly, the results for the homogenized powder indicate about 80% as sulfate species and 20% as hopeite, but in this case the identified sulfate species was zinc ammonium sulfate. While both zinc sulfate species have a similar spectrum, they have distinct differences (e.g. higher white line intensity and a peak around 9684 eV for zinc ammonium sulfate) and using the other species resulted in significantly worse fits. This suggests that the difference was likely not due to uncertainty in the XANES LCF analysis, but that contact of zinc sulfate with the monoammonium phosphate fertilizer matrix during dry grinding of the fertilizer resulted in conversion of zinc sulfate to zinc ammonium sulfate.
Speciation of zinc in residual granules
Residual granules were retrieved from the soil after 4 weeks of incubation. The edge steps of the XANES spectra indicated that the amount of Zn remaining in the granules without acid treatment was higher than in those with acid treatment. For accurate quantification of how treatment and soil properties affect the amount of residual Zn in the granule, a separate experiment was conducted (see below).
While the XANES spectra of the non-incubated granules showed clear differences between treatments (Fig. 2), the XANES spectra for the residual granules were much more similar, although there were some differences depending on soil and treatment (Figure S4). In all residual granules, the speciation was dominated by Zn phosphates (Table 3). For the control treatment, zinc ammonium phosphate was the main species, while hopeite (zinc phosphate) was the main species in the treatments with acid coating. This agrees with the results for the original (non-incubated) granules for which a shift from zinc ammonium phosphate to zinc phosphate was seen upon acid treatment. In the original granules, zinc (ammonium) sulfate was a major species (45 or 77%) in the acid-treated granules (Table 2). However, no sulfate species were detected in the residual granules after incubation, except for a minor fraction (8%) in Treatment (2) for the Bordertown soil. These results suggest that Zn in the sulfate species diffused away from the granule.
Speciation of zinc in the soil after granule incubation
Figure 3 shows the distribution of Zn in the soil after 1 or 4 weeks of incubation. In both soils, the acid treatments increased the diffusion of Zn in the soil, with most diffusion for Treatment (3), in which both ZnO and acid were coated on the granule. There was more diffusion for the Bordertown soil than the Monarto soil (likely related to the soil pH), and more diffusion for the longer incubation time. For the Monarto soil, the concentrations at the granule application site appeared to be lower than in the zone adjacent to the granule for treatments 2 and 3 after 4 weeks of incubation. We hypothesize that most Zn diffused out of the granule, followed by adsorption or precipitation in the soil near the granule. The diffusion patterns were also determined using a previously developed visualization technique in which Zn is captured on a filter paper (Degryse et al. 2015) (Figure S5). This visualization method reflects the dissolved Zn concentration, while the XRF measurements reflect total Zn concentrations, but very similar patterns were obtained using both methods.
For selected regions starting in the center of the Petri dish and moving to the periphery, from high to background Zn concentrations, 2D XANES maps were collected. Only the results for the 4-week incubation are shown, as the 1-week incubation gave very similar results. For each treatment, three XANES spectra were extracted based on the Zn concentrations, roughly corresponding to the zone with Zn concentrations > 100 mg kg−1, 50–100 mg kg−1, and 20–50 mg kg−1 (Figure S6). For a given soil, there were no distinguishable differences in the spectra among the three fertilizer treatments and the LCF yielded very similar results. Therefore, the spectra were merged for the three fertilizer treatments, yielding three spectra for each soil for the high, medium or low Zn zone (Fig. 4). To fit these spectra, a “soil Zn” spectrum (derived from unamended soil) was included as a reference in the LCF analysis (fits shown in Figure S7). This soil-Zn spectrum showed close resemblance to the spectrum of ferrihydrite-adsorbed Zn; the best fit for the Monarto soil was 100% ferrihydrite-adsorbed Zn and for the Bordertown soil 82% ferrihydrite-adsorbed and 18% ZnSO4.7H2O. In the zones with the higher Zn concentrations (> 50 mg kg−1), the Zn speciation in the soil was dominated by Zn phosphates (> 60%), hopeite only in the Bordertown soil and also some scholzite in the Monarto soil (Table 4). Gahnite (up to 19%) was identified close to the granule application site. In the zones with the lower Zn concentration (20–50 mg kg−1), the contribution of Zn phosphates was less (45–52%), while the proportion of soil Zn increased.
Normalized Zn K-edge XANES spectra in Bordertown or Monarto soil after 4 weeks of incubation with Zn-fortified monoammonium phosphate granules, for regions of high (roughly > 100 mg kg−1), medium (50–100 mg kg−1) or low Zn concentrations (20–50 mg kg−1). The spectra were merged across the fertilizer treatments, as there were no discernible differences
Incubation experiments to assess Zn release from granule
The findings above indicated that the speciation of Zn in the soil near the granule was the same for the three fertilizer treatments, but that Zn diffused further away from the acid-treated granules. We hypothesized that this was due to more Zn being released from the granule. To test this hypothesis, an incubation experiment was set up to assess how fertilizer treatment and soil properties affected the release of Zn from the granule. The initial granule weight was circa 30 mg, the residual granule weight after 1 week of incubation was around 9 mg. This residual granule consists mostly of fertilizer impurities and elemental S (5% in original fertilizer). Iron and Al (respectively 1.0% and 0.8% in the original fertilizer) were fully recovered in the residual granule, indicating the low solubility of these impurities. Both soil and fertilizer treatment had a highly significant effect (P < 10–6) on the amount of Zn remaining in the granule (Fig. 5). The amount of Zn remaining in the granule after one week of incubation increased with increasing soil pH, which is known to be the main soil property controlling Zn solubility in soil (Sauvé et al. 2000). On average, 31% of added Zn remained in the granule for the acid Kangaroo Island soil compared to 61% for the calcareous Streaky Bay soil. The acid treatments strongly reduced the amount of Zn remaining in the granule; from 56–86% for the control treatment to 25–59% for the treatment with acid coating and 10–39% for the treatment with both acid and Zn in the coating.
Effect of soil and fertilizer treatment on the percentage of Zn remaining in the residual granule after one week of incubation. The soils are sorted from most acid (KI) to most alkaline (SB). The fertilizer treatments are (1) control; (2) acid coating; (3) both Zn and acid in the coating. Different capital letters indicate significant differences between the soils (two-way ANOVA, Duncan P ≤ 0.05). Different small letters indicate significant differences between the fertilizer treatments for a given soil (one-way ANOVA, Duncan P ≤ 0.05)
In a second experiment, the effect of acid rate (up to 1% H2SO4) was assessed in a calcareous soil (Sultanonu). The amount of Zn remaining in the granule after one week of incubation decreased from 91 to 51% with increasing acid rate (Fig. 6). The effect was most pronounced at the lower rates; there was no significant difference between the 0.75 and 1% H2SO4 coating.
Pot experiment
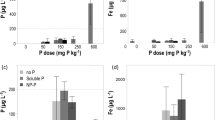
The Sultanonu soil was also used to assess the effect of acid coating rate on availability of Zn to plants. This soil is highly Zn deficient, so without Zn addition, the growth was severely restricted (dry matter yield of 0.5 g plant−1). Addition of ZnSO4 increased the dry matter yield to a maximum of 7.7 g plant−1 (Fig. 7a). These treatments with powdered ZnSO4 mixed through soil were included for reference, but they do not present a field-realistic application method. A clear response to the acid treatment of granules was observed, with the plants growing considerably taller for the higher rates (Figure S8). The dry matter yield increased by about 70% from 2.56 g plant−1 for the control to 4.32 g plant−1 at the 0.75% H2SO4 rate (Fig. 7b). There was no significant difference in yield between the 0.75 and 1% H2SO4 coating.
a Dry matter yield of corn as a function of added Zn rate in a Zn-deficient soil when Zn is added as powdered ZnSO4 mixed through soil or as Zn-fortified monoammonium phosphate granules. b Effect of granule acid coating rate on the dry matter yield for corn grown in a Zn-deficient soil. Different letters indicate significant differences between the acid coating rates (one-way ANOVA, Duncan P ≤ 0.05)
The Zn concentrations in the plant were fairly constant for the acid-coated treatments, ranging from 6.6 to 8.9 mg kg−1, with the highest Zn concentration for the control treatment with the lowest yield (Piper-Steenbjerg effect) (Steenbjerg 1951). As the Zn concentrations showed little variation across treatments, the acid coating had a similar effect on Zn uptake in the shoot as on yield, with Zn uptake increasing from 22 µg per plant for the control to 31 µg per plant at the highest coating rate.
Discussion
The speciation of Zn in the untreated Zn-fortified MAP granules was dominated by Zn phosphates (circa 70%), with the remainder accounted for by gahnite and ZnO. This is in agreement with the findings of Hettiarachchi et al. (2008), who also found that the speciation of Zn in the granule was dominated by Zn phosphate species. When a sulfuric acid coating (2% w:w) was applied to the granule, ~ 45% of the Zn was converted to zinc ammonium sulfate. This converted Zn was located in the outer shell (ca 0.5 mm deep). Da Silva et al. (unpublished results) evaluated a range of Zn-fortified MAP fertilizers and observed zinc ammonium sulfate in the samples with the lowest granulation pH. However, the maximal contribution was only ~ 20%, as granulation requires the pH to be maintained within a narrow window. The post-granulation acid treatment allows for more acidification and a higher proportion of sulfate species. Coating ZnO dissolved in sulfuric acid resulted in a coating with mainly zinc sulfate (ZnSO4.7H2O) and a minor fraction (~ 20%) of hopeite. The amount of acid was sufficient to convert all ZnO to Zn sulfate prior to coating, so the reaction with the fertilizer resulted in slight conversion of Zn sulfate to hopeite. These results indicate that acid treatment of ZnO prior to coating is more effective in converting the Zn to a soluble form than the post-granulation acid treatment, in which case the fertilizer matrix likely consumes some of the acid.
The speciation of Zn in the soil close to the granule application site, where Zn concentrations were elevated, was also dominated by Zn phosphate species (hopeite and scholzite). These precipitated species are not mobile, so would presumably have precipitated in the soil after Zn2+ was released from the granule. Phosphorus concentrations near a P fertilizer granule are highly elevated (McLaughlin et al. 2011), explaining the precipitation of Zn phosphates close to the application site. Closest to the application site, gahnite (ZnAl2O4) was also detected. Other studies have identified gahnite in hot spot areas (Gomes et al. 2023; Hettiarachchi et al. 2008). As Zn concentrations decreased with distance from the granule, the contribution of “soil Zn”, i.e. Zn with same speciation as the background Zn in soil (predominantly sorbed on Fe-(hydroxides) increased. For the different fertilizer treatments, there were no apparent differences in Zn speciation at a given concentration. However, both the XRF 2D maps (Fig. 3) and Zn visualization (Figure S5) indicated Zn concentrations were higher and Zn moved further away from the application site for the granules with acid treatment.
These differences in diffusion zone between the treatments could be explained by the differential release of Zn from the granule. For all treatments, nearly all Zn in the residual granule was present as Zn phosphates and oxides, but the amount of Zn remaining in the granule was different. A separate incubation experiment with four soils showed that 10–86% of the added Zn remained in the residual granule, depending on soil and fertilizer treatment. For the control granules (in which no sulfate species were detected), 44% of Zn was released from the granule in the acid soil compared to only 14% for the calcareous soil. For the acid-coated Zn-fortified granules (estimated to contain 45% of Zn as zinc ammonium sulfate), 75% of Zn was released in the acid soil and 41% in the calcareous soil. For the treatment in which granules were coated with both Zn and acid (estimated to contain ca 80% of Zn as zinc sulfate), 90% of Zn was released in the acid soil and 61% in the calcareous soil. Thus, it appears that all Zn in sulfate form was released from the granule in the acid soil, as well as a considerable part of the less soluble forms (phosphates and oxides) for all treatments. In the calcareous soil, a small portion of the oxides/phosphate species in the granule dissolved for the control treatment, but in the Zn + acid coated treatment, some of the Zn sulfate species were converted to less soluble forms and precipitated within the residual granule.
While the post-granulation acid treatment increased the release of Zn from the Zn-fortified granule, coating the same amount of Zn and acid onto the granule was even more effective. Both fertilizers had identical elemental composition, pH (pH 3.8 at L:S 250 L kg−1) and water-soluble Zn (> 90% of total Zn at L:S 250 L kg−1), but they differed in Zn distribution and speciation. Having the Zn at the outside of the granule in sulfate form allowed for more Zn to be released from the granule. However, having Zn in the coating makes it susceptible to losses during handling and transport. Even relatively little abrasion of the fertilizer could result in high losses of the coated micronutrient. Hence, having Zn throughout the granule may be preferable for practical reasons. Sulfuric acid is used in the production of phosphoric acid and hence often available on site in phosphate fertilizer production. Thus, a post-granulation sulfuric acid spray would be relatively easy to implement.
The pot trial with a highly Zn-deficient soil showed that the increased mobility of Zn in the soil for the acid-treated granules also resulted in enhanced plant availability. The dry matter yield increased by about 70% for a coating rate of 0.75% H2SO4 (Fig. 7b). No benefit was observed from further increasing the rate to 1% H2SO4. This aligned with the results of an incubation experiment in the same soil, in which the effect of acid coating rate (up to 1% H2SO4) on Zn release was assessed (Fig. 6). The largest effects were seen at the lower rates, while there was no significant difference between the 0.75 and 1% H2SO4 rate. Furthermore, while coating rates up to 1% showed little effect on physical quality of the fertilizers, higher coating rates (1.8% and more) substantially increased the moisture uptake of the granules and reduced the critical relative humidity (details not shown).
Even with the acid coating, the Zn-fortified MAP granules were not as effective in increasing plant yield as ZnSO4 mixed through soil, likely both related to the phosphate carrier and the difference in application of ZnSO4 as a a powder vs the Zn-fortified MAP as granules. The acid coating does not completely prevent the precipitation of Zn with P of the macronutrient carrier, but the difference in placement also strongly affects the availability of Zn. For instance, Ghosh (1990) showed that total Zn uptake in an acid soil was 33% for granular ZnSO4 and 24% for Zn-fortified MAP granules compared to a ZnSO4 solution mixed through soil. Thus, the effect of placement was more pronounced than the effect of the P carrier. Similar results were obtained by Goos et al. (2000), who found that availability of granular ZnSO4 was almost zero for the first crop grown in a calcareous soil, while ZnSO4 mixed through soil was highly effective. While the use of a fine ZnO/ZnSO4 powder or solution mixed through soil has been found to be highly effective both in this study and in literature, it is not a realistic application method in field conditions. It is noteworthy that several studies have found less differences in Zn availability between fertilizer treatments (either form or placement) in a second crop than in a first crop, particularly if soils were mixed/tilled prior to the second crop (Ghosh 1990; Goos et al. 2000; Slaton et al. 2005).
Overall, this study shows that an acid coating is an easy and effective way to increase the phytoavailability of Zn in Zn-fortified P fertilizers, especially in high pH soils. The acid treatment had a large effect on the Zn speciation in the granule, the percentage of Zn released from the granule and the diffusion distance in soil, but did not affect the speciation in soil. A 0.75% sulfuric acid granule coating increased corn yield by 70% in a Zn-deficient soil. Even when yield is not limited by Zn availability, acid coating of Zn-fortified P fertilizers can be helpful to increase Zn uptake and Zn accumulation in food crops, thus enhancing the nutritional quality of staple food crops such as corn.
Data Availability
Data is available upon request.
References
Alloway BJ (2008) Zinc in soils and crop nutrition. International Zinc Association Brussels, Belgium
Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 302:1–17
da Silva RC, Baird R, Degryse F, McLaughlin MJ (2018) Slow and fast-release boron sources in potash fertilizers: spatial variability, nutrient dissolution and plant uptake. Soil Sci Soc Am J 82:1437–1448
Degryse F, Baird R, McLaughlin M (2015) Diffusion and solubility control of fertilizer-applied zinc: chemical assessment and visualization. Plant Soil 386:195–204
Degryse F, Da Silva RC, Baird R, Cakmak I, Yazici MA, McLaughlin MJ (2020) Comparison and modelling of extraction methods to assess agronomic effectiveness of fertilizer zinc. J Plant Nutr Soil Sci 183:248–259
Degryse F, McLaughlin MJ (2014) A method to visualize diffusion of phosphorus from fertilizer and comparison with chemical measurements and modeling results. Soil Sci Soc Am J 78:832–842
Ghosh AK (1990) Chemistry and agronomic effectiveness of Zn-enriched fertilizer. PhD Thesis. University of Western Australia
Gomes FP, Soares MB, de Carvalho HWP, Sharma A, Hesterberg D, Alleoni LRF (2023) Zinc speciation and desorption kinetics in a mining waste impacted tropical soil amended with phosphate. Sci Total Environ 864:161009
Goos RJ, Johnson BE, Thiollet M (2000) A comparison of the availability of three zinc sources to maize (Zea mays L.) under greenhouse conditions. Biol Fertility Soils 31:343–347
Hettiarachchi GM, McLaughlin MJ, Scheckel KG, Chittleborough DJ, Newville M, Sutton S, Lombi E (2008) Evidence for different reaction pathways for liquid and granular micronutrients in a calcareous soil. Soil Sci Soc Am J 72:98–110
Howard DL, de Jonge MD, Afshar N, Ryan CG, Kirkham R, Reinhardt J, Kewish CM, McKinlay J, Walsh A, Divitcos J (2020) The XFM beamline at the Australian Synchrotron. J Synchrotron Radiat 27:1447–1458
Isaure M-P, Laboudigue A, Manceau A, Sarret G, Tiffreau C, Trocellier P, Lamble G, Hazemann J-L, Chateigner D (2002) Quantitative Zn speciation in a contaminated dredged sediment by μ-PIXE, μ-SXRF, EXAFS spectroscopy and principal component analysis. Geochim Cosmochim Acta 66:1549–1567
Martin A, Reeve R (1955) A rapid manometeic method for determining soil carbonate. Soil Sci 79:187–198
Matejovic I (1997) Determination of carbon and nitrogen in samples of various soils by the dry combustion. Commun Soil Sci Plant Anal 28:1499–1511
McKenzie N, Coughlan K, Cresswell H (2002) Soil physical measurements and interpretation for land evaluation. CSIRO Publishing, Collingwood
McLaughlin MJ, Degryse JLE, Baird RJ, Da Silva RC, Baylor BT, Peacock LA (2019) Acid treatment for fertilizers to increase zinc solubility and availability. U.S. Patent 10,487,016
McLaughlin MJ, McBeath TM, Smernik R, Stacey SP, Ajiboye B, Guppy C (2011) The chemical nature of P accumulation in agricultural soils—implications for fertiliser management and design: an Australian perspective. Plant Soil 349:69–87
Milani N, Hettiarachchi GM, Kirby JK, Beak DG, Stacey SP, McLaughlin MJ (2015) Fate of zinc oxide nanoparticles coated onto macronutrient fertilizers in an alkaline calcareous soil. PLoS ONE 10:e0126275
Mortvedt JJ (1991) Micronutrient Fertilizer Technology. Second edition. In: JJ Mortvedt (ed) Micronutrients in Agriculture. Soil Science Society of America, Madison, WI, USA
Mortvedt JJ, Giordano PM (1969) Extractability of zinc granulated with macronutrient fertilizers in relation to its agronomic effectivemess. J Agric Food Chem 17:1272–1275
Paterson D, De Jonge MD, Howard DL, Lewis W, McKinlay J, Starritt A, Kusel M, Ryan CG, Kirkham R, Moorhead G, Siddons DP (2011) The X-ray fluorescence microscopy beamline at the Australian synchrotron. AIP Conf Proc 1365:219–222
Ravel B, Newville M (2005) ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12:537–541
Rayment G, Higginson F (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press, Melbourne
Ryan C, Cousens D, Sie S, Griffin W, Suter G, Clayton E (1990) Quantitative pixe microanalysis of geological matemal using the CSIRO proton microprobe. Nucl Instrum Methods Phys Res B 47:55–71
Sauvé S, Hendershot W, Allen HE (2000) Solid-solution partitioning of metals in contaminated soils: dependence on pH, total metal burden, and organic matter. Environ Sci Technol 34:1125–1131
Slaton NA, Gbur EE, Wilson CE, Norman RJ (2005) Rice response to granular zinc sources varying in water-soluble zinc. Soil Sci Soc Am J 69:443–452
Steenbjerg F (1951) Yield curves and chemical plant analyses. Plant Soil 3:97–109
Wessells KR, Brown KH (2012) Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 7:e50568
Acknowledgements
Part of this research was undertaken using the XFM beamline at the Australian Synchrotron, Victoria Australia. We thank Juliane Reinhardt for her valuable support and guidance and the Australian Synchrotron for beamtime and travel funding. The authors also thank the Mosaic Company (Tampa, Florida) for their financial support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work was supported by the Mosaic Company (Tampa, Florida) and by the Australian Synchrotron (AS193/XFM/15505).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Tiina Roose.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Degryse, F., Harris, H., Baird, R. et al. Acid coating to increase availability of zinc in phosphate fertilizers. Plant Soil 495, 27–41 (2024). https://doi.org/10.1007/s11104-023-06323-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06323-w