Abstract
Aims
Increased subsoil water extraction through breeding of ‘designer’ root system architecture (RSA) may improve crop performance and resilience in the face of climate change (i.e. changing seasonal rainfall patterns). However, in many dryland environments, root systems face both water and nutrient scarcity (e.g. phosphorus (P)), with both resources often heterogeneously distributed in space and time. Under these conditions, interactions among RSA, nutrient distribution and soil water will determine crop performance, but remain poorly understood.
Methods
We grew two sorghum (Sorghum bicolor) genotypes defined by contrasting RSA (narrow or wide nodal root angle) in prepared soil cores with heterogeneous distributions of P and water along the soil profile. Plant growth and water use, shoot biomass, P uptake and root distribution were quantified in response to the different water × P combinations.
Results
Soil P placement and soil water distribution interactively determined plant growth and development in a genotype-dependent manner. The two sorghum genotypes shared common responses to P and water availability though varied for root and shoot traits and their relative responses to combined P and water stress.
Conclusions
Plant responses to the different water × P combinations were illustrative of the occurrence of spatio-temporal trade-offs between root architecture and efficient soil resource capture. The results suggest that the relative ability of crop root systems to effectively exploit soil profiles with greater resource availability will not necessarily be important for crop productivity in heterogeneous soil systems. Local environmental constraints should be considered when deploying genotypes with selected root architectural traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agriculture is challenged to sustainably increase yields over the coming decades to keep pace with global demand for agricultural products, whilst simultaneously addressing changing production scenarios due to climate change. Meeting this challenge requires the closing of yield gaps by increasing crop use efficiency of scarce resources such as water and nutrients (Mueller et al. 2012). A proposed approach is to develop crops with root system architectures (RSAs, the spatial and temporal distribution of roots in soil) that enhance crop exploration of the soil profile, and in that way improve the capture of soil resources from specific parts of the soil profile (Lynch 2019). For example, crops with deeper root systems may be better able to access deep subsoil water reserves (Lynch 2013). This improved access to water that would otherwise be out of reach could be important for crop success in future climates with shifting and more erratic rainfall patterns, including greater occurrence of in-season drought and off-season precipitation.
The development of crops with ‘designer’ RSA focuses on the targeted selection of root traits such as root angle, branching densities and length of root hairs, which are postulated to (collectively) deliver RSAs suited to overcome specific productivity constraints. In sorghum, a narrow nodal root angle is associated with a more vertical distribution of roots and traits for greater drought adaptation (Mace et al. 2012; Singh et al. 2012). Nodal root angle can be measured using rapid, high-throughput screening tools within a few weeks after seeding (Joshi et al. 2017), and has been observed to have moderate to high heritability, making it a selection target of interest for indirectly improving grain yield in water-limited environments (Mace et al. 2012; Singh et al. 2012). However, many RSA traits, including nodal root angle, have been identified and screened under controlled conditions; it is essential to validate the expression and improve understanding of the relationships between root traits and crop performance in realistic (future) production environments (Passioura 2010; Wasson et al. 2012).
Field-based experiments have shown that genetic traits selected at the seedling stage do not always correlate well with RSA of mature plants, or with crop yields (Bai et al. 2019; Rich et al. 2020; Severini et al. 2020). The expression of RSA traits can be strongly influenced by environmental conditions, such as soil structure, soil water, nutrient availability and temperature (Ober et al. 2021). It has therefore been argued that field screening will be unlikely to identify phenotypes with optimal RSA, because genotypes would also need to have local adaptation, vigour, disease resistance and so on (Lynch 2019). Furthermore, root phenotyping under field conditions can be difficult, with trenching or soil coring followed by root washing and image analysis being labour-intensive and time-consuming (Maeght et al. 2013). Although approaches such as minirhizotrons (Rasmussen et al. 2015; Svane et al. 2019), ingrowth cores (Steingrobe et al. 2000), and the core break method (Wasson et al. 2014) can save time, data collection and quality will be highly specific to seasonal conditions and variation. On the other hand, laboratory and greenhouse approaches such as root chambers and mesocosms may offer more control, but small pot sizes, 2D setups and soilless cultures are far removed from realistic soil conditions and inherently create artifacts. For instance, Yang et al. (2010) demonstrated that root space restrictions in pots smaller than 30 L can substantially change root–shoot biomass partitioning in sorghum and maize. Therefore, there is a need to investigate root traits and their interactions in clearly defined and simulated target environments that can translate laboratory research to relevant production environments.
Extensive, rainfed cropping systems represent a large proportion of arable agriculture—particularly in the tropics and subtropics—and are often characterised by multiple soil constraints that can be heterogeneously distributed through the soil profile and in time. For example, phosphorus (P) tends to be more concentrated in the top 0–5 or 0–10 cm layer of the soil profile in many cropping systems. This surface stratification of P can result in the spatial separation of P from reserves of soil water if the topsoil is vulnerable to rapid or frequent drying, with the implication that plant access to shallow P is reduced (Raymond et al. 2021; Strong et al. 1997). The separation of scarce resources will likely affect the performance of designer RSA (van der Bom et al. 2020), but few studies have investigated how the associated resource trade-offs affect the impact of RSA on crop performance. In addition to P stratification, deep subsurface placement of P fertilizers is an increasingly common management practice aimed at extending crop uptake of P by improving the co-location of it with subsoil water (Ma et al. 2009; Singh et al. 2005). Deep P placement has increased yields compared to conventional P placement, even in soils with relatively high topsoil P availability, though it may be hypothesised that crop access can also decrease if P is placed too far out of reach for the root system of juvenile plants (Nkebiwe et al. 2016). Currently there is only limited information about how resource distribution determines the benefits of RSA as a means of increasing productivity.
As the reliability of in-season rainfall decreases and climate variability increases, the success of rainfed cropping systems will be increasingly reliant on the ability of crops to access water and nutrients in deeper soil layers. However, as distributions of water and P are not always coincident, RSA selected to improve access to deep stored water may not be appropriate for the efficient acquisition of surface-stratified soil P reserves. Similarly, fertiliser application strategies such as deep P banding will only be effective if roots can find and exploit those bands in a timely manner. This paper describes results from a study using two sorghum (Sorghum bicolor L.) genotypes differing in RSA (defined by contrasting root angles) to investigate the interaction between crop root system and soil profile resource dynamics. We hypothesised that P placement and soil water dynamics interactively determine above and belowground genotypic responses. To investigate this hypothesis, we used a custom automated lysimeter platform to carefully simulate soil water dynamics over time, which we deployed into large, repacked soil cores with heterogeneous distributions of P fertiliser. The approach was designed to investigate interactions between crop root systems and water and nutrient resource dynamics that are increasingly characteristic of rainfed cropping systems experiencing changing climatic conditions.
Methods
Overview of the lysimeter platform
The lysimeter facility is located at The University of Queensland’s Gatton Campus (27′33′1″0.7″S 152′20′2″0.0″E), west of Brisbane, Australia. The platform is housed in a screenhouse with a UV-stabilised polyethylene cover that excludes rainfall and transmits approximately 70 to 80% of incident solar radiation. It has a capacity of 80 cores that consist of cylindrical polyvinyl chloride tubes, 300 mm diameter and 730 mm height, allowing relatively unrestricted plant and root growth until maturity for most field crops. The cores are divided over 10 enclosed boxes, laid out in a five by two pattern (Fig. 1A). The boxes are insulated and have a built-in air-conditioner to control soil temperatures, which can affect root and shoot growth and development (Clark and Reinhard 1991). Each box carries eight load cells on which the experimental soil cores are positioned (Fig. 1B and Fig. 1C), allowing the weights of each core to be continuously monitored. Each core is fitted with three irrigation probes that can inject moisture into the centre axis of the core cylinder at a flow rate of four L h−1, which can be placed at any desired depth of the soil profile. Irrigation is automated based on a pre-set minimum (trigger) and a maximum (target) weight. All cores are fitted with three RS485 output moisture/temperature probes, model BND-SNS that allow for continuous monitoring of soil water and soil temperature.
Overview of the lysimeter facility. A: the platform has a total capacity of 80 cores, divided over 10 enclosed and air-conditioned boxes, B: Top view of one lysimeter box, C: side view of an open lysimeter box showing four cores, each placed on an individual load cell, and connections for three individually controlled irrigation probes per core (shown between the cores). Measurements are in mm
Plant materials
The sorghum genotypes, SC1258-8 and SC1104-14E, were selected from a previously characterised diversity panel of 837 lines (Tao et al. 2017, 2020). The lines were screened for the root angles for the first flush of nodal roots, following the procedures described by Mace et al. (2012). SC1258-8 was observed to have a narrow nodal root angle and from hereon is referred to as ‘Narrow’ (Alan Cruickshank and Dave Jordan, personal communication). SC1104-14E was observed to have a wide root angle and from hereon is referred to as ‘Wide’. Based on these characteristics and their contrasting root morphologies in preliminary studies, these lines were expected to differ in the manner in which they explore the soil profile.
Experimental design
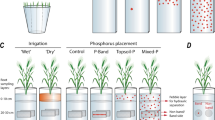
We evaluated the growth responses of the two sorghum genotypes to P placement and variable soil water conditions. The experiment was conducted with three P placements (Fig. 2):
-
1.
‘Banded-P’, for which a row of P granules was placed as a fertiliser band at 25 cm depth and 7 cm off-centre to the left side of the core;
-
2.
‘Topsoil-P’, for which fertiliser P was finely ground and dispersed throughout the top 10 cm soil layer;
-
3.
‘Mixed-P’, with finely ground fertiliser P throughout the entire soil profile.
The lysimeter experiment included five placement × irrigation treatments (Three phosphorus placements × ‘wet’ irrigation and two phosphorus placements (P-band, Topsoil-P) × ‘dry topsoil’ irrigation) For the dry topsoil treatment, topsoil irrigation was withheld from 4 weeks after seeding onward (orange shaded area). In-growth sampling bags (horizontal cylinders) were placed at pre-determined sampling layers to facilitate root quantification. Placement of P in each treatment is shown by the coloured dots
We included two irrigation patterns (Fig. 2):
-
1.
‘Wet’, for which all cores were maintained close to field capacity in all profile layers throughout the experiment; and
-
2.
‘Dry topsoil’, for which irrigation was withheld from the top 10 cm soil layer after the crop had established (after 28 days). Thus, the topsoil was allowed to dry out whilst water in the subsoil was maintained similar to the ‘Wet’ condition throughout the duration of the experiment. To achieve this, the lysimeter target weights were reduced and the topsoil irrigation point turned off, such that subsoil irrigation restarted when the reduced trigger target weight was reached.
In total, the experiment included five placement × irrigation treatments: Banded-P and Topsoil-P combined with both irrigation patterns, plus Mixed-P with the ‘Wet’ irrigation pattern, which can be interpreted as an indication of full growth and yield potential. This combination was tested for each of the two genotypes, creating ten treatments (five treatments × two genotypes), i.e., a fractional factorial design, that was randomized in incomplete blocks (considering that each lysimeter box had a maximum capacity of eight cores).
Soil preparations and fertiliser application
The soil type was a Grey Vertosol in the Australian classification (Isbell 2016), also referred to as a Vertisol in the FAO classification (FAO 2015). It was collected from a field cropped to grains near Hopeland, in the Western Downs Region, Queensland, Australia, from the 10–30 cm depth layer of the soil profile to ensure low P availability and the absence of fertiliser residuals. Selected soil properties are provided in Table 1. As intended, the soil had a very low P status with concentrations of labile P (Colwell-P) of 8 mg P kg−1 and sparingly soluble P (BSES-P) of 44 mg P kg−1, indicating that P deficiency was likely to severely limit the growth of crops.
The soil was air dried, crushed to < 1 cm, and basal fertiliser was applied using commercial grade fertilisers at the following rates: 250 mg CO(NH2)2 kg−1 (urea), 300 mg KCl kg−1 (muriate of potash), 69 mg (NH4)2SO4 kg−1 (ammonium sulfate). The fertilisers were ground to powder before mixing with the soil. Additionally, zinc sulfate heptahydrate fertiliser was applied as a foliar spray (1000 mg L−1 of ZnSO4∙7H2O) at 3 weeks and 5 weeks after plant emergence.
The P applied using the different placement methods was applied in the form of monoammonium phosphate (MAP, NH4H2PO4), at a rate of 0.45 g P core−1 for the Banded-P and the Topsoil-P. This application mimics a farmer practice of applying 60 kg P ha−1 in concentrated bands spaced 0.5 m apart. The Mixed-P treatment was established to achieve a P concentration throughout the soil profile equal to that of the fertilised Topsoil-P layer (viz. 45 mg P kg−1). Given the ammonium content of MAP, the basal (NH4)2SO4 applications to the Banded-P and the Topsoil-P treatments were adjusted to ensure equal amounts of N applied across all three fertiliser treatments.
A water retention curve was determined on samples from the processed soil using pressure plates equilibrated at 10, 30, 100, 500, and 1500 kPa (Dane and Hopmans 2002) and calculated according to the van Genuchten (1980) soil water characteristic model:
where θ is the gravimetric water content (g g−1), θs and θr are saturated and residual water contents (g g−1), h is the water matric potential (− kPa), and m and n (m = 1 − 1/n) are shape parameters related to the pore-size distribution. We obtained the water contents at the drained upper limit (-10 kPa, 0.38 g g−1) and the permanent wilting point (-1500 kPa, 0.17 g g−1) from the parameterized water retention function (Fig. S1) and calculated plant available water capacity as the difference between the two parameters.
Core assembly
Each lysimeter tube was lined with a custom 150 μm low-density polyethylene bag after which a five cm layer of expanded perlite was placed in the bottom to minimise water logging risk. Each tube was then filled with 60 kg of air-dry soil, resulting in a bulk density of ~ 1.25 g cm−3. A two cm depth layer of pebbles (0.3 to 0.5 cm diameter) was placed at 10 cm depth to prevent capillary movement of water from the subsoil to the topsoil (McLaren et al. 2013; van der Bom et al. 2023b). The irrigation probes and moisture sensors were inserted at depths of 5 cm, 20 cm, and 40 cm from the soil surface, distributing the water evenly throughout each core.
Four loose weave mesh bags (diameter 10 cm, length 25 cm, mesh size 2.5 mm) were filled with 2.5 kg of soil each and placed in each core to facilitate root sampling (Fig. 2). The mesh size allowed roots to grow into the bags and did not obstruct water movement. Given soil properties such as basal nutrient status and density inside and outside the bags was equal, this approach should provide reliable quantifications of root growth (Steingrobe et al. 2000). Two of the bags were placed at a depth of 20 to 30 cm and two cm off-centre to the ‘left’ or the ‘right’ of each lysimeter core. The location of the left bag corresponded to the location of the P band, such that the fertiliser granules for this treatment were placed through the centre of the soil-filled bag. The second set of bags was placed at 40–50 cm, and two cm off-centre in similar manner (‘Deep zone’).
Plant growth, harvest, and measurements
Each core was seeded with seven seeds into the topsoil layer, two cm below the soil surface. A small dose of water (≤ 50 mL) was added manually once a day to each core during establishment, with the goal of ensuring uniform germination and plant densities in each pot. Low germination percentages are a known challenge for these genotypes. All further irrigation requirements were automated through the lysimeter system. After two weeks the plants were thinned to one plant per core. However, some plants had to be transplanted between cores because of poor germination. The manual dosing of water ceased after four weeks, when automatic watering of the topsoil was also halted in the ‘Dry topsoil’ treatments.
Throughout the experiment core weights, irrigation data, volumetric soil water content (%) and soil temperature were collected by the automated system, with a time step of 10 min. Volumetric soil water content was converted to gravimetric soil content based on a sensor calibration curve collected prior to the experiment (Fig. S2). All data was manually checked for irregularities to ensure high quality data. Daily water use per plant was calculated as the decline in lysimeter weight from midnight to midnight, adjusted for any water applied during that period. The daily increase in plant dry mass was considered to be negligible relative to the amount of water used, considering final water use efficiency (WUE, the amount of biomass produced per unit of water applied) was lower than 4 g kg−1.
Plant height (cm) from the base to the whorl was recorded on a weekly basis from 20 days after seeding (DAS) until anthesis, providing a non-destructive measure of plant growth that is correlated with biomass increase (Hoffmann and Rooney 2014). Pests were controlled chemically when required and no significant outbreaks occurred.
The plants were harvested at anthesis of the main shoot, which should correspond to maximum root biomass i.e. before occurance of major root senescence during grain filling. All aboveground biomass was cut off above the soil surface and separated into the main stem, head, and tillers. For each core, the 10 cm topsoil and the ingrowth root bags were collected and the roots in each sample were carefully washed to remove any adhering soil. Root samples were stored separately in 33% v/v ethanol at 4 °C until root measurements could be made. The stubble was separated from the roots and root length (cm) and root diameter (mm) were determined by scanning the extracted roots of each sample on a flatbed scanner (Epson 12000 XL, 600 dots per inch) and analysed using WinRhizo Pro 2020 (Régent Instruments, Québec, Canada). All biomass samples were oven dried at 70 °C until weight stabilized, and their dry weights were recorded. The stubble was included in the aboveground biomass. Root length density (RLD, total length of roots per unit of soil volume, cm cm−3) was calculated based on the WinRhizo-acquired root length and the sample volume (7069 cm3 for the Topsoil sample and 1767 cm3 for a root bag) Where appropriate, root biomass data in each soil layer are presented on an equal volume basis, to account for the different sampling volumes between the topsoil and the subsoil mesh bags: root biomass sampled across the two bags in the soil layer was summed and then doubled, to correspond to the full 10 cm layer volume. Similarly, when presenting RLD for a full sampling layer this constitutes the average of the two bags in that soil layer. The proportion of root length was analysed within four root diameter classes, being ≤ 0.2 mm, 0.20–0.50 mm, 0.50–1.0 mm and > 1.0 mm, based on distributions of root diameter in sorghum populations (Singh et al. 2011). Phosphorus concentrations were determined separately for the main stems (incl. the heads) and tillers. The samples were finely ground and 0.5 g subsamples were digested in nitric acid and hydrogen peroxide using microwave-assisted closed vessel digestion, after which the P concentrations were analysed using inductively coupled plasma-optical emission spectroscopy (ICP-OES). Total P uptake was calculated by multiplying the measured P concentrations with the biomass dry matter.
Statistical analysis
Treatment effects on plant biomass and P uptake were analysed using linear mixed effects models, with fixed effects for the interaction between genotype, P placement and irrigation, and a random effect for the replication across incomplete blocks (i.e. eight cores per box). The contributions of the fixed effect parameters and their interactions were assessed using Maximum Likelihood and backward model reduction, starting with a full model including all crossed interactions. The final models were refitted using Restricted Maximum Likelihood, and pairwise comparisons were made using Estimated Marginal Means with Tukey adjustments. The analysis of root data was conducted with the sample layers (depth) included as a fixed effect. Family-wise error rates were calculated for each layer and genotype separately using Tukey's method. Relationships between shoot biomass and P uptake and water use were evaluated using linear regression.
Temporal measures of plant growth (i.e. water use and plant height) were evaluated with Nonlinear Mixed-Effects Models, with fixed effects for Genotype and Treatment (P placement × irrigation), and a random effect for plant identity, to account for the repeated measures. An exponential curve was fitted for cumulative water use:
In which \(a\) is the intercept and \(k\) represents the increase of water use per day i.e. the relative growth rate. A four-parameter logistic curve was fitted for plant height:
where \(d\) is the higher asymptote, \(c\) is the lower asymptote, \(e\) is the value producing a response half-way between \(d\) and \(c\), and \(b\) is the slope around the inflection point. The model fit was tested using Likelihood Ratio and pairwise comparisons were made for the fitted parameters using holm adjustments. Comparisons of drying days and Topsoil water contents among the Dry topsoil treatments were made using one-way analysis of variance.
All statistical analyses were conducted using R version 4.1.1 (R Core Team 2020) making extensive use of the nlme package (Pinheiro et al. 2020) and ggplot2 (Wickham 2009) for graphical operations. All models were checked for normality of residuals and homogeneity of variance using a combination of diagnostic plots and formal tests (Levene's test for homogeneity of variance, Shapiro–Wilk test for normality). Potential outliers were identified using Cook’s distance, in which those observations with a distance greater than four times the mean were classified as influential. Outlier removal was considered justified if a relevant observation had been recorded during the experiment (for instance, if a plant was transplanted and its early growth had clearly been affected by this procedure). Where required, analyses have been performed on log or square root-transformed data or with use of a heterogeneous variance structure to account for differences in variation between treatment combinations.
Results
Plant growth and development
Days to anthesis and final harvest varied from 65 to 86 d, depending on both genotype and P placement (P < 0.05, Table 2). The rate of phenological development was slower for plants growing in the deep banded P treatment, with the Narrow genotype (83 d) taking longer under these conditions than the Wide genotype (77–79 d). Plants growing in the Mixed P treatment tended to develop fastest (67–69 d), although the difference with the slightly slower Topsoil P treatment (72–74 d) was not significant. On average, the Narrow genotype produced more tillers than the Wide genotype (P < 0.001), and the proportion of the total aboveground biomass represented by tillers increased accordingly (P < 0.01) The P treatments affected tiller production in the order Topsoil P > Mixed P > Banded P (P < 0.001), although tillers in the Mixed-P treatment were larger and represented a greater contribution to total biomass (P < 0.001).
Both genotypes showed similar sigmoidal increases in plant height (Fig. 3A), but the Narrow genotype grew taller (P < 0.001) than the Wide genotype. The P placement and watering treatments caused differences in the rate of increase in plant height, but not in plant height at the anthesis harvest date. This was particularly evident in the Narrow genotype, with the rate of increase in plant height achieved in the order of Mixed-P Wet > Topsoil-P wet or Dry topsoil > Banded P wet or Dry topsoil.
The sorghum genotypes with contrasting root angles (SC1258-8 [Narrow] and SC1104-14E [Wide]) differed in their temporal growth dynamics, under the influence of the irrigation × phosphorus placement treatments. A: Mean cumulative water use, with data standardized to daily intervals (midnight to midnight). B: Mean plant height (cm) recorded weekly, commencing 20 days after sowing. Shadings indicate standard error of the mean. See Table S1 for model parameters
Placement of P fertiliser significantly affected aboveground biomass for both sorghum genotypes (P < 0.001; Table 2). Plants that received P fertiliser as a deep P band had a lower mean biomass than plants in the Topsoil-P and Mixed-P treatments. Although the differences between Topsoil-P and Mixed P were small in terms of biomass, plant P-uptake was greater in the Mixed-P treatment (P < 0.01), with this effect being similar among the two sorghum genotypes (Table 2). Plant P uptake did not differ significantly between the P-Band and Topsoil-P treatments. The effect of irrigation (topsoil drying) on shoot biomass and P uptake was variable and not significant. Within this, the different plant components (head, tillers, main stem) followed the same pattern as overall aboveground biomass (data not shown).
Plant water use
Cumulative water use from seeding to harvest was greater for the Narrow genotype (P < 0.01; Fig. 3B and Table 2). On average, plants growing under a drying topsoil tended to use less water than those that were well-watered (P < 0.05; Table 2). This difference was also reflected in the relative growth rate of water use, which tended to be lower for both drying topsoil treatments, and significantly so for Topsoil P (\(k\), P < 0.001; Table S1). Further, under equal soil water conditions, the relative growth rate was smaller for Banded P than for Topsoil P, and it tended to be greatest for Mixed P – although the difference to Topsoil P was not significant.
The soil sensors provided a measure of soil water in the soil profile, with a capacity to detect temporal changes and differences between soil layers (Fig. 4). The subsoil layers and the topsoil layers of the Wet treatments remained relatively constant throughout the experiment, and within the range to support plant water uptake. For the Dry topsoil treatments, withholding irrigation from 28 DAS resulted in a gradual depletion of water from the topsoil layer. On average, the reduced water target was reached sooner for Topsoil-P (23 d [Narrow] and 29 d [Wide]) than for Banded-P (39 d [Narrow], 40 d [Wide], P < 0.01). Despite the hydraulic discontinuity between the layers, the sensor data indicates some water must have moved upward once subsoil irrigation resumed (topsoil water content increased slightly at 51 d [Narrow] and 57 d [Wide]), but observations during the experiment and at time of harvest confirmed the 10-cm topsoil had dried out as intended.
Root distribution
Both sorghum genotypes had substantially greater root biomass in the 10 cm topsoil than in the subsoil layers (Fig. 5A, P < 0.001). Within the topsoil layer, P placement affected root biomass of both sorghum genotypes, with Topsoil-P resulting in greater biomass than deeper banded P (P < 0.05). The Wide genotype tended to produce fewer roots in the topsoil when this layer was allowed to dry out, but this difference was not significant.
The two sorghum genotypes (SC1258-8 [Narrow] and SC1104-14E [Wide]) differed in their belowground responses to the irrigation × phosphorus placement treatments. A: Root biomass distribution (g), B: root length density (cm of root per cm-3 of soil volume). Letters indicate significance across soil depth: layers sharing the same letter were not significantly different. For both subsoil layers, data are summarized from two sampling bags recovered from the band (LHS) or nonband (RHS) sides of the lysimeter cores. Subsoil root biomass was converted to correspond to a soil volume equivalent to a 10 cm profile layer, so that comparisons can be made to the 0–10 cm topsoil layer. C: overview of the significant factors and interactions for root biomass and root length density for each soil layer separately (G = Genotype, P = Placement, I = irrigation)
For both genotypes in the banded P treatment, irrigation regime affected root biomass in the upper subsoil (20–30 cm) (P < 0.05). Declining moisture in the top 10 cm in the Dry topsoil treatment increased root biomass production in the 20–30 cm layer for the Narrow genotype for both the P-band and Topsoil-P. In contrast, the Wide genotype decreased root biomass in this layer in the Dry topsoil treatment for the P-band and Topsoil-P placements. Further, averaged across both water management systems, root biomass in the P-band treatments was greater than in Mixed-P for the Wide genotype (P < 0.05).
In the lower subsoil (40 to 50 cm), the Dry topsoil treatments caused an increase in root biomass of the Narrow genotype (P < 0.05). Further, Mixed P showed the greatest root biomass for this genotype (P < 0.001), whereas no significant effects of irrigation or P placement were observed for the Wide genotype in this layer.
Root length density varied between 0.15 and 0.87 cm cm−3 with relatively similar magnitudes across the different layers (compared with the sharp decline in root biomass; Fig. 4A and B). For the Narrow genotype, the mean RLD amongst the different soil layers tended to be greater for the upper subsoil than for the deep subsoil, but this was strongly influenced by the response to the P-band treatment (Placement × Layer P < 0.05). For the wide genotype, irrigation was a major driver of variation, with the Wet treatments showing a greater mean RLD than their Dry topsoil counterparts (P < 0.05).
Within the separate layers, irrigation had a significant effect on RLD in the topsoil for the wide genotype (P < 0.05), but no differences between P placements could be observed. In the upper subsoil, RLD tended to be greater in the Narrow × Dry topsoil (P = 0.06) and the Wide × Wet (P < 0.05) combinations, which was similar to the trend for root biomass. Further, P placement had a clear effect on RLD of the wide genotype, in the order of P-Band > Topsoil-P > Mixed-P, with the Band × Wet combination being a major driver of this observation.
In the lower subsoil, differences in RLD among the treatments were small for the Wide genotype, whereas those for the Narrow genotype were more pronounced. Overall, Mixed-P resulted in the greatest RLD in this layer (P < 0.05). Secondly, RLD in this layer was greater under the Dry topsoil treatments (P < 0.05).
Locally, root biomass and RLD responses to the P-Band treatment in the Narrow genotype tended to be governed by increases on the side of the fertiliser band, i.e. root biomass and RLD were greater for the sampling site that included the P band (Fig. 6). However, owing to relatively large variation, these differences between the ‘Band-side’ and ‘non-band’ side were not significant.
Root diameter classes
The greatest relative proportion of roots amongst the diameter classes was in the finest class (< 0.2 mm) and decreased as root diameter increased. The distribution of roots amongst the different classes changed between the topsoil and the subsoil layers (Fig. 7), with both genotypes showing a greater proportion of thicker roots in the topsoil (Layer × Root Class P < 0.001). On average, the Dry topsoil treatments tended to shift the overall distribution for the Narrow genotype towards finer roots although this response was variable and primarily reflected as a smaller proportion of roots in the thicker 0.5–1.0 mm and > 1.0 mm classes (Irrigation × Root Class P < 0.01). A similar pattern was visible in the Wide treatment where P was banded, this was not statistically significant.
Proportion of root length in different root-diameter classes for two sorghum genotypes (SC1258-8 [Narrow] and SC1104-14E [Wide]) affected by the irrigation × phosphorus placement treatments across three sampling layers. MW = Mixed-P Wet, TW = Topsoil-P Wet, TD = Topsoil-P Dry topsoil, BW = P-Band Wet, BD = P-Band Dry topsoil. Significance: G = Genotype, I = Irrigation
Discussion
The study objective was to explore how genotypes with contrasting RSA respond to heterogeneously distributed soil resources—a common constraint in many dryland systems under no- till management. Conditions in which water and other soil resources are spatially disjunct in the soil profile (i.e. multiple constraints) will become increasingly common in future climates. Therefore the current approach aimed at enabling investigation of interactions between crop root systems and their interactions in clearly defined and simulated target environments. The genotypes in this study shared a common genetic background whilst differing in root angle, making them effective model phenotypes to explore the physiological impact of RSA on resource acquisition from heterogeneous soil environments (Alan Cruickshank and Dave Jordan, personal communication). However, these genotypes were not near-isogenic lines, meaning the genetic backgrounds were not fully identical. Inevitably the distinct genotypes would possess characteristics with adaptive influence; therefore the genotypes are suitable to evaluate relative treatment effects (e.g. compared with their potential under the mixed treatment), but care should be taken when interpreting absolute yield levels.
We tested root-mediated responses to realistic approximations of contrasting P fertilisation strategies (surface enrichment versus subsoil banding) in soil volumes that allowed relatively unconstrained root development and a stable temperature environment. Although some studies have previously observed trade-offs for water and stratified P for distinct genotypes with contrasting root systems (Ho et al. 2005; van der Bom et al. 2023b), our results expand this observation by demonstrating that crop access to P influences genotype performance through its effects on growth, physiological development, and water use, and by demonstrating that system management (i.e. fertiliser placement) can play a role to improve genotype performance by modifying access to P in space and time.
Plant responses to phosphorus placement
When considering the individual phenotypes, nodal root angle is presumed to shift the distribution of roots in the soil profile, delivering a more vertical (Narrow) or horizontal (Wide) RSA (Singh et al. 2012). In this manner, a wide root angle should deliver better scavenging for shallowly located P (Lynch 2011). In the current study, the Mixed-P treatment provided a measure of ‘optimal’ growth conditions in which P was available throughout the profile and there was no soil moisture limitation: roots would have encountered fertiliser P regardless of their location in the soil profile. In contrast, in the Topsoil-P treatment the primary source of P was located in a single topsoil layer; under these conditions the Narrow phenotype showed a proportionally greater reduction in P uptake than the Wide phenotype. Secondly, when comparing Banded-P and Topsoil-P, P uptake by the Wide genotype tended to be lower when P was banded, whereas it was similar between either treatment for the Narrow phenotype. Whilst not conclusive on their own, together these observations support the hypothesis that narrow or wide root distributions may have different abilities to exploit shallow or deep P placement strategies.
In general, P uptake at time of flowering was substantially less for Topsoil-P than for Mixed P and there were clear differences in the relative growth rates between the P treatments. This agrees with previous observations that P deficiency decreases the photosynthetic rate of sorghum plants (Ripley et al. 2004), resulting in reduced ATP production and CO2 fixation (Carstensen et al. 2018). The growth rate around anthesis can have a strong impact on final grain number (and subsequently, yields (van Oosterom and Hammer 2008)), highlighting the importance of P nutrition in modifying crop growth and development. Similarly, the amounts of P supplied in the Topsoil-P and the P-band treatments were equal, but plants grown with banded P showed a slower increase of plant height and later point of inflection (Fig. 3). This is an indication that plants growing under the P-Band treatment experienced greater initial P scarcity, which was further supported by the reduced biomass under this treatment. The low concentration of P in the top 10 cm of the soil would have meant plant access to P was delayed because roots had to grow through the low-P layer first, whereas Topsoil-P provided early P access close to the seed. Secondly, the P band was applied as a single row of granules, resulting in a smaller surface area that is in contact with the soil compared with the ground fertiliser that was dispersed in the topsoil. This would have reduced the probability of root to P fertilizer contact, which is an important determinant for P uptake, particularly as the distance of application from the seed increases (Eghball and Sander 1989).
The slower resource accumulation with banded P resulted in a smaller number of tillers and substantially increased days to anthesis. The former was consistent with the hypothesis that tiller production in sorghum is regulated by internal plant competition for assimilates (Kim et al. 2010), with reduced tiller number affecting grain yields by influencing canopy size and the potential number of grain-bearing panicles (Lafarge et al. 2002). A smaller number of tillers should benefit the plant by sparing available P such that individual tillers can fulfill their life cycle and produce seeds. The latter characteristic, days to anthesis, was consistent with observations that P deficiency may delay flowering and crop maturation by as much as 30 to 50% (Sahrawat et al. 1995). This slower development may have positive or negative effect on yields depending on the growth environment, that is, depending on how canopy development and demand for water synchronize with the available supply (Hammer et al. 2014). For example, in favourable water conditions a larger canopy with more time to develop would generate greater yields. But if water supply was low, greater tillering (larger canopy) and longer time to flowering would deplete more of the available water reserves and leave less available for post-anthesis growth (Borrell et al. 2014a, b). Considering post-anthesis water use is a major determinant of grain yields in environments with terminal drought stress, pre-anthesis sparing of water can substantially improve sorghum grain yields (Hammer 2006; Manschadi et al. 2006).
Plant responses to water distribution
In the current experiment ample water was available in the ‘Wet’ treatment, and differences in total water use were small among the different P placements. However, from the evolution of water use it is clear that the lack of differences was governed by the increasingly extended times to flowering for Topsoil P and Banded P, illustrating the influence of plant P access on water use through its effect on crop development. Similarly, in the Dry topsoil treatments, the topsoil layer dried quicker for Topsoil-P than for Banded-P. Here, greater plant P access in the Topsoil-P treatment supported a more rapid growth rate, and thus water demand, which would have increased the speed at which water from the topsoil layer was used up.
In the Dry topsoil treatments, soil water was maintained at field capacity in the soil layers below 10 cm once the core weights reached the new target. It was expected that this irrigation treatment would have a limited direct effect on plant growth, but rather an indirect effect by modifying plant access to shallowly placed P. In other words, topsoil drying was expected to cause greater relative reductions in biomass and P uptake when P was primarily located in this layer (Topsoil-P) compared with deeper in the soil profile (Banded-P). However, no such interaction between irrigation and P placement was observed. This may reflect the evolution of water use, in which effect of drying on P availability may not have become pronounced until late in the experiment, and plants would have had ample time to take up P during early growth. At the same time, contrary to expectations, topsoil drying reduced the total water use and the relative growth rates. Therefore, even though ample water would have been available in the subsoil, the reduced irrigation in the topsoil must have limited the capacity of plants to extract water. Under these conditions, total water use for the Narrow genotype was between 91 and 92% of the corresponding Wet treatments, whereas that of the Wide genotype was between 79 to 87%. A similar relative pattern was also observed with the reduction in plant biomass, which was associated with reduced root growth in the 0–10 cm topsoil layer for the Wide genotype. These results lend support to the hypothesis that a Narrow phenotype should maintain access to water from deeper profile layers (Hammer et al. 2009; Manschadi et al. 2006; Singh et al. 2012). However, differences in biomass potential meant the Narrow phenotype tended to have a larger overall root system, which could also have improved its potential to take up water below 10 cm; therefore, whilst supportive, these observations require further scrutiny using contrasting plant materials with similar potential biomass production.
The Narrow phenotype produced more roots in the Dry topsoil treatment, and more fine roots in deeper soil layers. This is consistent with many studies that have reported drought-induced increases of root growth in the subsurface soil layers (Alahmad et al. 2019; Benjamin and Nielsen 2006; Wang et al. 2009), so it was surprising that this pattern was not apparent for the Wide genotype. One explanation may be the differences in water demand due to differing canopy development and tiller addition, as indicated by total water use and the slopes of cumulative water use. A second possibility is that the Dry topsoil treatment had a greater effect on biomass accumulation of the Wide genotype, reducing its capacity to adapt. Regardless, this plasticity under drought and relatively stable productivity suggests a Narrow root angle may not have a major yield penalty in high rainfall seasons, provided ample P is available to support growth.
An unexpected outcome, and a possible study limitation, was that initiation of subsurface irrigation after topsoil drying slightly increased water content in the topsoil layer, despite the hydraulic separation of the pebble layer (Fig. 4). This could be due to capillary rise through the pebble layer, or due to hydraulic lift, i.e. the passive movement of water via plant roots into the surface layer with lower water potential (Caldwell et al. 1998). However, the former process is unlikely to generate the scale of change in moisture content, and hydraulic lift tends to buffer the initial drying of a soil rather than cause rewetting (Vetterlein and Marschner 1993; Wang et al. 2009), neither of which is consistent with the rapid increases in water content observed in the Dry topsoil. We therefore hypothesize that water application into the subsurface soil layer temporarily exceeded the hydraulic conductivity of the surrounding soil, such that free water could bridge the pebble layer shortly after irrigation. Although this indicates a need for methodological improvements to fine-tune future experimentation, the significant effect of irrigation on total water use, biomass and plant growth rate suggests that the slight increase of soil water near the end of the experiment was insufficient to impair the effect of the simulated topsoil drought.
Root plasticity and proliferation
Roots that encounter a P-rich zone or patch often respond by proliferating in this zone (Hodge 2004). Considering the highly concentrated nature of the P-band treatments, root proliferation may be expected around the enriched fertiliser band (van der Bom et al. 2023a), although the early P scarcity resulting from the low P topsoil would have slowed the ability to exploit that layer, as well as to potentially intercept any subsoil P bands. Production of roots on the side of the P band was not significantly different from the non-band side for the Wide genotype, suggesting that constrained early root development caused by low P, combined with the greater proportion of roots in the topsoil layer, limited the genotype’s ability to find and respond to the deep P bands. Conversely, the Narrow genotype tended to increase the production of (fine) roots in the vicinity of the band, although this was paired with very large variability. We hypothesise that this variability reflects differences in both the time to band interception (caused by the early P deficit) and the lower probability of root to P fertilizer contact (Eghball and Sander 1989) in the limited soil volume in contact with the fertiliser granules. The greater localised root proliferation by the Narrow genotype in the Dry topsoil treatment was also consistent with the observation that this treatment increased subsurface root growth, which would have increased the odds of an early interception of the fertiliser band.
Context and application
Many dryland production environments are defined by fluctuating moisture availability and distribution during the season. Agronomic practices in these systems are typically designed to optimize water capture, support crop growth during dry periods and synchronize crop water use with key yield-determining growth stages. For example, conservation agriculture principles are among the dominant management approaches in north-eastern Australia (Kirkegaard et al. 2014). Although such approaches deliver clear advantages for water retention, they also increase the occurrence of topsoil nutrient stratification. Subsequent separation of soil nutrient and water reserves prevents farmers from attaining water-limited yield potentials (Angus et al. 2019). The practice of deep fertiliser banding aims to address this issue and has been particularly successful in years with sparser or irregular rainfall (Bell et al. 2012), in which crop access to stratified nutrients was likely more restricted. All of these factors, i.e. climate (rainfall), soil nutrient distribution and management, determine the ‘ecological niche’ that is likely to influence the success of genotypes with selected root traits.
Within this setting, the success of deep fertiliser banding relies on the ability of plants to proliferate roots in and around the fertiliser. Our results show that RSA may influence crops’ ability to find and respond to this heterogeneously located resource, with temporal differences having potential effects on resource accumulation, water use, and phenological development. It is important to note that the deep P bands in the current experiment were the primary source of P in an otherwise low-P environment. This is in contrast with the common field environments where some topsoil P would be available for early uptake, either as stratified dispersed nutrients or as ‘starter-P’ fertiliser applications in or near the seeding trench, which may be hypothesised to improve early growth rates and the resulting soil root exploration to find the deeper P bands (van der Bom et al. 2023a).
Conclusions
Overall, from a nutrient perspective the results highlight the role of P in modifying crop growth and development, and the consequence of these modifications on crop water use and nutrient uptake. From a water perspective, different soil water conditions, RSA and management (i.e. fertiliser placement) will affect the plants’ ability to take up P (or other nutrients). Subsequently, the impact of these interacting factors and their feedback loops will be a major determining factor for the ability of genotypes to deliver benefits for crop productivity and resilience against climate change stress. To better understand these interactions, further studies involving realistic root-soil interactions are needed to clarify how RSA is expressed in complex target environments in which multiple stressors are likely to occur simultaneously.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RSA:
-
Root System Architecture
- RLD:
-
Root Length Density
- P:
-
Phosphorus
- WUE:
-
Water use Efficiency
- DAS:
-
Days after Seeding
References
Alahmad S, El Hassouni K, Bassi FM, Dinglasan E, Youssef C, Quarry G, Aksoy A, Mazzucotelli E, Juhasz A, Able JA, Christopher J, Voss-Fels KP, Hickey LT (2019) A Major Root Architecture QTL Responding to Water Limitation in Durum Wheat. Front Plant Sci 10:436. https://doi.org/10.3389/fpls.2019.00436
Angus J, Bell M, McBeath T, Scanlan C (2019) Nutrient-management challenges and opportunities in conservation agriculture. In: J Pratley, J Kirkegaard (eds) Australian agriculture in 2020: From conservation to automation. Australian Society for Agronomy, Wagga Wagga, Australia
Bai C, Ge Y, Ashton RW, Evans J, Milne A, Hawkesford MJ, Whalley WR, Parry MAJ, Melichar J, Feuerhelm D, Basler PB, Bartsch M (2019) The relationships between seedling root screens, root growth in the field and grain yield for wheat. Plant Soil 440:311–326. https://doi.org/10.1007/s11104-019-04088-9
Bell M, Lester D, Smith L, Want P (2012) Increasing complexity in nutrient management on clay soils in the northern grain belt – nutrient stratification and multiple nutrient limitations. In: I Yunusa (ed) Capturing opportunities and overcoming obstacles in Australian agronomy 16th Australian Agronomy Conference, Armidale, NSW
Benjamin JG, Nielsen DC (2006) Water deficit effects on root distribution of soybean, field pea and chickpea. Field Crops Res 97:248–253. https://doi.org/10.1016/j.fcr.2005.10.005
Borrell AK, Mullet JE, George-Jaeggli B, van Oosterom EJ, Hammer GL, Klein PE, Jordan DR (2014a) Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake. J Exp Bot 65:6251–6263. https://doi.org/10.1093/jxb/eru232
Borrell AK, van Oosterom EJ, Mullet JE, George-Jaeggli B, Jordan DR, Klein PE, Hammer GL (2014b) Stay-green alleles individually enhance grain yield in sorghum under drought by modifying canopy development and water uptake patterns. New Phytol 203:817–830. https://doi.org/10.1111/nph.12869
Caldwell MM, Dawson TE, Richards JH (1998) Hydraulic lift: consequences of water efflux from the roots of plants. Oecologia 113:151–161. https://doi.org/10.1007/s004420050363
Carstensen A, Herdean A, Schmidt SB, Sharma A, Spetea C, Pribil M, Husted S (2018) The Impacts of Phosphorus Deficiency on the Photosynthetic Electron Transport Chain. Plant Physiol 177:271–284. https://doi.org/10.1104/pp.17.01624
Clark RB, Reinhard N (1991) Effects of soil temperature on root and shoot growth traits and iron deficiency chlorosis in sorghum genotypes grown on a low iron calcareous soil. Plant Soil 130:97–103. https://doi.org/10.1007/BF00011862
Dane JH, Hopmans JW (2002) Pressure plate extractor. In: JH Dane, GC Topp (eds) Methods of soil analysis Part 4 Physical methods. Soil Science Society of America Inc., Madison, Wisconsin, USA
Eghball B, Sander DH (1989) Distance and Distribution Effects of Phosphorus Fertilizer on Corn. Soil Sci Soc Am J 53:282–287. https://doi.org/10.2136/sssaj1989.03615995005300010051x
FAO (2015) World reference base for soil resources 2014. International soil classification system for naming soils and creating legends for soil maps. Update 2015
Hammer G (2006) Pathways to prosperity: breaking the yield barrier in sorghum
Hammer GL, Dong Z, McLean G, Doherty A, Messina C, Schussler J, Zinselmeier C, Paszkiewicz S, Cooper M (2009) Can Changes in Canopy and/or Root System Architecture Explain Historical Maize Yield Trends in the U.S. Corn Belt? Crop Sci 49:299–312. https://doi.org/10.2135/cropsci2008.03.0152
Hammer GL, McLean G, Chapman S, Zheng B, Doherty A, Harrison MT, van Oosterom E, Jordan D (2014) Crop design for specific adaptation in variable dryland production environments. Crop Pasture Sci 65:614–626
Ho MD, Rosas JC, Brown KM, Lynch JP (2005) Root architectural tradeoffs for water and phosphorus acquisition. Funct Plant Biol 32:737–748. https://doi.org/10.1071/FP05043
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24. https://doi.org/10.1111/j.1469-8137.2004.01015.x
Hoffmann L, Rooney WL (2014) Accumulation of Biomass and Compositional Change Over the Growth Season for Six Photoperiod Sorghum Lines. BioEnergy Res 7:811–815. https://doi.org/10.1007/s12155-013-9405-5
Isbell R (2016) Australian Soil Classification. CSIRO Publishing
Joshi DC, Singh V, Hunt C, Mace E, van Oosterom E, Sulman R, Jordan D, Hammer G (2017) Development of a phenotyping platform for high throughput screening of nodal root angle in sorghum. Plant Methods 13:56. https://doi.org/10.1186/s13007-017-0206-2
Kim HK, van Oosterom E, Dingkuhn M, Luquet D, Hammer G (2010) Regulation of tillering in sorghum: environmental effects. Ann Bot 106:57–67. https://doi.org/10.1093/aob/mcq079
Kirkegaard JA, Conyers MK, Hunt JR, Kirkby CA, Watt M, Rebetzke GJ (2014) Sense and nonsense in conservation agriculture: Principles, pragmatism and productivity in Australian mixed farming systems. Agric, Ecosyst Environ 187:133–145. https://doi.org/10.1016/j.agee.2013.08.011
Lafarge TA, Broad IJ, Hammer GL (2002) Tillering in Grain Sorghum over a Wide Range of Population Densities: Identification of a Common Hierarchy for Tiller Emergence, Leaf Area Development and Fertility. Ann Bot 90:87–98. https://doi.org/10.1093/aob/mcf152
Lynch JP (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156:1041–1049. https://doi.org/10.1104/pp.111.175414
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112:347–357. https://doi.org/10.1093/aob/mcs293
Lynch JP (2019) Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytol 223:548–564. https://doi.org/10.1111/nph.15738
Maeght J-L, Rewald B, Pierret A (2013) How to study deep roots—and why it matters. 4. https://doi.org/10.3389/fpls.2013.00299
Ma Q, Rengel Z, Rose T (2009) The effectiveness of deep placement of fertilisers is determined by crop species and edaphic conditions in Mediterranean-type environments: a review. Soil Res 47:19–32. https://doi.org/10.1071/sr08105
Mace ES, Singh V, Van Oosterom EJ, Hammer GL, Hunt CH, Jordan DR (2012) QTL for nodal root angle in sorghum (Sorghum bicolor L. Moench) co-locate with QTL for traits associated with drought adaptation. Theor Appl Genet 124:97–109. https://doi.org/10.1007/s00122-011-1690-9
Manschadi AM, Christopher J, deVoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments J. Funct Plant Biol 33:823–837. https://doi.org/10.1071/FP06055
McLaren TI, Bell MJ, Rochester IJ, Guppy CN, Tighe MK, Flavel RJ (2013) Growth and phosphorus uptake of faba bean and cotton are related to Colwell-P concentrations in the subsoil of Vertosols. Crop Pasture Sci 64:825–833. https://doi.org/10.1071/CP13025
Mueller ND, Gerber JS, Johnston M, Ray DK, Ramankutty N, Foley JA (2012) Closing yield gaps through nutrient and water management. Nature 490: 254–257. http://www.nature.com/nature/journal/v490/n7419/abs/nature11420.html#supplementary-information
Nkebiwe PM, Weinmann M, Bar-Tal A, Müller T (2016) Fertilizer placement to improve crop nutrient acquisition and yield: A review and meta-analysis. Field Crops Res 196:389–401. https://doi.org/10.1016/j.fcr.2016.07.018
Ober ES, Alahmad S, Cockram J, Forestan C, Hickey LT, Kant J, Maccaferri M, Marr E, Milner M, Pinto F, Rambla C, Reynolds M, Salvi S, Sciara G, Snowdon RJ, Thomelin P, Tuberosa R, Uauy C, Voss-Fels KP, Wallington E, Watt M (2021) Wheat root systems as a breeding target for climate resilience. Theor Appl Genet 134:1645–1662. https://doi.org/10.1007/s00122-021-03819-w
Passioura JB (2010) Scaling up: the essence of effective agricultural research. Funct Plant Biol 37:585–591
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2020) nlme: Linear and Nonlinear Mixed Effects Models. R package version 31–148
Rasmussen IS, Dresbøll DB, Thorup-Kristensen K (2015) Winter wheat cultivars and nitrogen (N) fertilization—Effects on root growth, N uptake efficiency and N use efficiency. Eur J Agron 68:38–49. https://doi.org/10.1016/j.eja.2015.04.003
Rayment GE, Lyons DJ, Shelley B (2011) Soil chemical methods - Australasia : Australasia. CSIRO Publishing, Victoria, Australia
Raymond N, Kopittke PM, Wang E, Lester D, Bell MJ (2021) Does the APSIM model capture soil phosphorus dynamics? A case study with Vertisols. Field Crops Res 273:108302. https://doi.org/10.1016/j.fcr.2021.108302
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rich SM, Christopher J, Richards R, Watt M (2020) Root phenotypes of young wheat plants grown in controlled environments show inconsistent correlation with mature root traits in the field. J Exp Bot 71:4751–4762. https://doi.org/10.1093/jxb/eraa201
Ripley BS, Redfern SP, Dames J (2004) Quantification of the photosynthetic performance of phosphorus-deficient Sorghum by means of chlorophyll-a fluorescence kinetics. S Afr J Sci 100:615–618. https://doi.org/10.10520/EJC96177
Sahrawat KL, Rego TJ, Burford JR, Rahman MH, Rao JK, Adam A (1995) Response of sorghum to fertilizer phosphorus and its residual value in a Vertisol. Fertil Res 41:41–47. https://doi.org/10.1007/BF00749519
Severini AD, Wasson AP, Evans JR, Richards RA, Watt M (2020) Root phenotypes at maturity in diverse wheat and triticale genotypes grown in three field experiments: Relationships to shoot selection, biomass, grain yield, flowering time, and environment. Field Crops Res 255:107870. https://doi.org/10.1016/j.fcr.2020.107870
Singh DK, Sale PWG, Routley RR (2005) Increasing phosphorus supply in subsurface soil in northern Australia: Rationale for deep placement and the effects with various crops. Plant Soil 269:35–44. https://doi.org/10.1007/s11104-004-2475-6
Singh V, van Oosterom EJ, Jordan DR, Hunt CH, Hammer GL (2011) Genetic Variability and Control of Nodal Root Angle in Sorghum. Crop Sci 51:2011–2020. https://doi.org/10.2135/cropsci2011.01.0038
Singh V, van Oosterom EJ, Jordan DR, Hammer GL (2012) Genetic control of nodal root angle in sorghum and its implications on water extraction. Eur J Agron 42:3–10. https://doi.org/10.1016/j.eja.2012.04.006
Steingrobe B, Schmid H, Claassen N (2000) The use of the ingrowth core method for measuring root production of arable crops — influence of soil conditions inside the ingrowth core on root growth. J Plant Nutr Soil Sci 163:617–622. https://doi.org/10.1002/1522-2624(200012)163:6%3c617::AID-JPLN617%3e3.0.CO;2-0
Strong WM, Best EK, Cooper JE (1997) Phosphate fertiliser residues in wheat-growing soils of the Western Downs, Queensland. Soil Res 35:341–354. https://doi.org/10.1071/s96038
Svane SF, Jensen CS, Thorup-Kristensen K (2019) Construction of a large-scale semi-field facility to study genotypic differences in deep root growth and resources acquisition. Plant Methods 15:26. https://doi.org/10.1186/s13007-019-0409-9
Tao Y, Mace ES, Tai S, Cruickshank A, Campbell BC, Zhao X, Van Oosterom EJ, Godwin ID, Botella JR, Jordan DR (2017) Whole-Genome Analysis of Candidate genes Associated with Seed Size and Weight in Sorghum bicolor Reveals Signatures of Artificial Selection and Insights into Parallel Domestication in Cereal Crops. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.01237
Tao Y, Zhao X, Wang X, Hathorn A, Hunt C, Cruickshank AW, van Oosterom EJ, Godwin ID, Mace ES, Jordan DR (2020) Large-scale GWAS in sorghum reveals common genetic control of grain size among cereals. Plant Biotechnol J 18:1093–1105. https://doi.org/10.1111/pbi.13284
van der Bom FJT, Williams A, Bell MJ (2020) Root architecture for improved resource capture: trade-offs in complex environments. J Exp Bot 71:5752–5763. https://doi.org/10.1093/jxb/eraa324
van der Bom FJT, Williams A, Borrell AK, Raymond NS, Bell MJ (2023a) Phosphorus management is key to effective deployment of root ideotypes in complex soil environments. Plant Soil. in press: PLSO-D-23–00178
van der Bom FJT, Williams A, Raymond NS, Alahmad S, Hickey LT, Singh V, Bell MJ (2023b) Root angle, phosphorus, and water: Interactions and effects on durum wheat genotype performance in drought-prone environments. Plant Soil. https://doi.org/10.1007/s11104-023-05966-z
van Genuchten MT (1980) A Closed-form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. Soil Sci Soc Am J 44:892–898. https://doi.org/10.2136/sssaj1980.03615995004400050002x
van Oosterom EJ, Hammer GL (2008) Determination of grain number in sorghum. Field Crops Res 108:259–268. https://doi.org/10.1016/j.fcr.2008.06.001
Vetterlein D, Marschner H (1993) Use of a microtensiometer technique to study hydraulic lift in a sandy soil planted with pearl millet (Pennisetum americanum [L.] Leeke). Plant Soil 149:275–282. https://doi.org/10.1007/BF00016618
Wang X, Tang C, Guppy CN, Sale PWG (2009) The role of hydraulic lift and subsoil P placement in P uptake of cotton (Gossypium hirsutum L.). Plant Soil 325:263–275. https://doi.org/10.1007/s11104-009-9977-1
Wasson AP, Richards RA, Chatrath R, Misra SC, Prasad SVS, Rebetzke GJ, Kirkegaard JA, Christopher J, Watt M (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 63:3485–3498
Wasson AP, Rebetzke GJ, Kirkegaard JA, Christopher J, Richards RA, Watt M (2014) Soil coring at multiple field environments can directly quantify variation in deep root traits to select wheat genotypes for breeding. J Exp Bot 65:6231–6249. https://doi.org/10.1093/jxb/eru250
Wickham H (2009) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York
Yang Z, Hammer G, van Oosterom E, Rochas D, Deifel K (2010) Effects of pot size on growth of maize and sorghum plants. In: B George-Jaeggli, DJ Jordan (eds) 1st Australian Summer Grains Conference. Grains Research and Development Corporation, Gold Coast, Australia
Acknowledgements
We would like to acknowledge Tim Rossignol and Bec Archer of the School of Agriculture and Food Science for valuable technical assistance during the preparation and conducting of the experiment. We thank Celso Cordova, Andrew Hughes and Ismail Garba for their help with experimental preparations and the harvesting and processing of plant samples. Plant materials were provided by Alan Cruickshank and from the Department of Agriculture and Fisheries (DAF), QLD and Dave Jordan from the University of Queensland. We thank Alan Cruickshank, David Jordan and Andrew Borrell for valuable discussions during the course of the project.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions The project was funded by the Grains Research and Development Corporation of Australia (GRDC Project no. UOQ1805-005RTX).
Author information
Authors and Affiliations
Contributions
FB, AW, MB designed the experiment; MB, RS, GM designed the concept facility, RS built the facility and GM built the software; FB, NR conducted the experiment, measurements and analyses, FB drafted the first manuscript; All authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
RS is the founder of Biosystems Engineering, which builds advanced plant growing and phenotyping platforms. For this reason, there may be a conflict of interest in order to show that the developed platform is able to accurately simulate various moisture dynamics. It should be noted that RS has provided technical support during the experiment but was not involved in the overall experimental aims and design or its data analysis.
Additional information
Responsible Editor: Honghua He.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Bom, F., Williams, A., Raymond, N. et al. Spatio-temporal distribution of water and phosphorus determine growth of sorghum genotypes with contrasting nodal root angle. Plant Soil 499, 37–54 (2024). https://doi.org/10.1007/s11104-023-06073-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06073-9