Abstract
Purpose
Selection for root traits has become a target in (pre-)breeding programs aiming at improving crop ability to capture soil resources. However, the benefit of selected traits in heterogeneous target environments will depend on spatial and temporal interactions between root systems, the soil environment (fertility and water supply) and management (fertiliser placement).
Methods
We assessed growth and phosphorus acquisition of durum wheat (Triticum durum L.) lines defined by contrasting seminal root angles (41° ‘Narrow’ vs 82° ‘Wide’), in response to different soil phosphorus placements and seasonal water dynamics. Responses were evaluated in clear pots (seedlings), rhizoboxes (late-tillering stage) and a custom lysimeter system (flowering stage).
Results
The Narrow genotype increased deep soil exploration (down to 50 cm) during early growth, with a tendency to more rapidly acquire phosphorus placed as a deep fertiliser band (25 cm depth). However, genotypic differences in shoot biomass or phosphorus uptake were not apparent at anthesis, in part due to phosphorus-induced changes in phenological development. Contrastingly, the wide genotype increased root growth in the topsoil (0–10 cm) when phosphorus was stratified in this layer and produced greater biomass at anthesis under these conditions. Shoot and root biomass and phosphorus uptake decreased when the topsoil dried out, with the greatest effect observed for phosphorus stratified in this layer.
Conclusion
The benefits of the selected root angle trait strongly depend on nutrient and water distributions and dynamics in the target environment. Ideotype breeding efforts and farmer selection of genotypes should consider the context in which genotypes will be deployed.
Highlight
The interaction between root system architecture and heterogeneous distributions of phosphorus and available water determine the relative performance of durum wheat genotypes with contrasting root angles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selection of root functional traits is considered an important opportunity for developing crops with improved capacity to capture soil resources (Lynch 2019). Several root traits have been proposed to improve yields in water-limited environments, such as steeper root growth angle (Manschadi et al. 2008), reduced production of crown or lateral roots (Gao and Lynch 2016), and reduced xylem vessel diameter (Richards and Passioura 1989). Evidence suggests these traits may deliver a root system architecture (RSA) of deeper roots with minimal additional metabolic cost, which would improve plant ability to take up water from deeper soil layers. This relationship with deep RSA in combination with heritability makes them of interest as ‘proxy traits’ in (pre-)breeding programs to rapidly screen large numbers of plants (Christopher et al. 2013). For example, seminal root growth angle of wheat seedlings can be measured on plants only a few days old and has been correlated with vertical rooting distribution at a later growth stage (Manschadi et al. 2008; Oyanagi et al. 1993). However, correlations between lab and field root traits are often inconsistent (Bai et al. 2019; Rich et al. 2020; Severini et al. 2020), likely in part due to differences in soil and rainfall conditions.
In the past three decades, soil nutrient reserves in the dryland cropping environments of North-East Australia have declined substantially, with crop demand increasingly met by fertilisers. Conservation agriculture practices such as minimal tillage and stubble retention have become widespread and have increased grain yields through conserving soil water, but they have also led to increased topsoil stratification of nutrients with low mobility, such as phosphorus (P) (Angus et al. 2019; Kirkegaard et al. 2014). Crop access to stratified P frequently becomes limiting because the topsoil layer is prone to rapid drying (Raymond et al. 2021; Strong et al. 1997). Therefore, the agronomic practice of ‘deep banding’ P fertiliser (at 0.2 to 0.3 m depth) is increasingly used to improve crop P uptake. This approach relies on the placement of P in soil layers that stay moist for longer, and on roots proliferating in and achieving enhanced P uptake from the highly enriched soil volume around the concentrated band (Ma et al. 2009; Singh et al. 2005). Deep banding of P has elicited yield responses in many soils including some with a high topsoil P availability, though responses have often been more pronounced in seasons with sparse or irregular rainfall, in which crop access to topsoil resources was more restricted (Bell et al. 2012).
The exact benefit of selected RSA traits in these dryland systems will depend on the resulting capacity of the plant to take up several scarce resources, with root systems likely to face trade-offs such as for the uptake of P and water. For example, a deep RSA may improve uptake of water from deeper layers, but this may be at the expense of root growth and uptake of P from the topsoil layer (Lynch 2019). Few studies have investigated how crops with selected root traits (such as root angle) respond to such conditions, though there is some evidence that RSA will impact crop performance in environments with combined water and P stress (Ho et al. 2005). Furthermore, little is known about what trade-offs are involved with reliance on P placed in deep bands, and if these differ between genotypes with contrasting RSA. Therefore, for effective deployment of genotypes with selected root traits, it is critical to understand their response to realistic target environments, including the complex interactions and trade-offs that may affect overall crop performance (van der Bom et al. 2020).
This paper integrates results from three experiments that were conducted to elucidate the responses of selected genotypes with contrasting RSA (defined by seminal root angle) to P placement and soil water availability. Critical to this research was the development of a durum wheat (Triticum durum L.) nested association mapping (NAM) population which led to the identification of a major quantitative trait loci (QTL) qSRA-6A that modulates seminal root angle (Alahmad et al. 2019). In particular, identification of durum lines with different alleles for the qSRA-6A QTL enabled targeted investigation of how genotypes with ‘narrow’ or ‘wide’ root angles function in environments defined by multiple constraints, with minimal confounding effects of other traits related to water or P adaptation and phenological development. Durum wheat is typically grown under rainfed conditions in the semiarid regions of the world, with crop yields strongly influenced by the availability of soil water and in-season precipitation patterns. Selection of varieties with a narrow root angle has been suggested to increase the resilience of durum wheat genotypes in these environments (Alahmad et al. 2020). We conducted short-term (seedling stage and late-tillering stage) and longer-term (up to anthesis) experiments under (semi-)controlled conditions with two genotypes defined by contrasting root angles (wide/narrow). Our objective was to understand how these proposed contrasting root morphologies responded to soil environments in which P placement and seasonal water dynamics varied, and to assess the implications for growth and P acquisition.
Materials and methods
Plant materials
In previous studies, large genetic variability was observed for root angle in durum wheat varieties (El Hassouni et al. 2018). A panel of 14 genotypes known for having drought adaptation characteristics were evaluated for seminal root angle under controlled conditions using the ‘clear pot’ method (Richard et al. 2015) and root angle of the mature crown under field conditions using a ‘shovelomics’ approach (Trachsel et al. 2011). A nested association mapping population (NAM) was then developed by crossing a subset of these lines into the ‘reference’ Australian durum varieties Jandaroi and DBA Aurora. Segregating NAM lines were progressed to the genetically stable F6 generation (Alahmad et al. 2019, 2022). From these NAM lines, 393 recombinant inbred lines (RILs) were selected based on their agronomic similarities in the field and subsequently evaluated for seminal root angle and root system biomass, as well as used for a genome-wide association study. From this population, we strategically selected two durum wheat lines that had a strongly divergent seminal root angle (‘Wide’ vs ‘Narrow’) whilst presenting similar root system biomass and aboveground growth and phenological development. The two lines were included in a preliminary rhizobox screening with potting mix as the growth medium, in which contrasting seminal root angle and associated effects on root distribution were observed (Alahmad et al. 2019). QAF0512 had a seminal root angle of 41° and represented haplotype1 of the major root angle QTL qSRA-6A, and based on these characteristics is referred to as ‘Narrow’ in plant (pre-)breeding programs that target this proxy trait. QAF0411 had a seminal root angle of 82° and represented haplotype 2 and is referred to as ‘Wide’. Although these lines had different pedigrees (QAF0512 = Jandaroi × Fastoz8, and QAF0411 = Aurora × Fastoz7), they were considered the most suitable for studying the effects of seminal root angle and its interactions with the soil environment because of their previously observed contrasting RSA as well as critical similarities in root system biomass and aboveground phenological development (e.g. tiller number, growth rate, and time to flowering). The similar aboveground phenology was considered of importance, given common relationships exist between above and belowground phenology, e.g. tillering intensity has been observed to relate to root length, root biomass, and root depth at maturity (Hendriks et al. 2016).
Soil
A grey vertosol soil (Isbell 2016) was collected from the 10–30 cm depth soil layer of a field cropped to grains and cotton near Hopelands, in the Western Downs Region, Queensland, Australia. In the FAO classification, this is a Vertisol (FAO 2015). Concentrations of labile P (Colwell-P) and sparingly soluble P (BSES-P) were 7 mg P kg−1 and 44 mg P kg−1, respectively, indicating a low P status that was likely to severely limit crop growth (Table 1). The soil was air-dried and crushed with a jaw crusher to obtain a maximum particle size of approximately 8 mm. Water holding capacity (WHC) was defined as the gravimetric water content at the drained upper limit (-10 kPa, 0.38 g g−1).
Greenhouse clear-pot experiment
The seminal root angle of the two genotypes was re-phenotyped in the experimental soil using the same ‘clear pot’ method in which they were originally screened (Alahmad et al. 2019; Richard et al. 2015). Briefly, clear pots were filled with the experimental soil and seeds of each genotype were carefully placed in the pots such that the embryo faced the wall of the pot with the radical pointing downward (Fig. 1A). Seeds were placed in a randomized complete block design (RCBD) with 12 replicates per genotype. Plants were grown in the glasshouse for five days (22/17 °C day/night [12 h]) after which the seminal roots were photographed for subsequent analysis.
Schematic overview of the experiments and experimental treatments. A: The clear-pot experiment evaluated seedling root angle in the experimental soil after 5 days. B: The rhizobox experiment was conducted with four P fertiliser placements; plants were grown until late tillering. C: The lysimeter experiment included two irrigation treatments and four P fertiliser placements. For the ‘Dry’ irrigation treatment, topsoil irrigation was withheld from 28 days onward; plants were grown until anthesis. D: Schematic representation of the placement of the deep P-Band at 25 cm depth, through the centre of the ‘left’ root sampling bag. The root bags were placed at pre-determined sampling layers to facilitate root quantifications. The orange zone in the irrigation treatments indicates the topsoil drying zone. Coloured dots illustrate the placement of P fertiliser in each treatment
Greenhouse rhizobox experiment
A rhizobox experiment was conducted in a temperature-controlled glasshouse (22/17 °C day/night [12 h]) with plants grown in rhizoboxes that were 60 cm high, 40 cm wide, and 4 cm thick. The experiment was a randomised block design with five replicates. Batches of 10.0 kg of air-dry soil were supplied with basal nutrients (except P) in liquid solution at the following concentrations (mg kg−1 soil) for each element: 100.0 N, 166.2 K, 123.7 S, 20.4 Ca, 40.0 Mg, 2.0 Zn, 36.2 Cl, 3.4 Mn, 0.5 Cu, 0.1 Mo (modified from Raymond et al. 2018). Soils were dried after application and then thoroughly mixed to ensure homogeneous nutrient distribution, after which the rhizoboxes were filled with the prepared soils.
The P placement treatments included (Fig. 1B):
-
1.
control: from which fertiliser P was completely withheld;
-
2.
P-band: P fertiliser placed at 25 cm depth and 10 cm off-centre;
-
3.
Topsoil P: P fertiliser applied throughout the top 10 cm of the rhizobox; and
-
4.
Mixed P: P fertiliser dispersed throughout the entire soil volume.
All P fertiliser was ground before application. The rhizobox was considered as a ‘slice’ taken from the field directly below the plant, with the P-band treatment applied as a single spot containing 100 mg P. This was equivalent to a 50 kg P ha−1 band under field conditions. For the Topsoil P treatment, the same amount of fertiliser was mixed through to the top 10 cm of soil, resulting in a concentration of 60 mg P kg−1 in this zone. For the Mixed treatment, P was applied as 60 mg kg−1 throughout the entire profile, representing a high P control. The P fertiliser was applied as either monocalcium P (MCP, often referred to as triple superphosphate) or as mono-ammonium P (MAP). Farmers in North-East Australia generally use MAP, which contains 120 mg g−1 of N. The MCP fertiliser does not contain any N and therefore allowed for testing whether the effects of the MAP bands could be attributed to the fertiliser P content i.e. if the basal fertilisation conditions isolated the P response as intended. To ensure equivalent N between the two P fertiliser treatments, the rhizoboxes fertilised with MCP received additional an application of ammonium sulfate. We observed that the responses to MCP and MAP were highly correlated and not significantly different (data not shown), so only the combined mean is presented. The experimental approach was therefore assumed to have reliably achieved the intended purpose of targeting the response to P.
All rhizoboxes were watered to 80% WHC. Three seeds were sown in the centre of each box. After the seedlings had emerged, the plants were thinned to one plant per box. Soil water content was maintained between 70–80% of WHC by intermittent topsoil watering throughout the experiment.
All plants were grown for 38 days, with destructive harvesting corresponding to the late tillering stage. At harvest, tillers were counted and all aboveground biomass was cut at the base of the stem. The front of each rhizobox was removed and a pinboard with a similar dimension to the chamber was pushed into the soil so that the soil could be carefully washed away, exposing the intact root system (previously described by Singh et al. 2010). After all soil had been removed, the RSA was photographed with a digital camera mounted on a tripod. Photo quality was optimised in Adobe Photoshop to ensure high-quality analysis of root properties (contrast, black and white, touch-up of minor scratches on the pinboard).
Semi-controlled lysimeter experiment
The experiment was conducted in the lysimeter facility at the nursery at The University of Queensland Gatton campus. Briefly, the system consists of ten closed, air-conditioned containers, each capable of carrying eight lysimeter cores (300 mm diameter × 730 mm height). For each core, the system continuously monitors weight, temperature, and soil water status (by use of three temperature/moisture probes inserted in the soil profile). Soil water dynamics are automatically controlled by the use of three irrigation probes, from which each core is independently irrigated using custom software.
The experiment was conducted with four replicates in a randomised block design with complete factorials. The experiment was conducted with two irrigation patterns (Fig. 1C):
-
1.
‘Wet’, for which all cores were maintained at ~ 80% of WHC in all profile layers throughout the experiment; and
-
2.
‘Dry’, for which topsoil drought was allowed to develop after four weeks,once the crop had established (mid tillering). Water in the subsoil was maintained like the ‘Wet’ condition for the duration of the experiment.
Each core was filled with 60 kg of air-dry soil. A 1.5 cm layer of pebbles was placed at 10 cm as a hydraulic break between the subsoil and the topsoil. Four root bags (diameter 10 cm, length 25 cm, mesh size 2.5 mm) were filled with 2.5 kg of soil each and placed at different locations in the core: two at a depth of 20–30 cm and two at 40–50 cm; placed ‘Left’ and ‘Right’ off-centre to facilitate harvesting and measurement of roots at the end of the experiment (Fig. 1D). The mesh size of approximately 2.5 cm allowed roots to grow into the bags and did not obstruct water.
Like the rhizobox experiment, the experiment included four P placements (Fig. 1C), with the same amount of P added in both the P-band and Topsoil P treatments:
-
1.
Control, from which P fertiliser was completely withheld;
-
2.
P-band, with P fertiliser placed in the middle of the ‘Left’ mesh bag at 25 cm;
-
3.
Topsoil P, with 60 mg fertiliser P kg−1 dispersed throughout the top 10 cm layer; and
-
4.
Mixed P, 60 mg fertiliser P kg−1 dispersed throughout the entire soil profile
All P in this experiment was applied as MAP. Basal nutrients, except P, were supplied at the following rates (mg kg−1 soil) of each element: 154 N, 150 K, 37 S, 2 Zn, 150 Cl, using commercial fertilisers in the forms of urea, muriate of potash, ammonium sulfate, and zinc sulfate heptahydrate. The urea application was 120 mg kg−1 of N, with the balance applied as a combination of MAP and ammonium sulfate, depending on the amount of P fertiliser applied to each treatment. All fertilisers were ground before application.
Each core was seeded with ten seeds, which were thinned to seven plants per core after emergence. The number of seven plants per core corresponded to a commercial planting density of 100 plants m−2. Plants were grown for 68 days until approximately anthesis (treatment dependent). At harvest, all heads were counted and shoots were cut at the base of the stem. The 10 cm topsoil layer (to the pebble layer) and the mesh bags were collected from each core and stored at 4 °C until roots could be extracted. The collected soil samples were soaked until the soil was fully saturated, after which the soil was carefully washed away to recover the roots. The root crowns of the top 0–10 cm sample were photographed for root angle analysis. Roots were stored in 50% (v/v) ethanol at 4 °C until further morphology measurements could be made.
Measurements
Root angles were measured from photographs using Fiji (Schindelin et al. 2012). For the clear-pot experiment, the angle between the first pair of seminal roots was measured at 3 cm distance from the seed (Richard et al. 2015), whereas for the rhizobox and lysimeter experiments the outer angle of the nodal roots was measured. All other root data including root length and diameter classes were obtained using WinRHIZO Pro 2019a (Regent Instruments, Inc., Quebec City, QC). For the lysimeter experiment, the samples were scanned on a flatbed scanner (Epson expression 10000xl, Epson, CA) prior to the analysis. Root length density (RLD, total length of roots per unit of soil volume, cm cm−3) and specific root length (total length of roots per unit of root weight, m g−1) were calculated based on the WinRhizo-acquired root length, the sample volume and the root biomass. Where appropriate, root biomass data in each soil layer of the lysimeter experiment are presented on an equal volume basis, to account for the different sampling volumes between the topsoil layer and the subsoil mesh bags. For the rhizobox experiment, we report the projected root area (cm2), defined as the root area, measured using WinRhizo, on each root systems photo (after washing on the pinboard). The projected area is closely correlated with the root length (cm) but provided better relative comparisons than the calculated root lengths. We observed that root lengths tended to be slightly underestimated for the largest root systems and in areas of root proliferation due to overlap among roots (Fig. 2, Suppl. materials).
Example of (plastic) root system architectural responses to the different phosphorus placements (‘Narrow’ genotype). See Fig. 4 for the distribution of the projected root areas across the soil layers
For both experiments, shoot and root biomass were oven-dried at 65 °C until stable weight, after which biomass dry matter was determined. Shoot biomass samples were finely ground, and a 0.5 g subsample was digested in nitric acid and hydrogen peroxide using microwave-assisted closed vessel digestion, after which the P concentrations were analysed using inductively coupled plasma-optical emission spectroscopy (ICP-OES). Total P uptake was calculated by multiplying the measured P concentrations with the biomass dry matter.
Statistical analyses
Treatment effects on above and belowground biomass and P uptake were analysed for each genotype using linear mixed-effects models, with fixed effects for the P placement and irrigation treatments, and a random effect for the replication across blocks. The contributions of the fixed effect parameters were assessed using Maximum Likelihood and backward model reduction. The final models were refitted using Restricted Maximum Likelihood, and pairwise comparisons were made using Estimated Marginal Means with Tukey adjustments. Relationships between projected root area in the rhizobox experiment and root length and RLD in the lysimeter experiment were evaluated using linear regression, with these relationships specifically tested for the equivalent wet treatments. Differences in root angle were evaluated using a two sample t-test for the clear-pot experiment and with analysis of variance for the rhizobox and lysimeter experiments.
For the lysimeter experiment, the irrigation system sporadically closed slightly slower than intended, resulting in some experimental cores receiving more water than the intended target. This could have potentially resulted in local and temporary saturated conditions, but the impact of this on the overall experiment was likely minimal due to a combination of factors. First, the upper target weight was set at 80% of the WHC of the soil, so it was unlikely that the cores would become fully saturated from the additional water. Second, the high crop water demand later in the experiment meant any of the ‘excess’ water was rapidly used up, as illustrated by irrigation being triggered several times each day. Therefore, we used Cook's distance as a statistical approach to identify if any observations potentially influenced the results of the experiment. We conservatively classified observations with a Cook’s distance greater than four times the mean as influential. Subsequently, outlier removal was considered only if there was both a statistical indication and a relevant record related to the experimental conditions (i.e. the combined irrigation logs, core weights, and water sensor data). Specifically, in one instance, a core was removed due to the likelihood of saturated conditions early in the experiment.
All statistical analyses were conducted using R version 4.1.1 (R Core Team 2020) making extensive use of the tidyverse family of packages (Wickham et al. 2019), nlme (Pinheiro et al. 2020), and emmeans (Lenth 2021). All models were checked for normality of residuals and homogeneity of variance using a combination of diagnostic plots and formal tests (Levene's test for homogeneity of variance, Shapiro–Wilk test for normality). Where required, analyses have been performed on log or square root-transformed data, or with use of a heterogeneous variance structure to account for differences in variation between treatment combinations.
Results
Root angle across the three experiments
Root angle differed between the two genotypes in the clear pot experiment (P < 0.05), though the difference was less pronounced than in the original NAM screening (Table 2). Overall, root angle was variable in the rhizobox and lysimeter experiment, with no significant differences between the two genotypes in either of the experiments. In particular, the measured root angle for the narrow genotype was substantially greater in the current experiments compared with the root angle as measured in the original and the clear-pot screenings.
Rhizobox experiment: Above and belowground responses to phosphorus placement up to the late-tillering stage
We observed clear responses to P placement, with a strong increase in shoot biomass and tiller number, particularly for topsoil P and mixed P, and a smaller but significant response to banded P (P < 0.05, Fig. 3A and B). The aboveground responses were not significantly different between the genotypes, although the wide genotype tended to be smaller than the narrow one when P was banded at 25 cm. Root biomass largely followed the same pattern as observed for shoot biomass, in which the Narrow genotype tended to have greater total root biomass in response to the P-Band than the Wide genotype, though not significantly so.
The genotypic difference in response to the P-band was significant in terms of total P uptake (P < 0.01, Fig. 3C), i.e. the Narrow genotype took up more P from the P band than the Wide one. In general, P uptake followed a similar pattern to plant biomass.
Rhizobox experiment: Root distribution responses to phosphorus placement up to the late-tillering stage
For both genotypes, projected root area in the 0–10 cm layer was greatest when P was placed in this zone and not elsewhere in the profile (i.e. Topsoil P, P < 0.001; Figs. 2 & 4, Table 3). This was in stark contrast to Mixed P, which produced the lowest topsoil root area amongst all treatments. Overall, projected root area showed a marked increase between 10 and 30 cm for both Topsoil P and Mixed P (P < 0.05). For all treatments, root area declined in layers > 30 cm deep, but overall a clear fertilisation effect remained relative to the control (P < 0.05).
Projected root area (visible area of roots [cm.2] on the pinboard after washing) for each 10 cm increment of soil depth in the rhizoboxes. Error bars indicate the standard error of the mean. For statistical information and pairwise comparisons see Table 3
For the narrow genotype, a clear increase in projected root area in response to P placed as a deep band at 25 cm could be observed (P < 0.05). For the wide genotype, there was no difference between the control and the P-band in that zone. Furthermore, root area of the Narrow genotype was generally greater than that of the Wide genotype in the 30–40 cm and 40–50 cm layers, except when no P was applied (i.e. Control, P < 0.05). Conversely, in the topsoil (0–10 cm) the Wide genotype had an overall greater projected root area than the Narrow one (P < 0.001).
Lysimeter experiment: Aboveground responses to phosphorus placement and topsoil drying up to anthesis
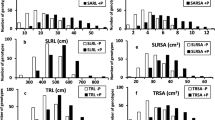
In this experiment, we observed clear interactions between genotype, P placement, and soil water (Table 4). The placement of P fertiliser had a significant effect on aboveground biomass for both durum genotypes, with shoot growth increasing in the order of Control < P-Band < Topsoil P < Mixed P (P < 0.001, Fig. 5A). This increase was reflected in tiller number in a similar order (P < 0.001, Fig. 5B).
Aboveground growth responses in the lysimeter experiment. A: shoot biomass, B: total number of tillers, C: total number of flowering spikes at time of harvest, D: total phosphorus uptake. Error bars indicate the standard error of the mean. Bars sharing a common letter are not significantly different (Tukey adjusted α < 0.05, F = fertiliser placement, G = genotype, I = Irrigation). See Table 4 for statistical information
Under well-watered conditions, the Wide genotype grew larger than the Narrow one when P was applied as Topsoil P (P < 0.001), even though this difference in biomass was not reflected in total P uptake (Fig. 5D). Conversely, the narrow genotype tended to grow larger and take up more P than the wide genotype with Mixed P, although not significantly so. When P was banded, both genotypes produced a similar amount of biomass and took up similar amounts of P. However, even though on average the genotypes had a similar number of total tillers, for this treatment, a substantial proportion of tillers of the Wide genotype were not yet flowering (Fig. 5C).
In general, a drying topsoil reduced shoot growth, tiller production and P uptake compared to the well-watered treatments, with differences in magnitude depending on where P was placed in the profile. The greatest declines were observed for Mixed P (on average 26%, from 156 to 115 g core−1) and particularly Topsoil P (on average 37%, from 119 to 75 g core−1). Within this, the proportionally largest reduction was observed for the Wide genotype growing under the Topsoil-P treatment, for which topsoil drying resulted in a 46% loss in biomass production, compared with 25% for the Narrow genotype (P < 0.001). Subsequently, the genotypic differences in biomass production that were accentuated by the topsoil P placement effectively disappeared when the topsoil dried out (P < 0.001). Similarly, on average plants took up only 46% of P under the dry Topsoil P placement compared with its wet counterpart, making it the greatest relative reduction of P uptake when the topsoil was allowed to dry (P < 0.001). In contrast, topsoil drying reduced P uptake to approximately 80% for the P-band and 75% for the control, with these reductions not statistically significant.
Lysimeter experiment: Root responses to phosphorus placement and topsoil drying up to anthesis
Across all treatments, the majority of sampled root biomass was located in the topsoil (0–10 cm, Fig. 6A). On average, the Wide genotype had more root biomass and greater RLD in the top 10 cm layer than the Narrow one (P < 0.001), but specific root length (SRL, m g−1) was not significantly different. The placement of P fertiliser had a significant effect on root biomass for both durum genotypes, with the largest increases when P was applied as Topsoil P or Mixed P (P < 0.001), and Topsoil P also had the greatest RLD (P < 0.001). The application of P reduced SRL for all treatments (relative to the control), with the greatest decrease observed for Mixed P (P < 0.001). Topsoil drying had a clear effect on all measured parameters, leading to reductions in root biomass and RLD (P < 0.001), and increasing SRL (P < 0.001).
Belowground responses in the lysimeter study. A: Root biomass distribution (g), B: root length density (cm of root cm−3 of soil volume), C: specific root length (m of root length g−1 of root biomass). For both subsoil layers, data are summarised from two sampling bags recovered from the band and non-band sides of the lysimeter cores. Subsoil root biomass was converted to correspond to a soil volume equivalent to a 10 cm profile layer, so that comparisons can be made to the 0–10 cm topsoil layer. Error bars indicate the standard error of the mean. See Tables 4 and 5 for statistical information
In the 20 to 30 cm layer, root biomass was substantially smaller than that in the top 10 cm layer, but clear differences in response to P fertiliser could still be observed. Root biomass was greatest for Mixed P and Topsoil P, followed by the P-band treatment (P < 0.001). Furthermore, RLD increased in response to P fertiliser application, but it was not significantly different between the different placements (P < 0.001). On average, SRL was greatest for the control and the P-Band treatments (P < 0.001). The P band treatment showed a clear spatial trend within the 20–30 cm layer, with both genotypes showing increased root biomass, RLD and SRL on the side where the fertiliser band was placed, relative to the non-band side (P < 0.001, Fig. 7). No such trend was observed for any of the other treatments.
In the lower subsoil (40–50 cm), root biomass and RLD were in the order of Control < P-band < Topsoil-P < Mixed-P (P < 0.001), which was similar for both genotypes. On average, SRL was greatest for the P-band treatments (P < 0.001), although not significantly different from the control. When exposed to a drying topsoil, root biomass in this deep soil layer significantly increased in all except the Mixed P treatment (P < 0.001). This was also reflected in RLD, which broadly increased as a result of the dry treatment (P < 0.05).
Correlation of root data across different growth stages
The projected root area at the late-tillering stage in the rhizobox experiment was linearly related to root biomass at anthesis in the lysimeter study for the two subsoil layers (P < 0.001, 20–30 cm and 40–50 cm), but not the 0–10 cm layer (Fig. 8A). Similarly, root area was linearly related to RLD at anthesis (in the lysimeter experiment) for all three of the sampled soil layers (P < 0.01, Fig. 8B).
Projected root area at late-tillering (cm2, visible root area on the pinboard after root washing in the rhizobox experiment) was linearly correlated with A: Root biomass (g) and B: Root Length Density (cm cm.−3) at anthesis (lysimeter experiment). Datapoints represent means of equivalent treatments between the two experiments. Root data for the subsoil layers (20–30 cm and 40–50 cm) in the lysimeter experiment were summed for the two sampling bags in each layer. Stars indicate significance: *P < 0.05, **P < 0.01
Discussion
This study demonstrated that interactions between P distribution and water dynamics in the target environment were the main driver of root systems growth and function, even if seminal root angle affected (early) soil exploration. Most previous studies on root angle in wheat have focused on genetic control and/or screening of variation (Christopher et al. 2013; Manschadi et al. 2008; Richard et al. 2018, 2015), or have considered functioning in environments primarily defined by water limitation (Alahmad et al. 2020; El Hassouni et al. 2018). Studies that investigated the role of RSA on crop use of soil P have mainly been conducted with maize and common bean, using cultivars with different overall root systems (Ho et al. 2005; Liao et al. 2001; Zhu et al. 2005), recombinant inbred lines for traits such as lateral branching or crown root numbers (Jia et al. 2018; Sun et al. 2018; Zhu and Lynch 2004), or modelling approaches (Miguel et al. 2015; Postma et al. 2014; Postma and Lynch 2012; Rangarajan et al. 2018). The genotypes used in this study were selected because of their previously observed contrasting seminal root angle, and associated root distribution at depth, whilst being similar in root system biomass and aboveground phenological development (e.g. tiller number, growth rate, and time to flowering). For these characteristics they were considered a suitable model to evaluate the impact and function of RSA traits under realistic target conditions, and to provide essential insight into the Genotype × Environment × Management (G × E × M) interactions that determine crop performance. Understanding these interactions is an important step to identify how cropping systems are most likely to benefit from crop improvement programmes that target RSA (Passioura 2010; van der Bom et al. 2020; Wasson et al. 2012).
Root angle modified root distribution primarily during early growth
Selection for a narrow root angle is aimed at delivering a more vertical root system with a greater ability to extract soil water at depth under (terminal) drought conditions (Alahmad et al. 2020). A wide root angle would therefore be expected to do the opposite and lead to a shallower root system. This has been proposed to improve P uptake because concentrations of labile P tend to be greater in the topsoil layer in most soil profiles (Lynch and Brown 2001). Our results support the hypothesis that root angle will change root distribution, with the wide genotype showing greater projected root area in the topsoil during early growth. Similarly, the narrow genotype delivered a greater root area at depth in accordance with its selection (Alahmad et al. 2019), although this pattern was not as evident at anthesis. On average the wide genotype had a greater root biomass and RLD in the topsoil layer, but the differences between the two genotypes were only marginal at depth (Fig. 6, Table 3).
It is often observed that laboratory-based screenings correlate with RSA of juvenile plants, but not with RSA at a later growth stage (Bai et al. 2019; El Hassouni et al. 2018; Watt et al. 2013). Drivers of this inconsistency may include biotic and abiotic factors, including seasonal rainfall distribution and stored soil water, oxygen and nutrient availability (Rich and Watt 2013). However, in the current experiments, soil water dynamics were controlled and thus the Mixed-P treatment with non-drying topsoil should have provided ample water and nutrition for the plants to grow close to their genetic potential. One possible reason we did not see more pronounced genotypic differences in subsoil root distribution in the lysimeter study may be an artefact of soil processing prior to filling the soil cores. The combination of finely ground, tightly packed soil that is continually moist with a high bulk density (not directly measured in our studies) may limit subsoil aeration and deep root distribution (Chen et al. 2014; Jin et al. 2013). This has previously been shown to diminish differences in deep rooting between rice genotypes defined by distinct root angles (Ramalingam et al. 2017), but the studies that made these conclusions used heavy machinery to create even ‘medium’ compaction, which would presumably have led to a much greater bulk density than in the current experiments. The reduction in root biomass moving down the soil profile (Fig. 6) may thus simply reflect the typical shift in root biomass and size with increasing soil depth, with the increasing SRL indicating roots in the subsoil were generally thinner than those in the topsoil layer.
A second explanation for the inconsistency in root distribution patterns between the early and anthesis growth stages may be that an adjustment of RSA took place as the plant developed from tillering, through stem elongation, and to anthesis. This is supported by the measurements of root angle in the rhizoboxes, in which no clear differences were observed between the two genotypes. How root traits change during the plant’s life cycle is still poorly understood (Rich et al. 2020), but there could be benefits in plastic genotypes that change root development over their lifespan. For example, a genotype able to explore the topsoil during early growth and deeper soil layers later in the growing season may confer benefits in dryland systems with stratified P, where early exploration of the topsoil before it dries out would allow uptake of immobile nutrients before they become inaccessible. On the other hand, the large variation of root angle among the different experiments (Table 4) also emphasises the difficulties of obtaining consistent, reliable measurements of root angle in soil at later growth stages, with the measurement of outer angle of the nodal roots illustrating the inability to identify and measure the first pair of seminal roots as measured in the clear-pot screening. Furthermore, seminal root angle was different in soil than in the original screening and phenotyping done in potting mix, with the result being that in soil the phenotypes were less extreme and differences between them were less pronounced. This suggests that the soil environment can influence the RSA phenotype and that genotypic differences may be smaller in soil than in greenhouse or laboratory media. Although selection for root traits using rapid, high-throughput screening tools and growth media can provide benefits for screening purposes, it remains necessary to validate the phenotypes in realistic environments.
Access to phosphorus was a greater driver for crop growth than root angle
In our experiments, basal fertilisers were applied throughout the soil profile to isolate P as the most limiting plant nutrient. Following the Sprengel-Liebig Law of the Minimum (see van der Ploeg et al. 1999), the the addition of P in the mixed P treatment should provide an indication of potential above and below-ground plant growth. The Topsoil P and P-Band treatments each received an equal amount of P, but the placement as a fertiliser band substantially reduced crop biomass, regardless of RSA, and there were several contributors to this response. Firstly, application of P in the Topsoil P treatment would have provided the juvenile root system immediate access to P, whereas young plants were deprived of P in the deeper P-band placement until their root system had grown through the low-P zone and encountered the P band. Secondly, the dispersed placement of P throughout the 10 cm soil layer meant the volume of soil enriched with P fertiliser and available for contact with roots was orders of magnitude greater than when P was placed in a discrete row of granules. The smaller contact area reduces the probability of efficient root exploitation of P fertiliser and would thus have limited P uptake (Eghball and Sander 1989). As a result, early crop growth and development were reduced until roots were able to extract P from the P band and improve plant P status, but the early P deficit was too large for the crop to still achieve growth close to its potential (Grant et al. 2001). Additionally, it may also be hypothesised that a maximum root density may have been achieved locally in the vicinity of the P band, limiting P uptake by increased root competition with roots of the same plant and of neighbouring plants (Ge et al. 2000; Lynch 2013). The effects of this intense competition may be accentuated by rapid localised depletion of soil water, with rapid drying as a result of root activity further limiting the ability of roots to extract more P from the band.
For both genotypes, root biomass generally declined in the layers below the topsoil, but a clear fertilisation effect remained relative to the control. This was particularly the case for the Topsoil P and Mixed P treatments, in which the application of fertiliser P improved root biomass and RLD at depth (lysimeter study) and projected root area (rhizobox study) relative to the control. Specifically, in the control treatment in the rhizoboxes, the Narrow genotype increased the projected root area compared with that of the Wide genotype, from approx. 19 cm2 to 28 cm2. Although this difference was significant, this increase was overshadowed by the effect of the fertiliser applications, with Mixed P doubling (for Wide) and tripling (for Narrow) the projected root areas compared with those oberved in the control treatment. In other words, the Narrow genotype particularly expressed an increased root growth at depth when it had access to P; and the effect of plant access to P was a proportionally greater driver of root distribution compared with the effect of genetic differences between the genotypes. We interpret this as improved crop P access supporting greater vegetative growth, in turn leading to an overall larger root system. In effect, improving plant access to P in a P limited environment may do more to promote deep rooting than choice of a (narrow) genotype. Subsequently, improved root growth will have important implications for water extraction, with a greater and deeper root system increasing potential for water extraction in a drying soil.
Root angle modified spatio-temporal access to phosphorus, but benefits are context-dependent
In the topsoil-P treatment, the increased topsoil exploration by the wide genotype was associated with a small increase in biomass at late tillering, and a significantly greater shoot biomass at anthesis. This suggests that the wide root angle conferred a benefit for improved early growth under stratified P conditions. This is further supported by the differences in shoot biomass between the Topsoil-P vs Mixed-P treatments. In the Mixed-P treatment, P was homogeneously distributed, which would have allowed roots of either genotype to take up P anywhere in the soil profile and thus for the plants to grow to their potential. In contrast, low subsoil P availability in the Topsoil-P treatment would have limited P uptake by roots growing in deeper layers, which presumably led to the reduced biomass as observed for the Narrow genotype. On the other hand, tissue P concentrations and total P uptake under Topsoil P were reduced to similar levels for both genotypes, suggesting P availability remained a limiting factor in this treatment, and that the relative benefit of shallow roots was limited under these conditions. In part, this may also be because the narrow genotype was able to respond plastically and achieve a high root density in the topsoil later in the growing season.
Compared to the Mixed treatment, the decline in total plant biomass under Topsoil-P was relatively small, but there was a pronounced difference in P uptake. Due to its low mobility in soil, P tends to be rapidly depleted in the rhizosphere by root uptake. This establishes a P-diffusion gradient that can supply P from the bulk soil to the root surface, but low diffusion rates in soil limit the amount of P that can be accessed in this manner (Richardson et al. 2009). Much of plant P uptake, therefore, occurs by young roots growing into unexplored soil that has not yet been exploited. In the Topsoil-P treatment, the high initial P availability in the top 10 cm would have allowed rapid early root P accumulation, depleting P from the soil solution in their immediate vicinity. As plant growth continued, new roots would have grown into deeper layers (> 10 cm depth) with lower P availability, limiting the capacity to take up additional P compared with young roots growing into high-P soil in the Mixed-P treatment.
When P was banded, the narrow genotype had a greater projected root area in the layer with the P band, a greater total P uptake at late tillering, and it tended to have greater total plant biomass at late tillering. Furthermore, although at anthesis shoot biomass, P uptake and total tiller number were similar between the two genotypes, a substantial proportion of tillers of the wide genotype were not yet flowering. Under conditions of P stress, cereals tend to slow the rate of development from emergence to anthesis, which enhances the chances of producing viable seeds (Elliott et al. 1997; Rodríguez et al. 1999). The differences in early growth and phenotypic delay at anthesis indicate that the narrow genotype was able to take up P from the banded fertiliser sooner than the wide genotype, presumably because its steeper root system allowed it to reach the P band more rapidly. Acute P deficiency tends to irreversibly reduce tillering, proportionally to the duration of P deficiency (Carstensen et al. 2018; Elliott et al. 1997; Rodríguez et al. 1999). Thus, substantial delays in access to the banded P fertiliser would have further reduced tiller number closer to that of the control treatment.
These data indicate that even if total P uptake at flowering or harvest is similar, RSA may still have important implications for crop success through effects on crop development. In particular, the balance between crop water demand and seasonal supply is an important determinant of crop yields (Hammer et al. 2014). Longer vegetative growth duration may support greater yields if water is plentiful, but it may also increase the risk of terminal drought, limiting plant capacity to fill grain in dryer years (Hammer 2006; Manschadi et al. 2006). On the other hand, an increased period of vegetative growth may also provide the crop with more time to develop roots that extend deeper into the soil profile, which may increase the potential to extract water from the soil during grain filling (Lilley and Kirkegaard 2007).
Topsoil drying diminished genotypic impact of root distribution
The premise for deep banding of P is that topsoil drying (i.e. limited rainfall) restricts plant access to stratified shallow P fertiliser applications (Raymond et al. 2021; Strong et al. 1997), with deep bands allowing continued crop P uptake under these conditions (Singh et al. 2005). Accordingly, topsoil drying reduced crop growth for both genotypes, but there were important interactions with both genotype and P placement. When P was located in the top 10 cm soil layer, drying caused the largest reduction in biomass in the wide genotype, which was associated with reduced root biomass and RLD in this layer. As a result, topsoil drying limited the genotypic advantage over the narrow genotype as was observed under ‘wet’ irrigation. This agrees with the assumption that root architectural trade-offs exist for soil exploration for P and water when they are differentially localised in the soil profile, and that shallow root systems may be relatively more prone to drought sensitivity (Ho et al. 2004; Lynch 2011). However, ample subsoil water was available in this experiment and the wide genotype was able to sustain similar levels of crop growth to the narrow genotypes in all topsoil drying conditions.
Amongst all treatments, the response to drying by plants growing in the low-P control was an exception, with plant and root biomass not showing a substantial decrease as a result of the drying topsoil. We explain this as plants growing in the control treatment being severely P deprived, which already strongly limited their growth under the well-watered conditions. Plant transpiration demand would have been much reduced because of the high P stress, such that the topsoil water reserves sufficed to supply water until well after irrigation was stopped.
When P was banded, the reductions in biomass and P uptake due to topsoil drying were proportionally smaller, such that total P uptake was greater under banded P than under topsoil P. Similar responses to deep P bands have previously been observed on soils with high topsoil P availability, with these responses often being more pronounced in seasons with sparse or irregular rainfall (Bell et al. 2012). In these conditions, deep P bands that stay moist for longer can sustain crop P uptake when access to the topsoil is limited, though the difference between the P-band and Topsoil-P treatments with constant moist irrigation indicate that in this low-P soil, a single deep band could not compensate for P availability in the topsoil.
The drying profile forced plants to produce more roots below the topsoil to find moisture. In particular, root biomass and RLD increased in the 40–50 cm layer. This is consistent with many studies that have reported drought-induced increases of root growth in the subsurface (Alahmad et al. 2019; Benjamin and Nielsen 2006; Wang et al. 2009). This trend included the plants growing in the Topsoil P treatments, which were able to quickly adapt and allocate resources to root growth in subsoil layers. Furthermore, drying increased SRL in the topsoil, but not in layers below. Greater SRL has previously been associated with maintaining plant productivity under water scarcity, as a result of improved water acquisition despite reduced plant carbon investment (Lynch et al. 2005).
Plastic responses to phosphorus placement
For both genotypes, the projected root area at late tillering and root biomass and RLD at anthesis were greatest in the 0–10 cm topsoil layer when P was placed in this zone and not elsewhere in the profile (i.e. the Topsoil P treatment). The ability of root systems to deploy roots and increase RLD in nutrient-rich zones is an important adaptation to enhance the capture of scarce resources (Hodge 2004), and studies of P responses in wheat roots have shown proliferation specifically in zones with fertiliser P application and P bands (Ma et al. 2007; Officer et al. 2009; Rose et al. 2009). Similarly, root responses at anthesis showed a relative increase of root biomass, RLD and SRL in the 20–30 cm layer in the P-band treatment. In combination with the rhizobox observations, this is consistent with the general understanding that proliferation responses involve the initiation of a large number of lateral roots in nutrient-rich zones (Hodge 2004).
It has previously been questioned whether plastic root proliferation responses may affect the broader distribution of roots (van der Bom et al. 2020). For example, if fertiliser P is placed as a shallow band, a plastic response in this zone may potentially lead to a relatively poorer exploration of other (deeper) soil domains. However, our data indicate that P-induced root proliferation is a local response that does not reduce the deployment of roots elsewhere in the soil profile. For example, at anthesis, plants responding to a deep-P band tended to have greater root biomass and RLD in the top 0–10 cm soil layer and at depth (40–50 cm) compared with those growing in the control treatments. Hence, the proliferation of roots around the P band allowed plants to take up more P and boosted plant growth such that it led to an overall larger root system. Under field conditions, this ability of plant root systems to locally respond to patches of high P availability and improve vegetative growth may also have benefits in terminal drought environments, in which a greater root system may allow plants to access deeper soil water that would otherwise be out of reach. Though ultimately the potential benefits will also depend on the seasonal conditions such as the amount of in-crop rainfall and its synchrony with crop phenology.
Conclusions
Genotypes with selected root traits may confer advantages in some environments, but the exact benefits will strongly depend on the interactions of the plant root system with its environment (e.g. water, nutrient availability and distribution) and crop system management (e.g. fertiliser management, planting density). In the current study, (early) P access was the major driver for crop growth, with P scarcity affecting crop growth, phenology, and the relative contributions of root angle to crop resource uptake. Further, the responses to topsoil drying were illustrative of the occurrence of spatio-temporal trade-offs between efficient resource capture and pre-defined root ideotypes. The results suggest that the relative ability of crop root systems to effectively exploit zones or patches of high P availability – such as stratified P or a deep P fertiliser band—will be important for crop productivity in complex systems with multiple constraints, although water availability (either seasonal or due to root competition) may limit the relative effectiveness of crops relying on this strategy. Deployment of genotypes with ‘designer’ RSA should consider the local environmental constraints and need to efficiently capture multiple scarce resources in synchrony with crop phenology.
Data Availability
The data generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RSA:
-
Root system architecture
- RLD:
-
Root length density
- SRL:
-
Specific root length
- P:
-
Phosphorus
References
Alahmad S, El Hassouni K, Bassi FM, Dinglasan E, Youssef C, Quarry G, Aksoy A, Mazzucotelli E, Juhasz A, Able JA, Christopher J, Voss-Fels KP, Hickey LT (2019) A Major Root Architecture QTL Responding to Water Limitation in Durum Wheat. Front Plant Sci 10:436. https://doi.org/10.3389/fpls.2019.00436
Alahmad S, Kang Y, Dinglasan E, Mazzucotelli E, Voss-Fels KP, Able JA, Christopher J, Bassi FM, Hickey LT (2020) Adaptive Traits to Improve Durum Wheat Yield in Drought and Crown Rot Environments. Int J Mol Sci 21:5260. https://doi.org/10.3390/ijms21155260
Alahmad S, Kang Y, Dinglasan E, Jambuthenne D, Robinson H, Tao Y, Able JA, Christopher J, Voss-Fels KP, Bassi FM, Hickey LT (2022) A multi-reference parent nested-association mapping population to dissect the genetics of quantitative traits in durum wheat. Genet Resour Crop Evol 15:1–5. https://doi.org/10.1007/s10722-022-01515-2
Angus J, Bell M, McBeath T, Scanlan C (2019) Nutrient-management challenges and opportunities in conservation agriculture. In: J Pratley, J Kirkegaard (eds) Australian agriculture in 2020: From conservation to automation. Australian Society for Agronomy, Wagga Wagga, Australia
Bai C, Ge Y, Ashton RW, Evans J, Milne A, Hawkesford MJ, Whalley WR, Parry MAJ, Melichar J, Feuerhelm D, Basler PB, Bartsch M (2019) The relationships between seedling root screens, root growth in the field and grain yield for wheat. Plant Soil 440:311–326. https://doi.org/10.1007/s11104-019-04088-9
Bell M, Lester D, Smith L, Want P (2012) Increasing complexity in nutrient management on clay soils in the northern grain belt – nutrient stratification and multiple nutrient limitations. In: I Yunusa (ed) Capturing opportunities and overcoming obstacles in Australian agronomy 16th Australian Agronomy Conference, Armidale, NSW.
Benjamin JG, Nielsen DC (2006) Water deficit effects on root distribution of soybean, field pea and chickpea. Field Crops Res 97:248–253. https://doi.org/10.1016/j.fcr.2005.10.005
Carstensen A, Szameitat AE, Frydenvang J, Husted S (2018) Chlorophyll a fluorescence analysis can detect phosphorus deficiency under field conditions and is an effective tool to prevent grain yield reductions in spring barley (Hordeum vulgare L.). Plant Soil 434:79–91. https://doi.org/10.1007/s11104-018-3783-6
Chen YL, Palta J, Clements J, Buirchell B, Siddique KHM, Rengel Z (2014) Root architecture alteration of narrow-leafed lupin and wheat in response to soil compaction. Field Crops Res 165:61–70. https://doi.org/10.1016/j.fcr.2014.04.007
Christopher J, Christopher M, Jennings R, Jones S, Fletcher S, Borrell A, Manschadi AM, Jordan D, Mace E, Hammer G (2013) QTL for root angle and number in a population developed from bread wheats (Triticum aestivum) with contrasting adaptation to water-limited environments. Theor Appl Genet 126:1563–1574. https://doi.org/10.1007/s00122-013-2074-0
Eghball B, Sander DH (1989) Distance and Distribution Effects of Phosphorus Fertilizer on Corn. Soil Sci Soc Am J 53:282–287. https://doi.org/10.2136/sssaj1989.03615995005300010051x
El Hassouni K, Alahmad S, Belkadi B, Filali-Maltouf A, Hickey LT, Bassi FM (2018) Root System Architecture and Its Association with Yield under Different Water Regimes in Durum Wheat. Crop Sci 58:2331–2346. https://doi.org/10.2135/cropsci2018.01.0076
Elliott DE, Reuter DJ, Reddy GD, Abbott RJ (1997) Phosphorus nutrition of spring wheat (Triticum aestivum L.). 1. Effects of phosphorus supply on plant symptoms, yield, components of yield, and plant phosphorus uptake. Aust J Agric Res 48:855–867. https://doi.org/10.1071/a96159
FAO (2015) World reference base for soil resources 2014. International soil classification system for naming soils and creating legends for soil maps. Update 2015.
Gao Y, Lynch JP (2016) Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). J Exp Bot 67:4545–4557. https://doi.org/10.1093/jxb/erw243
Ge Z, Rubio G, Lynch JP (2000) The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: results from a geometric simulation model. Plant Soil 218:159–171. https://doi.org/10.1023/A:1014987710937
Grant CA, Flaten DN, Tomasiewicz DJ, Sheppard SC (2001) The importance of early season phosphorus nutrition. Can J Plant Sci 81:211–224. https://doi.org/10.4141/p00-093
Hendriks PW, Kirkegaard JA, Lilley JM, Gregory PJ, Rebetzke GJ (2016) A tillering inhibition gene influences root–shoot carbon partitioning and pattern of water use to improve wheat productivity in rainfed environments. J Exp Bot 67:327–340. https://doi.org/10.1093/jxb/erv457
Ho MD, McCannon BC, Lynch JP (2004) Optimization modeling of plant root architecture for water and phosphorus acquisition. J Theor Biol 226:331–340. https://doi.org/10.1016/j.jtbi.2003.09.011
Ho MD, Rosas JC, Brown KM, Lynch JP (2005) Root architectural tradeoffs for water and phosphorus acquisition. Funct Plant Biol 32:737–748. https://doi.org/10.1071/FP05043
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24. https://doi.org/10.1111/j.1469-8137.2004.01015.x
Isbell R (2016) Australian Soil Classification. CSIRO Publishing.
Jia X, Liu P, Lynch JP (2018) Greater lateral root branching density in maize improves phosphorus acquisition from low phosphorus soil. J Exp Bot 69:4961–4970. https://doi.org/10.1093/jxb/ery252
Jin K, Shen J, Ashton RW, Dodd IC, Parry MA, Whalley WR (2013) How do roots elongate in a structured soil? J Exp Bot 64:4761–4777. https://doi.org/10.1093/jxb/ert286
Kirkegaard JA, Conyers MK, Hunt JR, Kirkby CA, Watt M, Rebetzke GJ (2014) Sense and nonsense in conservation agriculture: Principles, pragmatism and productivity in Australian mixed farming systems. Agric Ecosyst Environ 187:133–145. https://doi.org/10.1016/j.agee.2013.08.011
Lenth RV (2021) emmeans: Estimated Marginal Means, aka Least-Squares Means R package version 1.7.0.
Liao H, Rubio G, Yan X, Cao A, Brown KM, Lynch JP (2001) Effect of phosphorus availability on basal root shallowness in common bean. Plant Soil 232:69–79. https://doi.org/10.1023/A:1010381919003
Lilley JM, Kirkegaard JA (2007) Seasonal variation in the value of subsoil water to wheat: simulation studies in southern New South Wales. Aust J Agric Res 58:1115–1128. https://doi.org/10.1071/ar07046
Lynch JP (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156:1041–1049. https://doi.org/10.1104/pp.111.175414
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112:347–357. https://doi.org/10.1093/aob/mcs293
Lynch JP (2019) Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytol 223:548–564. https://doi.org/10.1111/nph.15738
Lynch JP, Brown KM (2001) Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant Soil 237:225–237. https://doi.org/10.1023/a:1013324727040
Lynch JP, Ho MD, Phosphorus L (2005) Rhizoeconomics: Carbon costs of phosphorus acquisition. Plant Soil 269:45–56. https://doi.org/10.1007/s11104-004-1096-4
Ma Q, Rengel Z, Bowden B (2007) Heterogeneous distribution of phosphorus and potassium in soil influences wheat growth and nutrient uptake. Plant Soil 291:301–309. https://doi.org/10.1007/s11104-007-9197-5
Ma Q, Rengel Z, Rose T (2009) The effectiveness of deep placement of fertilisers is determined by crop species and edaphic conditions in Mediterranean-type environments: a review. Soil Research 47:19–32. https://doi.org/10.1071/sr08105
Manschadi AM, Christopher J, deVoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments J Functional Plant Biology 33:823–837. https://doi.org/10.1071/FP06055
Manschadi AM, Hammer GL, Christopher JT, deVoil P (2008) Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303:115–129. https://doi.org/10.1007/s11104-007-9492-1
Miguel MA, Postma JA, Lynch JP (2015) Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiol 167:1430–1439. https://doi.org/10.1104/pp.15.00145
Officer SJ, Dunbabin VM, Armstrong RD, Norton RM, Kearney GA (2009) Wheat roots proliferate in response to nitrogen and phosphorus fertilisers in Sodosol and Vertosol soils of south-eastern Australia. Soil Res 47:91–102
Oyanagi A, Nakamoto T, Wada M (1993) Relationship between Root Growth Angle of Seedlings and Vertical Distribution of Roots in the Field in Wheat Cultivars. Jpn J Crop Sci 62:565–570. https://doi.org/10.1626/jcs.62.565
Passioura JB (2010) Scaling up: the essence of effective agricultural research. Funct Plant Biol 37:585–591
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2020) nlme: Linear and Nonlinear Mixed Effects Models. R package version 31–148.
Postma JA, Dathe A, Lynch JP (2014) The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol 166:590–602. https://doi.org/10.1104/pp.113.233916
Postma JA, Lynch JP (2012) Complementarity in root architecture for nutrient uptake in ancient maize/bean and maize/bean/squash polycultures. Ann Bot 110:521–534. https://doi.org/10.1093/aob/mcs082
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ramalingam P, Kamoshita A, Deshmukh V, Yaginuma S, Uga Y (2017) Association between root growth angle and root length density of a near-isogenic line of IR64 rice with DEEPER ROOTING 1 under different levels of soil compaction. Plant Production Science 20:162–175. https://doi.org/10.1080/1343943x.2017.1288550
Rangarajan H, Postma JA, Lynch JP (2018) Co-optimization of axial root phenotypes for nitrogen and phosphorus acquisition in common bean. Ann Bot 122:485–499. https://doi.org/10.1093/aob/mcy092
Raymond N, Kopittke PM, Wang E, Lester D, Bell MJ (2021) Does the APSIM model capture soil phosphorus dynamics? A case study with Vertisols. Field Crops Res 273:108302 https://doi.org/10.1016/j.fcr.2021.108302
Raymond NS, Jensen LS, Müller Stöver D (2018) Enhancing the phosphorus bioavailability of thermally converted sewage sludge by phosphate-solubilising fungi. Ecol Eng 120:44–53. https://doi.org/10.1016/j.ecoleng.2018.05.026
Rich SM, Christopher J, Richards R, Watt M (2020) Root phenotypes of young wheat plants grown in controlled environments show inconsistent correlation with mature root traits in the field. J Exp Bot 71:4751–4762. https://doi.org/10.1093/jxb/eraa201
Rich SM, Watt M (2013) Soil conditions and cereal root system architecture: review and considerations for linking Darwin and Weaver. J Exp Bot 64:1193–1208. https://doi.org/10.1093/jxb/ert043
Richard C, Christopher J, Chenu K, Borrell A, Christopher M, Hickey L (2018) Selection in Early Generations to Shift Allele Frequency for Seminal Root Angle in Wheat. Plant Genome 11 https://doi.org/10.3835/plantgenome2017.08.0071
Richard CA, Hickey LT, Fletcher S, Jennings R, Chenu K, Christopher JT (2015) High-throughput phenotyping of seminal root traits in wheat. Plant Methods 11:13. https://doi.org/10.1186/s13007-015-0055-9
Richards RA, Passioura JB (1989) A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain yield in rain-fed environments. Aust J Agric Res 40:943–950
Richardson AE, Hocking PJ, Simpson RJ, George TS (2009) Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci 60:124–143. https://doi.org/10.1071/cp07125
Rodríguez D, Andrade FH, Goudriaan J (1999) Effects of phosphorus nutrition on tiller emergence in wheat. Plant Soil 209:283–295. https://doi.org/10.1023/a:1004690404870
Rose TJ, Hardiputra B, Rengel Z (2009) Wheat, canola and grain legume access to soil phosphorus fractions differs in soils with contrasting phosphorus dynamics. Plant Soil 326:159–170. https://doi.org/10.1007/s11104-009-9990-4
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Severini AD, Wasson AP, Evans JR, Richards RA, Watt M (2020) Root phenotypes at maturity in diverse wheat and triticale genotypes grown in three field experiments: Relationships to shoot selection, biomass, grain yield, flowering time, and environment. Field Crops Res 255:107870 https://doi.org/10.1016/j.fcr.2020.107870
Singh DK, Sale PWG, Routley RR (2005) Increasing phosphorus supply in subsurface soil in northern Australia: Rationale for deep placement and the effects with various crops. Plant Soil 269:35–44. https://doi.org/10.1007/s11104-004-2475-6
Singh V, van Oosterom EJ, Jordan DR, Messina CD, Cooper M, Hammer GL (2010) Morphological and architectural development of root systems in sorghum and maize. Plant Soil 333:287–299. https://doi.org/10.1007/s11104-010-0343-0
Strong WM, Best EK, Cooper JE (1997) Phosphate fertiliser residues in wheat-growing soils of the Western Downs, Queensland. Soil Res 35:341–354. https://doi.org/10.1071/s96038
Sun B, Gao Y, Lynch JP (2018) Large Crown Root Number Improves Topsoil Foraging and Phosphorus Acquisition. Plant Physiol 177:90–104. https://doi.org/10.1104/pp.18.00234
Trachsel S, Kaeppler SM, Brown KM, Lynch JP (2011) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 341:75–87. https://doi.org/10.1007/s11104-010-0623-8
van der Bom FJT, Williams A, Bell MJ (2020) Root architecture for improved resource capture: trade-offs in complex environments. J Exp Bot 71:5752–5763. https://doi.org/10.1093/jxb/eraa324
van der Ploeg RR, Böhm W, Kirkham MB (1999) On the Origin of the Theory of Mineral Nutrition of Plants and the Law of the Minimum. Soil Sci Soc Am J 63:1055–1062. https://doi.org/10.2136/sssaj1999.6351055x
Wang X, Tang C, Guppy CN, Sale PWG (2009) The role of hydraulic lift and subsoil P placement in P uptake of cotton (Gossypium hirsutum L.). Plant Soil 325:263–275. https://doi.org/10.1007/s11104-009-9977-1
Wasson AP, Richards RA, Chatrath R, Misra SC, Prasad SVS, Rebetzke GJ, Kirkegaard JA, Christopher J, Watt M (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 63:3485–3498
Watt M, Moosavi S, Cunningham SC, Kirkegaard JA, Rebetzke GJ, Richards RA (2013) A rapid, controlled-environment seedling root screen for wheat correlates well with rooting depths at vegetative, but not reproductive, stages at two field sites. Ann Bot 112:447–455. https://doi.org/10.1093/aob/mct122
Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M, Pedersen T, Miller E, Bache S, Müller K, Ooms J, Robinson D, Seidel D, Spinu V, Takahashi K, Vaughan D, Wilke C, Woo K, Yutani H (2019) Welcome to the Tidyverse. J Open Source Softw 4:1686. https://doi.org/10.21105/joss.01686
Zhu J, Kaeppler SM, Lynch JP (2005) Topsoil foraging and phosphorus acquisition efficiency in maize (Zea mays). Funct Plant Biol 32:749–762. https://doi.org/10.1071/FP05005
Zhu J, Lynch JP (2004) The contribution of lateral rooting to phosphorus acquisition efficiency in maize (Zea mays) seedlings. Funct Plant Biol 31:949–958. https://doi.org/10.1071/FP04046
Acknowledgements
We would like to acknowledge Tim Rossignol and Bec Archer of the School of Agriculture and Food Science for valuable technical assistance during the preparation and conducting of the experiment. We thank Celso Cordova, Andrew Hughes and Meghan Barnard for their help with experimental preparations, harvesting and processing of plant samples. Soil collection was supported by David Lester at the Department of Agriculture and Fisheries (DAF), Queensland.
Funding
This research was made possible by funding from the Grains Research and Development Corporation of Australia (Project no. UOQ1805-005RTX). Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
Conceptualisation: FB, AW, LH, MB; Methodology: FB, AW, VS, MB; Investigation: FB, NR, SA, VS; Resources: SA and LH; Formal Analysis: FB, NR, SA; Writing – Original Draft Preparation: FB; Writing – Review & Editing: all.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Yinglong Chen.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Bom, F.J.T., Williams, A., Raymond, N.S. et al. Root angle, phosphorus, and water: Interactions and effects on durum wheat genotype performance in drought-prone environments. Plant Soil (2023). https://doi.org/10.1007/s11104-023-05966-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-023-05966-z