Abstract
Photosynthetic fluorescence emission spectra measurement at the temperature of 77 K (–196°C) is an often-used technique in photosynthesis research. At low temperature, biochemical and physiological processes that modulate fluorescence are mostly abolished, and the fluorescence emission of both PSI and PSII become easily distinguishable. Here we briefly review the history of low-temperature chlorophyll fluorescence methods and the characteristics of the acquired emission spectra in oxygen-producing organisms. We discuss the contribution of different photosynthetic complexes and physiological processes to fluorescence emission at 77 K in cyanobacteria, green algae, heterokont algae, and plants. Furthermore, we describe practical aspects for obtaining and presenting 77 K fluorescence spectra.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Abbreviations
- Chl:
-
chlorophyll
- LHC:
-
light-harvesting complex
- PSI:
-
photosystem I
- PSII:
-
photosystem II
References
Alboresi A., Le Quiniou C., Yadav S.K.N. et al.: Conservation of core complex subunits shaped the structure and function of photosystem I in the secondary endosymbiont alga Nannochloropsis gaditana.–New Phytol. 213: 714–726, 2017.
Allen J.F.: Protein phosphorylation in regulation of photosynthesis.–BBA-Bioenergetics 1098: 275–335, 1992.
Andrizhiyevskaya E.G., Chojnicka A., Bautista J.A. et al.: Origin of the F685 and F695 fluorescence in photosystem II.–Photosynth. Res. 84: 173–180, 2005.
Ben-Shem A., Frolow F., Nelson N.: Crystal structure of plant photosystem I.–Nature 426: 630–635, 2003.
Berkaloff C., Caron L., Rousseau B.: Subunit organization of PSI particles from brown algae and diatoms: polypeptide and pigment analysis.–Photosynth. Res. 23: 181–193, 1990.
Bibby T.S., Nield J., Barber J.: Iron deficiency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria.–Nature 412: 743–745, 2001a.
Bibby T.S., Nield J., Barber J.: Three-dimensional model and characterization of the iron stress-induced CP43′-photosystem I supercomplex isolated from the cyanobacterium Synechocystis PCC 6803.–J. Biol. Chem. 276: 43246–43252, 2001b.
Biggins J., Bruce D.: Regulation of excitation energy transfer in organisms containing phycobilins.–Photosynth. Res. 20: 1–34, 1989.
Bína D., Gardian Z., Herbstová M., Litvín R.: Modular antenna of photosystem I in secondary plastids of red algal origin: a Nannochloropsis oceanica case study.–Photosynth. Res. 131: 255–266, 2017.
Björkman O., Demmig B.: Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins.–Planta 170: 489–504, 1987.
Boardman N., Thorne S., Anderson J.: Fluorescence properties of particles obtained by digitonin fragmentation of spinach chloroplasts.–P. Natl. Acad. Sci. USA 56: 586–593, 1966.
Boehm M., Romero E., Reisinger V. et al.: Investigating the early stages of Photosystem II assembly in Synechocystis sp. PCC 6803 isolation of CP47 and CP43 complexes.–J. Biol. Chem. 286: 14812–14819, 2011.
Boehm M., Yu J., Reisinger V. et al.: Subunit composition of CP43-less photosystem II complexes of Synechocystis sp. PCC 6803: implications for the assembly and repair of photosystem II.–Philos. T. R. Soc. B 367: 3444–3454, 2012.
Boekema E.J., Dekker J.P., van Heel M.G. et al.: Evidence for a trimeric organization of the photosystem I complex from the thermophilic cyanobacterium Synechococcus sp.–FEBS Lett. 217: 283–286, 1987.
Boekema E.J., Hifney A., Yakushevska A.E., Piotrowski M.: A giant chlorophyll-protein complex induced by iron deficiency in cyanobacteria.–Nature 412: 745–758, 2001.
Bonaventura C., Myers J.: Fluorescence and oxygen evolution from Chlorella pyrenoidosa.–BBA-Bioenergetics 189: 366–383, 1969.
Brewster D.: On the colours of natural bodies.–Earth Env. Sci. T. R. So. 12: 538–545, 1834.
Brody S.: New excited state of chlorophyll.–Science 128: 838–839, 1958.
Burnap R.L., Troyan T., Sherman L.A.: The highly abundant chlorophyll-protein complex of iron-deficient Synechococcus sp. PCC7942 (CP43 [prime]) is encoded by the isiA gene.–Plant Physiol. 103: 893–902, 1993.
Busch A., Nield J., Hippler M.: The composition and structure of photosystem Iassociated antenna from Cyanidioschyzon merolae.–Plant J. 62: 886–897, 2010.
Butler W.L.: Chlorophyll fluorescence: a probe for electron transfer and energy transfer.–In: Trebst A., Avron M. (ed.): Photosynthesis I. Pp. 149–167. Springer, Berlin–Heidelberg 1977.
Cardona T.: Reconstructing the origin of oxygenic photosynthesis: Do assembly and photoactivation recapitulate evolution?–Front. Plant Sci. 7: 257, 2016.
Cavalier Smith T.O.M.: Principles of protein and lipid targeting in secondary symbiogenesis: euglenoid, dinoflagellate, and sporozoan plastid origins and the eukaryote family tree.–J. Eukaryot. Microbiol. 46: 347–366, 1999.
Chen M., Blankenship R.E.: Expanding the solar spectrum used by photosynthesis.–Trends Plant Sci. 16: 427–431, 2011.
Chen M., Li Y., Birch D., Willows R.D.: A cyanobacterium that contains chlorophyll f–a redabsorbing photopigment.–FEBS Lett. 586: 3249–3254, 2012.
Cho F., Spencer J.: Emission spectra of Chlorella at very low temperatures (−269° to −196°).–Biochim. Biophys. Acta 126: 174–176, 1966.
Cho F.: Low-temperature (4–77° K) spectroscopy of Anacystis; temperature dependence of energy transfer efficiency.–BBABioenergetics 216: 151–161, 1970a.
Cho F.: Low-temperature (4–77° K) spectroscopy of Chlorella; temperature dependence of energy transfer efficiency.–BBABioenergetics 216: 139–150, 1970b.
Clayton R.K.: Photosynthesis: Physical Mechanisms and Chemical Patterns. Vol. 4. Pp. 19–35. Cambridge University Press, Cambridge 1980.
Cordón G.B., Lagorio M.G.: Re-absorption of chlorophyll fluorescence in leaves revisited. A comparison of correction models.–Photochem. Photobio. S. 5: 735–740, 2006.
Croce R., Zucchelli G., Garlaschi F.M., Jennings R.C.: A thermal broadening study of the antenna chlorophylls in PSI-200, LHCI, and PSI core.–Biochemistry 37: 17355–17360, 1998.
Dau H.: Molecular mechanisms and quantitative models of variable photosystem II fluorescence.–Photochem Photobiol 60: 1–23, 1994a.
Dau H.: New trends in photobiology: Short-term adaptation of plants to changing light intensities and its relation to Photosystem II photochemistry and fluorescence emission.–J. Photoch. Photobio. B 26: 3–27, 1994b.
de Marsac N.T.: Phycobiliproteins and phycobilisomes: the early observations.–Photosynth. Res. 76: 193–205, 2003.
Dekker J., Hassoldt A., Petterson A. et al.: On the nature of the F695 and F685 emission of photosystem II.–In: Mathis P. (ed.): Photosynthesis: From Light to Biosphere, Vol III. Pp. 53–56. Kluwer, Dordrecht 1995.
Dietzel L., Bräutigam K., Steiner S. et al.: Photosystem II supercomplex remodeling serves as an entry mechanism for state transitions in Arabidopsis.–Plant Cell 23: 2964–2977, 2011.
Dong C., Tang A., Zhao J. et al.: ApcD is necessary for efficient energy transfer from phycobilisomes to photosystem I and helps to prevent photoinhibition in the cyanobacterium Synechococcus sp. PCC 7002.–BBA-Bioenergetics 1787: 1122–1128, 2009.
Drop B., Webber-Birungi M., Yadav S.K.N. et al.: Lightharvesting complex II (LHCII) and its supramolecular organization in Chlamydomonas reinhardtii.–BBA-Bioenergetics 1837: 63–72, 2014.
Duysens L., Sweers H.: Mechanism of two photochemical reactions in algae as studied by means of fluorescence.–In: Tamiya H. (ed.): Studies on Microalgae and Photosynthetic Bacteria. Pp. 353–372. University of Tokyo Press, Tokyo 1963.
Eaton-Rye J.J., Sobotka R.: Assembly of the photosystem II membrane-protein complex of oxygenic photosynthesis.–Front. Plant Sci. 8: 884, 2017.
El Bissati K., Delphin E., Murata N. et al.: Photosystem II fluorescence quenching in the cyanobacterium Synechocystis PCC 6803: involvement of two different mechanisms.–BBABioenergetics 1457: 229–242, 2000.
Emerson R.: Dependence of yield of photosynthesis in long-wave red on wavelength and intensity of supplementary light.–Science 125: 746, 1957.
Erickson E., Wakao S., Niyogi K.K.: Light stress and photoprotection in Chlamydomonas reinhardtii.–Plant J. 82: 449–465, 2015.
Falk S., Samson G., Bruce D. et al.: Functional analysis of the iron-stress induced CP 43′ polypeptide of PS II in the cyanobacterium Synechococcus sp. PCC 7942.–Photosynth. Res. 45: 51–60, 1995.
Farkas D.L., Malkin S.: Cold storage of isolated class C chloroplasts optimal conditions for stabilization of photosynthetic activities.–Plant Physiol. 64: 942–947, 1979.
Förster T.: Delocalizing Excitation and Excitation Transfer. Modern Quantum Chemistry Istanbul Lectures. Pp. 93–137. Academic Press, New York 1965.
Franck F., Juneau P., Popovic R.: Resolution of the photosystem I and photosystem II contributions to chlorophyll fluorescence of intact leaves at room temperature.–BBA-Bioenergetics 1556: 239–246, 2002.
Frank H.A., Cua A., Chynwat V. et al.: Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis.–Photosynth. Res. 41: 389–395, 1994.
Galka P., Santabarbara S., Khuong T.T.H. et al.: Functional analyses of the plant photosystem I–light-harvesting complex II supercomplex reveal that light-harvesting complex ii loosely bound to photosystem ii is a very efficient antenna for photosystem I in state II.–Plant Cell 24: 2963–2978, 2012.
Gan F., Shen G., Bryant D.A.: Occurrence of far-red light photoacclimation (FaRLiP) in diverse cyanobacteria.–Life 5: 4–24, 2015.
Garczarek L., van der Staay G.W.M., Thomas J.C., Partensky F.: Isolation and characterization of Photosystem I from two strains of the marine oxychlorobacterium Prochlorococcus.–Photosynth. Res. 56: 131–141, 1998.
Gardian Z., Bumba L., Schrofel A. et al.: Organisation of photosystem I and photosystem II in red alga Cyanidium caldarium: encounter of cyanobacterial and higher plant concepts.–BBA-Bioenergetics 1767: 725–731, 2007.
Goldschmidt-Clermont M., Bassi R.: Sharing light between two photosystems: mechanism of state transitions.–Curr. Opin. Plant. Biol. 25: 71–78, 2015.
Gouterman M., Wagnière G.H., Snyder L.C.: Spectra of porphyrins: Part II. Four orbital model.–J. Mol. Spectrosc. 11: 108–127, 1963.
Govindjee, Björn L.O.: Evolution of the Z-scheme of photosynthesis: a perspective.–Photosynth. Res. 133: 5–15, 2017.
Govindjee, Ichimura S., Cederstrand C., Rabinowitch E.: Effect of combining far-red light with shorter wave light in the excitation of fluorescence in Chlorella.–Arch. Biochem. Biophys. 89: 322–323, 1960.
Govindjee, Yang L.: Structure of the red fluorescence band in chloroplasts.–J. Gen. Physiol. 49: 763–780, 1966.
Govindjee: Emerson enhancement effect and two light reactions in photosynthesis.–In: Kok B., Jagendorf A.T. (ed.): Photosynthetic Mechanisms of Green Plants. Pp 318. National Academy of Science–National Research Council Publication, Washington DC 1963.
Govindjee: Chlorophyll a fluorescence: a bit of basics and history.–In: Papageorgiou G.C., {ieGovindjee: Chlorophyll a Fluorescence: A Signature of Photosynthesis. Pp. 1–41. Springer, Dordrecht 2004.
Govindjee: Sixty-three years since kautsky: chlorophyll a fluorescence.–Aust. J. Plant Physiol. 22: 131–160, 1995.
Grouneva I., Rokka A., Aro E.-M.: The thylakoid membrane proteome of two marine diatoms outlines both diatom-specific and species-specific features of the photosynthetic machinery.–J. Proteome Res. 10: 5338–5353, 2011.
Gundermann K., Büchel C.: Structure and functional heterogeneity of fucoxanthin-chlorophyll proteins in diatoms.–In: Hohmann-Marriott M.F. (ed.): The Structural Basis of Biological Energy Generation. Pp. 31–27. Springer, Dordrecht 2014.
Havaux M., Guedeney G., Hagemann M. et al.: The chlorophyllbinding protein IsiA is inducible by high light and protects the cyanobacterium Synechocystis PCC6803 from photooxidative stress.–FEBS Lett. 579: 2289–2293, 2005.
Herbstová M., Bína D., Koník P. et al.: Molecular basis of chromatic adaptation in pennate diatom Phaeodactylum tricornutum.–BBA-Bioenergetics 1847: 534–543, 2015.
Hillier W., Babcock G.T.: Photosynthetic reaction centers.–Plant Physiol. 125: 33–37, 2001.
Hipkins M.F., Baker N.R.: Photosynthetic energy transduction: A practical approach.–In: Hipkins M.F., Baker N.R.: Photosynthesis: Energy Transduction, A Practical Approach. Pp 1. IRL Press, Arlington 1986.
Hirsch R.E., Rich M., Govindjee: A tribute to Seymour Steven Brody: in memoriam (November 29, 1927 to May 25, 2010).–Photosynth. Res. 106: 191–199, 2010.
Hofstraat J.W., Rubelowsky K., Slutter S.: Corrected fluorescence excitation and emission spectra of phytoplankton: toward a more uniform approach to fluorescence measurements.–J. Plankton. Res. 14: 625–636, 1992.
Hohmann-Marriott M.F., Blankenship R.E.: Evolution of photosynthesis.–Plant. Physiol. 154: 434–438, 2011.
Hohmann-Marriott M.F., Takizawa K., Eaton-Rye J.J. et al.: The redox state of the plastoquinone pool directly modulates minimum chlorophyll fluorescence yield in Chlamydomonas reinhardtii.–FEBS Lett. 584: 1021–1026, 2010.
Ikeda Y., Komura M., Watanabe M. et al.: Photosystem I complexes associated with fucoxanthin-chlorophyll-binding proteins from a marine centric diatom, Chaetoceros gracilis.–BBA-Bioenergetics 1777: 351–361, 2008.
Irrgang K.D., Boekema E.J., Vater J., Renger G.: Structural determination of the photosystem II core complex from spinach.–FEBS J. 178: 209–217, 1988.
Iwai M., Takahashi Y., Minagawa J.: Molecular remodeling of photosystem II during state transitions in Chlamydomonas reinhardtii.–Plant Cell 20: 2177–2189, 2008.
Järvi S., Suorsa M., Aro E.-M.: Photosystem II repair in plant chloroplasts–regulation, assisting proteins and shared components with photosystem II biogenesis.–BBABioenergetics 1847: 900–909, 2015.
Jordan P., Fromme P., Witt H.T., Klukas O.: Three-dimensional structure of cyanobacterial photosystem I at 2.5 angstrom resolution.–Nature 411: 909–917, 2001.
Joshua S., Mullineaux C.W.: Phycobilisome diffusion is required for light-state transitions in cyanobacteria.–Plant Physiol. 135: 2112–2119, 2004.
Juhas M., Büchel C.: Properties of photosystem I antenna protein complexes of the diatom Cyclotella meneghiniana.–J. Exp. Bot. 63: 3673–3681, 2012.
Kaňa R., Kotabová E., Lukeš M. et al.: Phycobilisome mobility and its role in the regulation of light harvesting in red algae.–Plant Physiol. 165: 1618–1631, 2014.
Karapetyan N.V., Bolychevtseva Y.V., Yurina N.P. et al.: Longwavelength chlorophylls in photosystem I of cyanobacteria: origin, localization, and functions.–Biochemistry 79: 213, 2014.
Kargul J., Nield J., Barber J.: Three-dimensional reconstruction of a light-harvesting complex I-photosystem I (LHCI-PSI) supercomplex from the green alga Chlamydomonas reinhardtii. Insights into light harvesting for PSI.–J. Biol. Chem. 278: 16135–16141, 2003.
Kautsky H., Hirsch A.: [New attempts for carbon dioxide assimilation.]–Naturwissenschaft 19: 964–964, 1931. [In German]
Keeling P.J.: The number, speed, and impact of plastid endosymbioses in eukaryotic evolution.–Annu. Rev. Plant Biol. 64: 583–607, 2013.
Komenda J., Sobotka R., Nixon P.J.: Assembling and maintaining the photosystem II complex in chloroplasts and cyanobacteria.–Curr. Opin. Plant Biol. 15: 245–251, 2012.
Kondo K., Ochiai Y., Katayama M., Ikeuchi M.: The membraneassociated CpcG2-phycobilisome in Synechocystis: a new photosystem I antenna.–Plant Physiol. 144: 1200–1210, 2007.
Krause G.H., Briantais J.M., Vernotte C.: Characterization of chlorophyll fluorescence quenching in chloroplasts by fluorescence spectroscopy at 77 K I. ΔpH-dependent quenching.–BBA-Bioenergetics 723: 169–175, 1983.
Krause G.H., Weis E.: Chlorophyll fluorescence as a tool in plant physiology.–Photosynth. Res. 5: 139–157, 1984.
Krey A., Govindjee: Fluorescence studies on a red alga, Porphyridium cruentum.–Biochim. Biophys. Acta. 120: 1–18, 1966.
Kyle D.J., Ohad I., Arntzen C.J.: Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes.–P. Natl. Acad. Sci. USA 81: 4070–4074, 1984.
La Roche J., van der Staay G.W.M., Partensky F. et al.: Independent evolution of the prochlorophyte and green plant chlorophyll a/b light-harvesting proteins.–P. Natl. Acad. Sci. USA 93: 15244–15248, 1996.
Lakowicz J.R.: Fluorescence polarization.–In: Lakowicz J.R. (ed): Principles of Fluorescence Spectroscopy Pp. 111–153. Springer, Boston 1983a.
Lakowicz J.R.: Quenching of fluorescence.–In: Lakowicz J.R. (ed): Principles of Fluorescence Spectroscopy. Pp. 257–301. Springer, Boston 1983b.
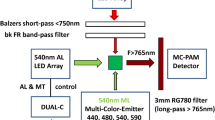
Lamb J., Forfang K., Hohmann-Marriott M.: A practical solution for 77 K fluorescence measurements based on LED excitation and CCD array detector.–PLoS ONE 10: e0132258, 2015.
Laudenbach D.E., Reith M.E., Straus N.A.: Isolation, sequence analysis, and transcriptional studies of the flavodoxin gene from Anacystis nidulans R2.–J. Bacteriol. 170: 258–265, 1988.
Lavaud J., Lepetit B.: An explanation for the inter-species variability of the photoprotective non-photochemical chlorophyll fluorescence quenching in diatoms.–BBA-Bioenergetics 1827: 294–302, 2013.
Lepetit B., Volke D., Szabó M. et al.: Spectroscopic and molecular characterization of the oligomeric antenna of the diatom Phaeodactylum tricornutum.–Biochemistry46: 9813–9822, 20
Ley A.C., Butler W.L.: Energy distribution in the photochemical apparatus of Porphyridium cruentum in state I and state II.–BBA-Bioenergetics 592: 349–363, 1980.
Li D., Xie J., Zhao J. et al.: Light-induced excitation energy redistribution in Spirulina platensis cells: “spillover” or “mobile PBSs”?–BBA-Bioenergetics 1608: 114–121, 2004.
Li H., Yang S., Xie J., Zhao J.: Probing the connection of PBSs to the photosystems in Spirulina platensis by artificially induced fluorescence fluctuations.–J. Lumin. 122-123: 294–296, 2007.
Li M., Semchonok D.A., Boekema E.J., Bruce B.D.: Characterization and evolution of tetrameric photosystem I from the thermophilic cyanobacterium Chroococcidiopsis sp TS-821.–Plant Cell 26: 1230–1245, 2014.
Li Y., Chen M.: Novel chlorophylls and new directions in photosynthesis research.–Funct. Plant Biol. 42: 493–501, 2015.
Litvin F.F., Krasnovsky A.A.: Investigation by fluorescence spectra of intermediate stages of chlorophyll biosynthesis in etiolated leaves.–Dokl. Acad. Nauk+ 117: 106–109, 1957.
Litvín R., Bína D., Herbstová M., Gardian Z.: Architecture of the light-harvesting apparatus of the eustigmatophyte alga Nannochloropsis oceanica.–Photosynth. Res. 130: 137–150, 20
Liu H., Roose J.L., Cameron J.C., Pakrasi H.B.: A genetically tagged Psb27 protein allows purification of two consecutive photosystem II (PSII) assembly intermediates in Synechocystis 6803, a cyanobacterium.–J. Biol. Chem. 286: 24865–24871, 2011.
Liu H., Zhang H., Niedzwiedzki D.M. et al.: Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria.–Science 342: 1104–1107, 2013.
Marx A., David L., Adir N.: Piecing together the phycobilisome.–In: Hohmann-Marriott M.F. (ed): The Structural Basis of Biological Energy Generation. Pp. 59–76. Springer, Dordrecht 2014.
Maxwell K., Johnson G.N.: Chlorophyll fluorescence–a practical guide.–J. Exp. Bot. 51: 659–668, 2000.
Mazor Y., Borovikova A., Nelson N.: The structure of plant photosystem I super-complex at 2.8 Å resolution.–Elife 4: e07433, 2015.
McConnell M.D., Koop R., Vasil’ev, S., Bruce D.: Regulation of the distribution of chlorophyll and phycobilin-absorbed excitation energy in cyanobacteria. A structure-based model for the light state transition.–Plant Physiol. 130: 1201–1212, 2002.
McCormac D.J., Marwood C.A., Bruce D., Greenberg B.M.: Assembly of Photosystem I and II during the early phases of lightinduced development of chloroplasts from proplastids in Spirodela oligorrhiza.–Photochem. Photobiol. 63: 837–845, 19
Miloslavina Y., Grouneva I., Lambrev P.H. et al.: Ultrafast fluorescence study on the location and mechanism of nonphotochemical quenching in diatoms.–BBA-Bioenergetics 1787: 1189–1197, 2009.
Minagawa J.: State transitions–the molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast.–BBA-Bioenergetics 1807: 897–905, 2011.
Miyashita H., Ikemoto H., Kurano N. et al.: Chlorophyll d as a major pigment.–Nature 383: 402, 1996.
Morosinotto T., Breton J., Bassi R., Croce R.: The nature of a chlorophyll ligand in Lhca proteins determines the far red fluorescence emission typical of photosystem I.–J. Biol. Chem. 278: 49223–49229, 2003.
Mukerji I., Sauer K.: Temperature Dependent Steady State and Picosecond Kinetic Fluorescence Measurements of a Photosystem I Preparation from Spinach. Pp. 30. Lawrence Berkeley Laboratory, Berkeley, 1988.
Müller N.: [Relationships between assimilation, absorption and fluorescence in the chlorophyll of the living leaf.]–Jahrb. Wiss. Bot. 9: 42–49, 1887. [In German]
Mullet J., Burke J., Arntzen C.: A developmental study of photosystem I peripheral chlorophyll proteins.–Plant Physiol. 65: 823–827 1980a.
Mullet J., Burke J., Arntzen C.: Chlorophyll proteins of photosystem I.–Plant Physiol. 65: 814–822, 1980b.
Mullineaux C.W., Allen J.F.: State 1-State 2 transitions in the cyanobacterium Synechococcus 6301 are controlled by the redox state of electron carriers between Photosystems I and II.–Photosynth. Res. 23: 297–311, 1990.
Mullineaux C.W.: Electron transport and light-harvesting switches in cyanobacteria.–Front. Plant Sci. 5: 7, 2014.
Mullineaux C.W.: Excitation energy transfer from phycobilisomes to photosystem I in a cyanobacterial mutant lacking photosystem II.–BBA-Bioenergetics 1184: 71–77, 1994.
Mullineaux C.W.: Excitation energy transfer from phycobilisomes to photosystem I in a cyanobacterium.–BBABioenergetics 1100: 285–292, 1992.
Mullineaux C.W.: Phycobilisome-reaction centre interaction in cyanobacteria.–Photosynth. Res. 95: 175–182, 2008.
Mulo P., Sakurai I., Aro E.-M.: Strategies for psbA gene expression in cyanobacteria, green algae and higher plants: from transcription to PSII repair.–BBA-Bioenergetics 1817: 247–257, 2012.
Murakami A.: Quantitative analysis of 77K fluorescence emission spectra in Synechocystis sp. PCC 6714 and Chlamydomonas reinhardtii with variable PS I/PS II stoichiometries.–Photosynth. Res. 53: 141–148, 1997.
Murata N., Nishimura M., Takamiya A.: Fluorescence of chlorophyll in photosynthetic systems. III. Emission and action spectra of fluorescence–three emission bands of chlorophyll a and the energy transfer between two pigment systems.–Biochim. Biophys. Acta 126: 234–243, 1966.
Murata N.: Control of excitation transfer in photosynthesis I. Light-induced change of chlorophyll a fluoresence in Porphyridium cruentum.–BBA-Bioenergetics 172: 242–251, 19
Murata N.: Control of excitation transfer in photosynthesis. IV. Kinetics of chlorophyll a fluorescence in Porphyra yezoensis.–BBA-Bioenergetics 205: 379–389, 1970.
Mysliwa-Kurdziel B., Barthélemy X., Strzalka K., Franck F.: The early stages of photosystem II assembly monitored by measurements of fluorescence lifetime, fluorescence induction and isoelectric focusing of chlorophyll-proteins in barley etiochloroplasts.–Plant Cell Physiol. 38: 1187–1196, 1997.
Nagao R., Takahashi S., Suzuki T. et al.: Comparison of oligomeric states and polypeptide compositions of fucoxanthin chlorophyll a/c-binding protein complexes among various diatom species.–Photosynth. Res. 117: 281–288, 2013.
Nagao R., Tomo T., Noguchi E. et al.: Purification and characterization of a stable oxygen-evolving Photosystem II complex from a marine centric diatom, Chaetoceros gracilis.–BBABioenergetics 1797: 160–166, 2010.
Nakatani H., Ke B., Dolan E., Arntzen C.: Identity of the photosystem II reaction center polypeptide.–BBABioenergetics 765: 347–352, 1984.
Natali A., Croce R.: Characterization of the major lightharvesting complexes (LHCBM) of the green alga Chlamydomonas reinhardtii.–PLoS ONE 10: e0119211, 2015.
Nickelsen J., Rengstl B.: Photosystem II assembly: from cyanobacteria to plants.–Annu. Rev. Plant Biol. 64: 609–635, 2013.
Nixon P.J., Barker M., Boehm M. et al.: FtsH-mediated repair of the photosystem II complex in response to light stress.–J. Exp. Bot. 56: 357–363, 2005.
Novoderezhkin V.I., Palacios M.A., van Amerongen H., van Grondelle R.: Excitation dynamics in the LHCII complex of higher plants: modeling based on the 2.72 Å crystal structure.–J. Phys. Chem. B 109: 10493–10504, 2005.
Owens T.G.: Dynamics and mechanism of singlet energytransfer between carotenoids and chlorophylls-light harvesting and nonphotochemical fluorescence quenching.–In: Murata N. (ed.): Research in Photosynthesis. Pp. 179–186. Kluwer Acad. Publ., Dordrecht 1992.
Pakrasi H.B., Goldenberg A., Sherman L.A.: Membrane development in the cyanobacterium, Anacystis nidulans, during recovery from iron starvation.–Plant Physiol. 79: 290–295, 1985a.
Pakrasi H.B., Riethman H.C., Sherman L.A.: Organization of pigment proteins in the photosystem II complex of the cyanobacterium Anacystis nidulans R2.–P. Natl. Acad. Sci. USA 82: 6903–6907, 1985b.
Park Y.I., Sandström S., Gustafsson P., Öquist G.: Expression of the isiA gene is essential for the survival of the cyanobacterium Synechococcus sp. PCC 7942 by protecting photosystem II from excess light under iron limitation.–Mol. Microbiol. 32: 123–129, 1999.
Passarini F., Wientjes E., Hienerwadel R., Croce R.: Molecular basis of light harvesting and photoprotection in CP24 unique features of the most recent antenna complex.–J. Biol. Chem. 284: 29536–29546, 2009.
Pfundel E., Pfeffer M.: Modification of photosystem I light harvesting of bundle-sheath chloroplasts occurred during the evolution of NADP-malic enzyme C4 photosynthesis.–Plant Physiol. 114: 145–152, 1997.
Qin X., Suga M., Kuang T., Shen J.-R.: Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex.–Science 348: 989–995, 2015.
Rabinowitch E., Govindjee: Photosynthesis. Pp. 273. John Wiley & Sons, Inc, New York 1969.
Riethman H.C., Sherman L.A.: Purification and characterization of an iron stress-induced chlorophyll-protein from the cyanobacterium Anacystis nidulans R2.–BBA-Bioenergetics 935: 141–151, 1988.
Rijgersberg C.P., Amesz J., Thielen A., Swager J.A.: Fluorescence emission spectra of chloroplasts and subchloroplast preparations at low temperature.–BBA-Bioenergetics 545: 473–482, 1979.
Ruban A.V., Calkoen F., Kwa S.L.S. et al.: Characterisation of LHC II in the aggregated state by linear and circular dichroism spectroscopy.–BBA-Bioenergetics 1321: 61–70, 1997.
Ruban A.V., Johnson M.P., Duffy C.D.P.: The photoprotective molecular switch in the photosystem II antenna.–BBABioenergetics 1817: 167–181, 2012.
Şener M., Strümpfer J., Hsin J. et al.: Förster energy transfer theory as reflected in the structures of photosynthetic lightharvesting systems.–ChemPhysChem. 12: 518–531, 2011.
Sétif P., Mathis P., Vänngård T.: Photosystem I photochemistry at low temperature. Heterogeneity in pathways for electron transfer to the secondary acceptors and for recombination processes.–BBA-Bioenergetics 767: 404–414, 1984.
Schlodder E., Falkenberg K., Gergeleit M., Brettel K.: Temperature dependence of forward and reverse electron transfer from A1-, the reduced secondary electron acceptor in photosystem I.–Biochemistry 37: 9466–9476, 1998.
Sjöback R., Nygren J., Kubista M.: Absorption and fluorescence properties of fluorescein.–Spectrochim. Acta A 51: 7–21, 1995.
Sobiechowska-Sasim M., Stoń-Egiert J., Kosakowska A.: Quantitative analysis of extracted phycobilin pigments in cyanobacteria–an assessment of spectrophotometric and spectrofluorometric methods.–J. Appl. Phycol. 26: 2065–2074, 2014.
Standfuss J., Kühlbrandt W.: The three isoforms of the lightharvesting complex II spectroscopic features, trimer formation, and functional roles.–J. Biol. Chem. 279: 36884–36891, 2004.
Strasser R.J., Tsimilli-Michael M., Srivastava A.: Analysis of the Chlorophyll a Fluorescence Transient.–In: Papageorgiou G.C., {ieGovindjee (ed.): Chlorophyll a Fluorescence: A Signature of Photosynthesis. Pp. 321–362. Springer, Dordrecht 2004.
Sugiura M., Inoue Y.: Highly purified thermo-stable oxygenevolving photosystem II core complex from the thermophilic cyanobacterium Synechococcus elongatus having His-tagged CP43.–Plant Cell Physiol. 40: 1219–1231, 1999.
Swingley W.D., Iwai M., Chen Y. et al.: Characterization of photosystem I antenna proteins in the prasinophyte Ostreococcus tauri.–BBA-Bioenergetics 1797: 1458–1464, 2010.
Takahashi S., Badger M.R.: Photoprotection in plants: a new light on photosystem II damage.–Trends Plant Sci. 16: 53–60, 2011.
Tang K., Ding W.-L., Höppner A. et al.: LCM: A light-harvesting pigment with a phytochrome chromophore.–P. Natl. Acad. Sci. USA 112: 15880–15885, 2015.
Tokutsu R., Minagawa J.: Energy-dissipative supercomplex of photosystem II associated with LHCSR3 in Chlamydomonas reinhardtii.–P. Natl. Acad. Sci. USA 110: 10016–10021, 2013.
Umena Y., Kawakami K., Shen J.-R., Kamiya N.: Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å.–Nature 473: 55–60, 2011.
van Wijk K.J., Bingsmark S., Aro E.-M., Andersson B.: In vitro synthesis and assembly of photosystem II core proteins. the D1 protein can be incorporated into photosystem II in isolated chloroplasts and thylakoids.–J. Biol. Chem. 270: 25685–25695, 1995.
Veith T., Büchel C.: The monomeric photosystem I-complex of the diatom Phaeodactylum tricornutum binds specific fucoxanthin chlorophyll proteins (FCPs) as light-harvesting complexes.–BBA-Bioenergetics 1767: 1428–1435, 2007.
Walters R.G., Horton P.: Resolution of components of nonphotochemical chlorophyll fluorescence quenching in barley leaves.–Photosynth. Res. 27: 121–133, 1991.
Watanabe M., Semchonok D.A., Webber-Birungi M.T. et al.: Attachment of phycobilisomes in an antenna–photosystem I supercomplex of cyanobacteria.–P. Natl. Acad. Sci. USA 111: 2512–2517, 2014.
Wei X., Su X., Cao P. et al.: Structure of spinach photosystem IILHCII supercomplex at 3.2 Å resolution.–Nature 534: 69–87, 2016.
Weis E.: Chlorophyll fluorescence at 77 K in intact leaves: characterization of a technique to eliminate artifacts related to self-absorption.–Photosynth. Res. 6: 73–86, 1985.
Wientjes E., van Stokkum I.H.M., van Amerongen H., Croce R.: The role of the individual Lhcas in photosystem I excitation energy trapping.–Biophys. J. 101: 745–754, 2011.
Yamamoto Y., Hori H., Kai S. et al.: Quality control of Photosystem II: reversible and irreversible protein aggregation decides the fate of Photosystem II under excessive illumination.–Front. Plant Sci. 4: 433, 2013.
Yokono M., Nagao R., Tomo T., Akimoto S.: Regulation of excitation energy transfer in diatom PSII dimer: How does it change the destination of excitation energy?–BBABioenergetics 1847: 1274–1282, 2015.
Young A.J., Frank H.A.: Energy transfer reactions involving carotenoids: quenching of chlorophyll fluorescence.–J. Photoch. Photobio. B 36: 3–15, 1996.
Author information
Authors and Affiliations
Corresponding author
Additional information
This review is dedicated to Govindjee. In addition to Govindjee’s original contributions to the field, we, the authors, are also very thankful to Govindjee for sharing historical context and personal connections, which contributed to researchers making their discoveries. In the case of 77 K fluorescence, the topic of this review, Govindjee was not only witness to its first implementation, but also went on to refine and extend the interpretation of this powerful measuring technique. The following short historical introduction into 77 K fluorescence often refers to information obtained from publications by Govindjee and coworkers.
Acknowledgements: Martin Hohmann-Marriott acknowledges support from the Research Council Norway (grant number 240741). Gunvor Røkke’s research was supported by a PhD fellowship from the NT faculty of the Norwegian University of Science and Technology–NTNU. Jacob Lamb acknowledges the support from the ENERSENSE research initiative, and his research was supported by a postdoctoral fellowship from the Norwegian University of Science and Technology–NTNU.
This article is published with open access at link.springer.com
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Lamb, J.J., Røkke, G. & Hohmann-Marriott, M.F. Chlorophyll fluorescence emission spectroscopy of oxygenic organisms at 77 K. Photosynthetica 56, 105–124 (2018). https://doi.org/10.1007/s11099-018-0791-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-018-0791-y