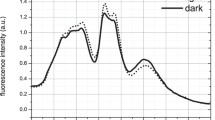
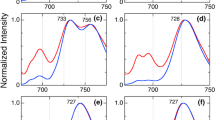
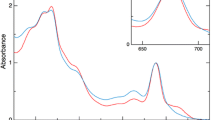
Abstract
The mechanism of excitation energy redistribution (state transition) in organisms containing phycobilins is reviewed. Recent measurements using time-resolved fluorescence spectroscopy in the picosecond range confirm that the state transition in cyanobacteria and red algae is controlled by changes in the kinetics of energy transfer from PS 2 to PS 1 (spillover) rather than by physical dislocation of the phycobilisome and reassociation between the two photosystems (mobile antenna model). Contrary to the analogous situation in higher plants, there is no compelling evidence for the involvement of a protein phosphorylation event in the rapid time range of the state transition, but a variety of data indicate that a membrane conformational change occurs that might change the relative distance between, and/or orientation of the two photosystems within the thylakoid. The state transition is most probably initiated by the redox state of the intersystem electron transport chain, and the conversion to state 1 is driven by coupled PS1 cyclic electron transport. The cryptomonads also undergo wavelength dependent changes in excitation energy distribution by a mechanism very similar to that observed in the red algae and cyanobacteria. However, the changes in energy distribution in this group are most likely related to a photoprotection mechanism for PS2 rather than to a state transition.
Similar content being viewed by others
Abbreviations
- APC:
-
allophycocyanin
- EF:
-
exoplasmic face
- PE:
-
phycoerythrin
- PC:
-
phycocyanin
- PF:
-
protoplasmic face
- LHC:
-
light harvesting chlorophyll a/b protein
- PBS:
-
phycobilisome
- LD:
-
linear dichroism
- RC:
-
reaction center
References
Alfonzo, R, Nelson, N and Racker, E (1980) A light-dependent protein kinase activity of chloroplasts. Plant Physiol. 65: 730–734.
Allen, JF and Holmes, NG (1986) A general model for regulation of photosynthetic function by protein phosphorylation. FEBS Lett 202: 175–181.
Allen, JF, Bennett, J, Steinback, KE and Arntzen, CJ (1981) Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature (London) 291: 25–29.
Allen, JF, Sanders, CE and Holmes, NG (1985) Correlation of membrane protein phosphorylation with excitation energy distribution in the cyanobacterium Synechococcus 6301. FEBS Lett 193: 271–275.
Amesz, J and Duysens, LNM (1962) Action spectrum, kinetics and quantum requirement of phosphopyridine nucleotide reduction and cytochrome oxidation in the blue-green alga Anacystis nidulans. Biochim Biophys Acta 64: 261–278.
Anderson, JM (1981) Consequences of spatial separation of photosystem 1 and 2 in thylakoid membranes of higher plant chloroplasts. FEBS Lett 124: 1–10.
Andersson, B and Anderson, JM (1980) Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta 593: 427–440.
Baker, NR and Webber, AN (1987) Interactions between photosystems. In: Callow, JA ed. Advances in Botanical Research, pp 2–56. London: Academic Press Publishers.
Bárány M, Bárány K, Barron JT, Kopp SJ, Doyle DD, Hager SR, Schlesinger DH, Homa F, Sayers ST and Janis RA (1981) Protein phosphorylation in live muscle. In: Rosen OR and Krebs EG eds. Protein phosphorylation, pp 869–886. Cold Spring Harbor Laboratory.
Barker, J (1982) Influence of surface charges on thylakoid structure and function. Ann Rev Plant Physiol 33: 261–295.
Barber, J (1983) Membrane conformational changes due to phosphorylation and the control of energy transfer in photosynthesis. Photobiochem Photobiophys 5: 181–190.
Barber (1986) Regulation of energy transfer by cations and protein phosphorylation in relation to thylakoid organization. Photosynth Res 10: 243–253.
Bennett, J (1977) Phosphorylation of chloroplast membrane polypeptides. Nature 269: 344–346.
Bennett, J (1979) Chloroplast phosphoproteins, the protein kinase of thylakoid membranes is light dependent. FEBS Lett 103: 342–344.
Bennett, J (1980) Chloroplast phosphoproteins: evidence for a thylakoid bound phosphoprotein phosphatase. Eur J Biochem 104: 85–89.
Bennett, J, Steinback, KE and Arntzen, CJ (1980) Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci USA 77: 5253–5257.
Biggins, J (1973) Kinetic behavior of cytochrome f in cyclic and noncyclic electron transport in Porphyridium cruentum. Biochem 12: 1165–1170.
Biggins, J (1983) Mechanism of the light state transition in photosynthesis. 1. Analysis of the kinetics of cytochrome f oxidation in state 1 and state 2 in the red alga Porphyridium cruentum. Biochim Biophys Acta 724: 111–117.
Biggins, J and Bruce, D (1985) Mechanism of the light state transition in photosynthesis. 111. Kinetics of the state transition in Porphyridium cruentum. Biochim Biophys Acta 806: 230–236.
Biggins, J and Bruce, D (1987) The relationships between protein kinase activity and chlorophyll a fluorescence changes in thylakoids from the cyanobacterium Synechococcus 6301. In: Biggins, J ed. Progress in Photosynthesis Research, Vol 2, pp 773–776. Dordrecht: Martinus Nijhoff publishers.
Biggins, J, Campbell, CL, Creswell, LL and Wood, EA (1984a) Mechanism of the light state transition in Porphyridium cruentum. In: Sybesma, C ed. Advances in Photosynthesis Research, Vol 2, pp 303–306. The Hague/Boston/Lancaster: Martinus Nijhoff/Dr W Junk Publishers.
Biggins, J, Campbell, CL and Bruce, D (1984b) Mechanism of the light state transition in photosynthesis. 11. Analysis of phosphorylated polypeptides in the red alga Porphyridium cruentum. Biochim Biophys Acta 767: 138–144.
Bonaventura, C and Myers, J (1969) Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta 189: 366–383.
Bowes, JM, Stewart, AC and Bendall, DS (1983) Purification of photosystem 11 particles from Phormidium laminosum using the detergent dodecyl-β-D-maltoside. Properties of the purified complex. Biochim Biophys Acta 725: 210–219.
Brody, SS, Porter, G, Tredwell, CJ and Barber, J (1981) Picosecond energy transfer in Anacystis nidulans. Photobiochem Photobiophys 2: 11–14.
Bruce, D and Biggins, J (1985) Mechanism of the light state transition in photosynthesis. V. 77K linear dichroism of Anacystis nidulans in state 1 and state 2. Biochim Biophys Acta 810: 295–301.
Bruce, D, Biggins, J, Steiner, T and Thewalt, M (1985) Mechanism of the state transition in photosynthesis. IV. Picosecond fluorescence spectroscopy of Anacystis nidulans and Porphyridium cruentum in state 1 and state 2 at 77K. Biochim Biophys Acta 806: 237–246.
Bruce, D, Biggins, J, Steiner, T and Thewalt, M (1986a) Excitation energy transfer in the cryptophytes. Fluorescence excitation spectra and picosecond time-resolved emission spectra of intact algae at 77K. Photochem Photobiol 44: 519–525.
Bruce, D, Hanzlik, CA, Hancock, LE, Biggins, J and Knox, RS (1986b) Energy distribution in the photochemical apparatus of Porphyridium cruentum: picosecond fluorescence spectroscopy of cells in state 1 and state 2 at 77K. Photosynth Res 10: 283–290.
Bruce, D, Biggins, J, Charbonneau, S and Thewalt, M (1987) Excitation energy transfer in Cryptomonas ovata. Preillumination dependent changes in 77K picosecond time resolved fluorescence emission spectra. In: Biggins, J ed. Progress in Photosynthesis Research Vol 2, pp 777–780. Dordrecht: Martinus Nijhoff Publishers.
Bryant DA (1986) The cyanobacterial photosynthetic apparatus: comparison of those of higher plants and photosynthetic bacteria. In: Platt T and Li WKW eds Photosynthetic picoplankton, pp 423–500. Canadian Bulletin of Fisheries and Aquatic Sciences 214.
Butler, WL (1976) Energy distribution in the photosynthetic apparatus of plants. Brookhaven Symp Biol 28: 338–346.
Butler WL (1977) Chlorophyll fluorescence. A probe for electron and energy transfer. In: Trebst A and Avron M eds Photosynthesis 1. Photosynthetic electron transport and photophosphorylation. Encyclopedia of Plant Physiology, pp 149–167.
Butler, WL (1978) Energy distribution in the photochemical apparatus of photosynthesis. Ann Rev Plant Physiol 29: 345–378.
Butler, WL and Kitajima, M (1975a) A tripartite model for chloroplast fluorescence. In: Avron, M ed Proceedings of the Third International Congress on Photosynthesis, pp 13–24. Amsterdam: Elsevier Publishers.
Butler, WL and Kitajima, M (1975b) Energy transfer between photosystem 11 and photosystem 1 in chloroplasts. Biochim Biophys Acta 396: 72–85.
Canaani, O (1986) Photoacoustic detection of oxygen evolution and state 1-state 2 transitions in cyanobacteria. Biochim Biophys Acta 852: 74–80.
Canaani, O (1987) Control of state 1-state 2 transitions in the blue-green alga Nostoc muscorum. In: Biggins, J ed Progress in Photosynthesis Research, Vol 2, pp 769–772. Dordrecht: Martinus Nijhoff Publishers.
Chereskin, BM, Clement-Metral, JD and Gantt, E (1985) Characterization of a purified photosystem 11-phycobilisome particle preparation from Porphyridium cruentum. Plant Physiol 77: 626–629.
Chiang, G and Dilley, RA (1987) Evidence for Ca2+-gated proton fluxes in chloroplast thylakoid membranes: Ca2+ controls a localized to delocalized proton gradient switch. Biochemistry 26: 4911–4916.
Chow, WS, Telfer, A, Chapman, DJ and Barber, J (1981) State 1-State 2 transition in leaves and its association with ATP-induced chlorophyll fluorescence quenching. Biochim Biophys Acta 638: 60–68.
Davis, RJ, Johnson, GL, Kelleher, DJ, Anderson, JK, Mole, JE and Czech, MP (1986) Identification of serine 24 as the unique site on the transferrin receptor phosphorylated by protein kinase. J Biol Chem 261: 9034–9041.
Dilley, RA, Theg, SM and Beard, WA (1987) Membrane-proton interactions in chloroplast bioenergetics: localized proton domains. Ann Rev Plant Physiol 38: 347–389.
Dilworth, MF and Gantt, E (1981) Phycobilisome-thylakoid topography on photosynthetically active vesicles of Porphyridium cruentum. Plant Physiol 67: 608–612.
Dominy, PJ and Williams, WP (1987) The role of respiratory electron flow in the control of excitation energy distribution in blue-green algae. Biochim Biophys Acta 892: 264–274.
Duysens LNM (1952) Transfer of excitation energy in photosynthesis. PhD Thesis: Utrecht.
Duysens, LNM and Amesz, J (1962) Function and identification of two photochemical systems in photosynthesis. Biochim Biophys Acta 64: 243–260.
Dwarte, D and Veske, M (1983) A freeze-fracture study of cryptomonad thylakoids. Protoplasma 117: 130–141.
Emerson, R and Lewis, CM (1942) The photosynthetic efficiency of phycocyanin in Chroococcus and the problem of carotenoid participation in photosynthesis. J Gen Physiol 25: 579–595.
Engelmann, TW (1983) Farbe und Assimilation. Bot Ztg 41: 1–6.
Engelmann, TW (1984) Untersuchungen Uber die quantitativen Beviehungen zwischen Absorption des Lichtes und Assimilation in Pflanzenzelle. Bot Ztg 42: 81–87.
Farchaus, JW, Widger, WR, Cramer, WA and Dilley, RA (1982) Kinase-induced changes in electron transport rates of spinach chloroplasts. Arch Biochem Biophys 217: 362–367.
Fork, DC and Satoh, K (1983) State 1-state 2 transitions in the thermophilic blue-green alga (cyanobacterium) Synechococcus lividus. Photochem Photobiol 37: 421–427.
Fork, DC and Satoh, K (1986) The control by state transitions of the distribution of excitation energy in photosynthesis. Ann Rev Plant Physiol 37: 335–361.
Fork, DC, Murata, N and Sato, N (1979) Effect of growth temperature on the lipid and fatty acid composition, and the dependence of temperature on light-induced redox reactions of cytochrome f and of light energy distribution in the thermophilic blue-green alga Synechococcus lividus. Plant Physiol 63: 524–530.
French, CS and Young, VK (1952) The fluorescence spectra of red algae and the transfer of energy from phycoerythrin to phycocyanin and chlorophyll. J Gen Physiol 35: 873–890.
Gantt, E (1979) Phycobiliproteins of cryptophyceae. In: Hutner, HS and Levandowsky, M eds Biochemistry and Physiology of Protozoa, 2nd Edition, pp 121–137. New York: Academic Press Publishers.
Gantt, E (1980a) Structure and function of phycobilisomes: light harvesting pigment complexes in red and blue-green algae. Int Rev Cytol 66: 45–80.
Gantt E (1980b) Photosynthetic cryptophytes. In: Cox ER ed Phytoflagellates, pp 381–405. Elsevier North Holland Publishers.
Gantt, E (1981) Phycobilisomes. Ann Rev Plant Physiol 32: 327–347.
Gantt, E and Lipshultz, CA (1973) Energy transfer in phycobilisomes from phycoerythrin to allophycocyanin. Biochim Biophys Acta 292: 858–861.
Gantt, E, Edwards, MR and Provasoli, L (1971) Chloroplast structure of the cryptophyceae. Evidence for phycobiliproteins within intrathylakoidal spaces. J Cell Biol 48: 280–290.
Gantt, E, Lipshultz, CA and Zilinskas, B (1976) Further evidence for a phycobilisome model from selective dissociation, fluorescence emission, immunoprecipitation, and electron microscopy. Biochim Biophys Acta 430: 375–388.
Giddings, TH and Staehelin, LA (1979) Changes in thylakoid structure associated with differentiation of heterocysts in the cyanobacterium Anabaena cylindrica. Biochim Biophys Acta 546: 373–382.
Giddings, TH, Wasmann, C and Staehelin, LA (1983) Structure of the thylakoids and envelope membranes of the cyanelles of Cyanophora paradoxa. Plant Physiol 71: 409–419.
Glazer, AN and Bryant, DA (1975) Allophycocyanin B (λ max 671, 618 nm). A new cyanobacterial phycobiliprotein. Arch Microbiol 104: 15–22.
Glazer, AN and Melis, A (1987) Photochemical reaction centers: structure, organization and function. Ann Rev Plant Physiol 38: 11–45.
Glazer, AN, Lundell, DJ, Yamanaka, G and Williams, RC (1983) The structure of a simple phycobilisome. Ann Microbiol (Inst Pasteur) 134B: 159–180.
Graan, T and Ort, DR (1982) Photophosphorylation associated with synchronous turnovers of the electron transport carriers in chloroplasts. Biochim Biophys Acta 682: 395–403.
Hallier, VW and Park, RB (1969) Photosynthetic light reactions in chemically fixed Anacystis nidulans, Chlorella pyrenoidosa, and Porphyridium cruentum. Plant Physiol 44: 535–539.
Harnishfeger, G and Codd, GA (1977) Liquid nitrogen fluorescence studies of the photosynthetic apparatus of blue-green algae. Br Phycol J 12: 225–236.
Haxo, FT and Blinks, LR (1950) Photosynthetic action spectra of marine algae. J Gen Physiol 33: 389–422.
Haxo, FT and Fork, DC (1959) Photosynthetically active accessory pigments of cryptomonads. Nature 184: 1051–1052.
Hafferle, P, Nies, M, Wehrmeyer, W and Schneider, S (1983) Picosecond time-resolved fluorescence study of the antenna system isolated from Mastocladus laminosum Cohn. 1. Functionally intact phycobilisomes. Photobiochem Photobiophys 5: 41–51.
Hirano, M, Satoh, K and Katoh, S (1980) Plastoquinone as a common link between photosynthesis and respiration in a blue-green alga. Photosynth Res 1: 149–162.
Holzwarth, AR (1986) Fluorescence lifetimes in photosynthetic systems. Photochem Photobiol 43: 707–725.
Holzwarth, AR, Wendler, J and Wehrmeyer, W (1983) Studies on chromophore coupling in isolated phycobiliproteins. 1. Picosecond fluorescence kinetics of energy transfer in phycocyanin 645 from Chroomonas sp.. Biochim Biophys Acta 724: 388–395.
Homann, P (1969) Cation effects on the fluorescence of isolated chloroplasts. Plant Physiol 44: 932–936.
Horton, P (1983) Control of chloroplast electron transport by phosphorylation of thylakoid proteins. FEBS Lett 152: 47–52.
Horton, P and Black, MT (1981) Light-induced redox changes in chloroplast cytochrome f after phosphorylation of membrane proteins. FEBS Lett 132: 75–77.
Horton, P and Black, MT (1982) On the nature of the fluorescence decrease due to phosphorylation of chloroplast membrane proteins. Biochim Biophys Acta 680: 22–27.
Horton, P, Allen, JF, Black, MT and Bennett, J (1981) Regulation of phosphorylation of chloroplast membrane polypeptides by the redox state of plastoquinone. FEBS Lett 125: 193–196.
Ingram, K and Hiller, RG (1983) Isolation and characterization of a major chlorophyll a/c2 light-harvesting protein from a Chroomonas species (Cryptophyceae). Biochim Biophys Acta 722: 310–319.
Jones, LW and Myers, J (1964) Enhancement in the blue-green alga, Anacystis nidulans. Plant Physiol 39: 938–946.
Jung, J, Song, PS, Paxton, RJ, Edelstein, MS, Swanson, R and Hazen, EE (1980) Molecular topography of the phycocyanin photoreceptor from Chroomonas species. Biochemistry 19: 24–32.
Katoh, T and Gantt, E (1979) Photosynthetic vesicles with bound phycobilisomes from Anabaena variablis. Biochim Biophys Acta 546: 383–393.
Kawamura, M, Mimuro, M and Fujita, Y (1979) Quantitative relationship between two reaction centers in the photosynthetic system of blue-green algae. Plant Cell Physiol 20: 697–705.
Khanna, R, Graham, J-R, Myers, J and Gantt, E (1983) Phycobilisome composition and possible relationship to reaction centers. Arch Biochem Biophys 224: 534–542.
Kirilovsky, D and Ohad, I (1986) Functional assembly in vitro of phycobilisomes with isolated photosystem 1 particles of eukaryotic chloroplasts. J Biol Chem 261: 12317–12323.
Kirilovsky, D, Kessel, M and Ohad, I (1983) In vitro reassociation of phycobiliproteins and membranes to form functional membrane-bound phycobilisomes. Biochim Biophys Acta 724: 416–426.
Kirschner, J and Senger, H (1986) Thylakoid protein phosphorylation in the red algae Porphyridium cruentum. In: Akoyunoglou, G and Senger, H eds Regulation of chloroplast differentiation, Plant Biology vol 2 pp 339–344. New York: AR Liss Inc. Publishers.
Kitajima, M and Butler, WL (1975) Excitation spectra for photosystem 1 and photosystem 11 in chloroplasts and the spectral characteristics of the distribution of quanta between the two photosystems. Biochim Biophys Acta 408: 297–305.
Kursar, TA and Alberte, RA (1983) Photosynthetic unit organization in a red alga. Plant Physiol 72: 409–414.
Kyle, DJ, Staehelin, LA and Arntzen, CJ (1983) Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation energy distribution in higher plants. Arch Biochem Biophys 222: 527–541.
Kyle, DJ, Kuang, TY, Watson, JL and Arntzen, CJ (1984) Movement of a subpopulation of the light-harvesting complex (LHC-11) from grana to stroma lamellae as a consequence of its phosphorylation. Biochim Biophys Acta 765: 89–96.
Larkum, AWD and Weyrauch, SK (1977) Photosynthetic action spectra and light-harvesting in Griffithsia monilis (Rhodophyta). Photochem Photobiol 25: 65–72.
Larsson, U, Jergil, B and Andersson, B (1983) Changes in the lateral distribution of the light-harvesting chlorophyll-a/b-protein complex induced by phosphorylation. Eur J Biochem 136: 25–29.
Larsson, UK, Ogren, E, Oquist, G and Andersson, B (1986) Electron transport and fluorescence studies on the functional interaction between phospho-LHC 11 and photosystem 1 in isolated stroma lamellae vesicles. Photobiochem Photobiophys 13: 29–39.
Lefort-Tran, H, Cohen-Bazire, G and Pouphil, M (1973) Les membranes photosynthétiques des algues à phycobiliprotéins observées après cryodécapage. J Ultrastruct Res 44: 199–209.
Ley, AC (1984) Effective absorbance cross sections in Porphyridium cruentum. Plant Physiol 74: 451–454.
Ley, AC and Butler, WL (1976) Efficiency of energy transfer from photosystem 11 to photosystem 1 in Porphyridium cruentum. Proc Natl Acad Sci USA 73: 3957–3960.
Ley AC and Butler WL (1977) The distribution of excitation energy between Photosystem 1 and Photosystem 11 in Porphyridium cruentum. In: Photosynthetic organelles, special issue of Plant Cell Physiol pp 33–46.
Ley, AC and Butler, WL (1980) Energy distribution in the photochemical apparatus of Porphyridium cruentum in state 1 and state 11. Biochem Biophys Acta 592: 349–363.
Ley, AC, Butler, WL, Bryant, DA and Glazer, AN (1977) Isolation and function of allophycocyanin B of Porphyridium cruentum. Plant Physiol 59: 974–980.
Lichtlé, C and Thomas, JC (1976) Étude ultrastructural des thylacoides des algues à phycobiliprotéines, comparaison des résultats obtenus par fixation classique et cryodécapage. Phycologia 15: 393–404.
Lichtlé, C, Jupin, H and Duval, JC (1980) Energy transfer from Photosystem 2 to Photosystem 1 in Cryptomonas rufescens (Cryptophyceae). Biochim Biophys Acta 591: 104–112.
Lichtlé, C, Duval, JC, Hauswirth, N and Spilar, A (1986) Freeze fracture study of the thylakoid organization of Cryptomonas rufescens (Cryptophyceae) according to illumination conditions. Photochem Photobiol 11: 159–171.
MacColl, R and Berns, DS (1978) Energy transfer studies on cryptomonad biliproteins. Photochem Photobiol 27: 343–349.
MacColl, R, Habig, W and Berns, DS (1973) Characterization of phycocyanin from Chroomonas species. J Biol Chem 248: 7080–7086.
Mandori, A and Melis, A (1984) Photochemical apparatus organization in Anacystis nidulans (Cyanophyceae). Plant Physiol 74: 67–71.
Mandori, A and Melis, A (1985) Phycobilisome-photosystem 11 association in Synechococcus 6301. FEBS Lett 181: 79–82.
Mandori, A and Melis, A (1986) Light quality regulates photosystem stoichiometry in cyanobacteria. In: Akoyunoglou, G and Senger, H eds Regulation of chloroplast differentiation, pp 653–662. New York: AR Liss Publishers.
Mandori, A, Alhadeff, M, Glazer, AN and Melis, A (1984) Photochemical apparatus organization in Synechococcus 6301 (Anacystis nidulans). Effect of phycobilisome mutation. Arch Microbiol 139: 117–123.
Maxwell, PC and Biggins, J (1976) Role of cyclic electron transport in photosynthesis as measured by the photoinduced turnover of P700 in vivo. Biochemistry 15: 3975–3981.
Melis, A and Brown, JS (1980) Stoichiometry of system 1 and system 11 reaction centers and of plastoquinone in different photosynthetic membranes. Proc Natl Acad Sci USA 77: 4712–4716.
Mildvan, AS (1970) Metals in enzyme catalysis. In: Boyer, PD ed. The Enzymes vol 2, pp 445–536. New York and London: Academic Press Publishers.
Mimuro, M and Fujita, Y (1977) Estimation of chlorophyll a distribution in the photosynthetic pigment systems 1 and 11 of the blue-green alga Anabaena variablis. Biochim Biophys Acta 459: 376–389.
Mimuro, M, Lipschultz, C and Gantt, E (1986) Energy flow in the phycobilisome core of Nostoc sp. (MAC): two independent terminal pigments. Biochim Biophys Acta 852: 126–132.
Mörschel, E and Wehremeyer, W (1975) Cryptomonad biliprotein: Phycocyanin 645 from a Chroomonas species. Arch Microbiol 105: 153–158.
Mörschel, E and Muhlethaler, K (1983) On the linkage of exoplasmic freeze-fracture particles to phycobilisomes. Planta 158: 451–457.
Mörschel, E and Shatz, GH (1987) Correlation of photosystem-II complexes with exoplasmic freeze-fracture particles of thylakoids of the cyanobacterium Synechococus sp. Planta 172: 145–154.
Mullet, JE (1983) The amino acid sequence of the polypeptide segment which regulates membrane adhesion (grana stacking) in chloroplasts. J Biol Chem 258: 9941–9948.
Mullineaux, CW and Allen, JF (1986) The state 2 transition in the cyanobacterium Synechococcus 6301 can be driven by the respiratory electron flow into the plastoquinone pool. FEBS Lett 205: 155–160.
Mullineaux, CW, Boult, M, Sanders, CE and Allen, JF (1986) Fluorescence induction transients indicate altered absorption cross section during light-state transitions in the cyanobacterium Synechococcus 6301. Biochim Biophys Acta 851: 147–150.
Murata, N (1969a) Control of excitation transfer in photosynthesis. 1. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta 172: 242–251.
Murata, N (1969b) Control of excitation transfer in photosynthesis 11. Magnesium-ion dependent distribution of excitation energy between two pigment systems in spinach chloroplasts. Biochim Biophys Acta 189: 171–181.
Myers, J, Graham, J-R and Wang, RT (1980) Light harvesting in Anacystis nidulans studied in pigment mutants. Plant Physiol 66: 1144–1149.
Ohki, K, Gantt, E, Lipschultz, CA and Ernst, MC (1985) Constant phycobilisome size in chromatically adapted cells of the cyanobacterium Tolypothrix tenuis, and variation in Nostoc sp.. Plant Physiol 79: 943–948.
Ohki, K, Okabe, Y, Murakami, A and Fujita, Y (1987) A comparative study of quantitative relationship between phycobiliproteins and photosystem 11 in cyanobacteria and red algae. Plant Cell Physiol 28: 1219–1226.
Olive, J, M'Bina, I, Vernotte, C, Astier, C and Wollman, FA (1986) Randomization of the EF particles in thylakoid membranes of Synechocystis 6714 upon transition from state 1 to state 2. FEBS Lett 208: 308–312.
Pakrasi, HB and Sherman, LA (1984) A highly active oxygen-evolving photosystem 11 preparation from the cyanobacterium Anacystis nidulans. Plant Physiol 74: 742–745.
Paone, DAM and Stevens, SE (1981) Nitrogen starvation and the regulation of glutamine synthetase in Agmenellum quadruplicatum. Plant Physiol. 67: 1097–110.
Peterson, RB, Dolan, E, Calvert, HE and Ke, B (1981) Energy transfer from the phycobiliproteins to photosystem 1 in vegetative cells and heterocysts of Anabaena variablis. Biochim Biophys Acta 634: 237–248.
Porter, G, Tredwell, CJ, Searle, GFW and Barber, J (1978) Picosecond time-resolved energy transfer in Porphyridium cruentum. Part 1. In the intact alga. Biochim Biophys Acta 501: 232–245.
Raps, S, Kycia, JH, Ledbetter, MC and Siegelman, HW (1985) Light intensity adaptation and phycobilisome composition of Microcystis aeruginosa. Plant Physiol 79: 983–987.
Redlinger, T and Gantt, E (1983) Photosynthetic membranes of Porphyridium cruentum. Plant Physiol 73: 36–40.
Ried, A and Reinhardt, B (1977) Distribution of excitation energy between photosystem 1 and photosystem 11 in red algae. 11. Kinetics of the transition between state 1 and state 2. Biochim Biophys Acta 460: 25–35.
Ried, A and Reinhardt, B (1980) Distribution of excitation energy between photosystem 1 and photosystem 11 in red algae. 111. Quantum requirements of the induction of a state 2-state 1 transition. Biochim Biophys Acta 592: 76–86.
Ried, A, Hessenberg, B, Metzner, H and Zeigler, R (1977) Distribution of excitation energy between photosystem 1 and photosystem 11 in red algae. 1. Action spectra of light reactions 1 and 11. Biochim Biophys Acta 459: 175–186.
Sanders, CE and Allen, JF (1987) The 18.5kD phosphoprotein of the cyanobacterium Synechococus 6301: A component of the phycobilisome. In: Biggins, J ed Progress in Photosynthesis Research Vol 2, pp 761–764. Dordrecht: Martinus Nijhoff Publishers.
Sanders, CE, Holmes, NG and Allen, JF (1986) Membrane protein phosphorylation in the cyanobacterium Synechococcus 6301. Biochem Soc Trans 14: 66–67.
Satoh, K and Fork, DC (1983a) A new mechanism for adaptation to changes in light intensity and quality in the red alga, Porphyra perforata.1.Relation to state 1-state 2 transitions. Biochim Biophys Acta 722: 190–196.
Satoh, K and Fork, DC (1983b) The relationship between state 11 to state 1 transitions and cyclic electron flow around photosystem 1. Photosynth Res 4: 245–256.
Satoh, K, Strasser, R and Butler, WL (1976) A demonstration of energy transfer from photosystem 11 to photosystem 1 in chloroplasts. Biochim Biophys Acta 440: 337–345.
Schatz, GH and Witt, HT (1984) Extraction and characterization of oxygen-evolving photosystem 11 complexes from the thermophilic cyanobacterium Synechococus spec.. Photobiochem Photobiophys 7: 1–14.
Schoumacher, RA, Shoemaker, RL, Halm, DR, Tallant, EA, Wallace, RW and Frizzell, RA (1987) Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature 330: 752–754.
Schuster, G, Owens, GC, Cohen, Y and Ohad, I (1984) Thylakoid polypeptide composition and light-independent phosphorylation of the chlorophyll a,b-protein in Prochloron, a prokaryote exhibiting oxygenic photosynthesis. Biochim Biophys Acta 767: 596–605.
Simmerman, HKB, Collins, JH, Theibert, JL, Wegener, AD and Jones, LR (1986) Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem 261: 13333–13341.
Snyder, UK and Biggins, J (1987a) Excitation energy distribution in Cryptomonas ovata. In: Biggins, J ed Progress in Photosynthesis Research Vol 2, pp 781–784, Dordrecht: Martinus Nijhoff Publishers.
Snyder, UK and Biggins, J (1987b) Excitation-energy distribution in the cryptomonad alga Cryptomonas ovata. Biochim Biophys Acta 892: 48–55.
Spear-Bernstein, L and Miller, K (1985) Are the photosynthetic membranes of cryptophyte algae inside out? Protoplasma 129: 1–9.
Staehelin, LA (1986) Chloroplast structure and supramolecular organization of photosynthetic membranes. In: Staehelin, LA and Arntzen, CJ eds Ency Plant Physiol, New Series, Vol 19, pp 1–84. Berlin, Heidelberg, New York and Tokyo: Springer-Verlag.
Staehelin, LA and Arntzen, CJ (1983) Regulation of chloroplast membrane function: protein phosphorylation changes in the spatial organization of membrane components. J Cell Biol 97: 1327–1337.
Stanier, RY (1974) The origins of photosynthesis in eukaryotes. Symp Soc Gen Microbiol 24: 219–240.
Steinback, KE, Bose, S and Kyle, DJ (1982) Phosphorylation of the light-harvesting chlorophyll protein regulates excitation energy distribution between photosystem 11 and photosystem 1. Arch Biochem Biophys 216: 356–361.
Sugden, PH, Holladay, LA, Reimann, EM and Corbin, JV (1976) Purification and characterization of the catalytic subunit of adenosine 3′:5′-cyclic monophosphate-dependent protein kinase from bovine liver. Biochem J 159: 409–422.
Sutter, GW and Holzwarth, AR (1987) A kinetic model for energy transfer in phycobilisomes. Biophys J 52: 673–683.
Tandeau de Marsac, N (1983) Phycobilisomes and complementary chromatic adaptation in cyanobacteria. Bull Inst Pasteur 81: 201–254.
Tel-Or, E and Malkin, S (1977) The photochemical and fluorescence properties of whole cells, spheroplasts and spheroplast particles from the blue-green alga Phormidium luridum. Biochim Biophys Acta 459: 157–174.
Telfer, A, Bottin, H, Barber, J and Mathis, P (1984) The effect of magnesium and phosphorylation of light-harvesting chlorophyll a/b-protein on the yield of P700 photooxidation in pea chloroplasts. Biochim Biophys Acta 764: 324–330.
Wang, RT, Stevens, CLR and Myers, J (1977) Action spectra for photoreactions 1 and 11 of photosynthesis in the blue-green alga Anacystis nidulans. Photochem Photobiol 25: 103–108.
Weaver, EC (1984) State 1-state 11 transitions in Porphyridium cruentum: observations on photosystem 1 by electron paramagnetic resonance spectroscopy. Photobiochem Photobiophys 7: 195–203.
Wendler, J and Holzwarth, AR (1987) State transitions in the green alga Scenedesmus obliquus probed by time-resolved fluorescence spectroscopy and global data analysis. Biophys J 52: 717–728.
Whitmarsh, J and Ort, DR (1984) Stoichiometries of electron transport complexes in spinach chloroplasts. Arch Biochem Biophys 231: 378–389.
Williams, WP and Allen, JF (1987) State 1/state 2 changes in higher plants and algae. Photosynth Res 13: 19–45.
Wollman, F-A (1979) Ultrastructural comparison of Cyanidium caldarium wild type and 111-C mutant lacking phycobilisomes. Plant Physiol 63: 375–381.
Yamanaka, G and Glazer, AN (1980) Dynamic aspects of phycobilisome structure. Phycobilisome turnover during nitrogen starvation in Synechococcus sp.. Arch Microbiol 124: 39–47.
Yamazaki, I, Mimuro, M, Murao, T, Yamazaki, T, Yoshihara, K and Fujita, Y (1984) Excitation energy transfer in the light harvesting antenna system of the red alga Porphyridium cruentum and the blue-green alga Anacystis nidulans: analysis of time-resolved fluorescence spectra. Photochem Photobiol 39: 233–240.
Zilinskas, BA (1982) Isolation and characterization of the central component of the phycobilisome core of Nostoc sp.. Plant Physiol 70: 1060–1065.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Biggins, J., Bruce, D. Regulation of excitation energy transfer in organisms containing phycobilins. Photosynth Res 20, 1–34 (1989). https://doi.org/10.1007/BF00028620
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00028620