Abstract
Molecular modelling concepts always prove to be an efficient technique for studying the interaction between various substances prior to experimental work. They were utilized for investigating the interaction of chitosan (Cs) and carboxymethyl cellulose (CMC) biopolymers with a modified graphene structure (G). Geometry optimization calculations were carried out using PM6 method. Results illustrate that the proposed interactions are all stable; however, the interaction site has no role in the resulting energy values. The calculated energies for the G-CMC interactions are quite lower than those for the G-Cs ones indicating quite higher stability for the former group. On contrary to energy, the proposed interaction active site has a significant part in determining total dipole moment (TDM) and hence reactivity of the structures. The calculated quantitative structure-activity relationship (QSAR) parameters show that the interaction of graphene with these biopolymers lowers its hydrophobicity. Modification of Cs and CMC with graphene has a significant positive impact on enhancing their electrical features. The resulting bandgap of the proposed structures is lower than half of their original values. Bandgap values of the G-CMC proposed structures are quite lower than those of the G-Cs ones. An experimental trial was carried out by printing a sixty-layer simple electrode of both biocomposite inks via InkJet printing technique. Then, Sheet resistance and charge mobility measurements were conducted. Results demonstrate that the sheet resistance of the G-Cs printed electrode is about five times higher than that of the G-CMC one. Such result was confirmed by the measured Hall Effect measurement which showed that the charge mobility in the G-CMC electrode is much greater than that in the G-Cs one. Both theoretical and experimental parts agree that the G-CMC biocomposite has much more electrical conductivity than the G-Cs, proposing it as a potential candidate for bio-electronic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Molecular modelling concepts are frequently utilized to investigate the studied structures on the molecular gaseous state. The constructed computational model aims also to boost the resulting experimental data on theoretical basis as it can investigate both physical and biological characteristics of the structures under investigation depending on computer software packages which are always able to offer valuable data in many interdisciplinary fields of science (Abdel-Karim et al. 2020; Morsy et al. 2020; Refaat et al. 2020). Not only do computational models play significant roles when the experimental work is inaccessible, but they can present also logic explanations for the already running infield work depending on theoretical aspects (Ezzat et al. 2019; Ibrahim et al. 2019). Conducting geometry optimization calculations is of great deal in describing chemical, physical and electronic features as well as quantifying geometrical parameters of chemical structures (Bayoumy et al. 2019). For example, having quantitative data of energy state enables researchers to assess structures’ stability and the favourable conditions for their formulation from their proposed candidates as well (El-Naggar et al. 2021). Furthermore, the calculated dipole moment plays a significant role in evaluating the reactivity of such structures (Ibrahim et al. 2011; Ibrahim and Mahmoud 2009; Sabry et al. 2018). HOMO/LUMO bandgap energy can offer a quantitative description of the electrical conductivity of chemical structures, since it represents the electron transfer processes from valence band to conduction one (El-Sayed et al. 2021). In addition, quantitative structure-activity relationship (QSAR) parameters are usually calculated for the optimized structures to investigate some of their physical and electronic features related to biological applications (Bulat et al. 2010; Fahim et al. 2019; Saleh et al. 2017). They frequently offer a positive promising way for investigating the chemical structures because they present a simple strategy for anticipating their biological reactivity.
Utilization of biopolymers and/or their derivatives has become a worldwide trend in the scientific community as they own several merits, such as being renewable materials, easy to be processed, cost effectiveness, eco-friendly and their promising potential for biological applications as well as industrial ones. Chitosan is always considered the second most abundant polymer in our planet after cellulose (Ali et al. 2012; Bayoumy et al. 2020b). It is the main derivative of chitin biopolymer which presents in the exoskeleton of crustaceans, crabs, shrimps…etc (El Knidri et al. 2018). A deacetylation process of chitin is frequently carried out to produce chitosan from chitin (Rivero et al. 2010; Tănase et al. 2014). It is well-known by its biocompatibility, biodegradability, being economically, and has a great ability to form composites with wide range of both natural and synthetic structures due to its various functional groups (Suginta et al. 2013). It owns both antibacterial and antimicrobial activities, as well (Jarmila and Eva 2011). Hence, it has been emerged in multiple applications (Aljawish et al. 2016; Rinaudo 2006), including biomedical as a drug delivery carrier (Agnihotri et al. 2004; Kim et al. 2006; Sabnis and Block 2000; Zhang et al. 2015), agricultural (Hadwiger 2013), and electrochemical applications (Hassan et al. 2014a). However, it has quite low conductivity. Like chitosan, carboxymethyl cellulose (CMC) is one of the common derivatives of cellulose biopolymer. It is formed by replacing the hydrogen atom in cellulose by a carboxymethyl group instead in order to be soluble in water. Due to its ease in processing and its viscous nature, it has been extensively enrolled in different appliances including 3D biocompatible scaffolds (Chen and Fan 2007; Singh et al. 2016), tissue engineering (Chen et al. 2020; Mohan et al. 2020; Verma et al. 2021; Zheng et al. 2021), bone-grafting implants (Matinfar et al. 2019; Sarkar et al. 2020; Sharmila et al. 2020) and wound healing dressings (Koneru et al. 2020; Sadeghi et al. 2020; Saladino et al. 2020). Contrary to chitosan, CMC has higher electrical conductivity, hence many reports have been published for its usage in enhancing electrical conductivity of various structures. For example, it was blended with polyaniline conducting polymer in order to enhance its electrical conductivity for biosensing applications (Basavaraja et al. 2012). CMC was reported to enhance conductivity as well as thermal stability of the formulated blends in high temperatures up to 500 K. Furthermore, CMC was emerged in the fabrication of Li-ion batteries (LIBs) (Lee and Oh 2013) due to its high viscosity to act as a binder to the active ingredients of anode. A composite was formulated of Li4Ti5O12 (LTO) and CMC to enhance its overall performance where it could support the binding affinity between LTOs as well as boost their ion conductivity.
Recently, nanotechnology is one of the uprising research trends. It always provides magic clues to control the preparation procedures of different chemical structures and manage their physicochemical features, as well (Bayoumy et al. 2020a). Hence, preparation of nanomaterials has become the first choice for numerous researchers, and it has been the main concern in many recent advanced applications (Maruyama et al. 2003; Ferreira et al. 2019). Not only do scientists concentrate on boosting preparation and characterization techniques of these nanomaterials, but they focus also on proposing the theories that can describe the interaction between chemical structures on this small scale (Thiruvengadam et al. 2018; Bayoumy et al. 2020b). Hence, various scientific publications have reported the utilization of nanomaterials in wide range of applications including medical and clinical applications (Singh and Amiji 2022; Phillips and Mousa 2022), environmental (Di Tinno et al. 2022; Nitschke and Marangon 2022), and sensing/biosensing ones (Sohrabi et al. 2022; Cancelliere et al. 2021). Carbon-based nanosized structures always succeed in surprising researchers due to their salient features for interdisciplinary fields. Such large category includes carbon nanotubes (CNTs) (Sakki et al. 2022), graphene (Hadad et al. 2021), fullerenes (Waite et al. 2021) and nano-diamonds (Mydlova et al. 2022). Graphene is frequently used in various applications due to its unique physicochemical features, such as electrical conductivity, flexibility, ease of functionalization…etc (Gamil et al. 2014). Therefore, it has been emerged in several electrochemical applications (Hassan et al. 2014b; Rashed and El-Moneim 2017). Therefore, to enhance the electrical conductivity of both chitosan and CMC polymers, some modifications need to be carried out. Several materials were reported to form composites with them, such as conducting polymers (polyaniline, polypyrrole) and carbonaceous materials (graphene, CNTs and GO). Such composites are urgently needed for various bio-electronic products. For example, a composite ink was formulated of chitosan, graphite and glycerol for printing conductive electrodes for biosensing uric acid (Camargo et al. 2022). In addition, graphene-chitosan composite was utilized to immobilize glucose oxidase enzyme (Kang et al. 2009). It was employed to detect glucose biomolecule with high accuracy. On the same manner, a bending sensor was fabricated of graphene-CMC composite (Liu et al. 2020). It was useful in detecting strains for wearable devices.
Regarding the fabrication technique, additive manufacturing (AM) or solid freeform (SFF) techniques have been intensively investigated in the fabrication procedures of the various components of electronic and bio-electronic devices in microscale and even in nanoscale (Zhu et al. 2016; Wei et al. 2017). Specifically, InkJet printing (IJP) is always considered one of the most utilized techniques in the manufacturing of flexible electronics (Pinto et al. 2022). This can be related to the ease of its processing steps where it only requires the homogenous dispersion of the effective material in the proper solvent media in order to have an ink formulation with the optimum rheological characteristics (Sajedi-Moghaddam et al. 2020). In addition, IJP has various salient features including its high-resolution capabilities to print elements in both 2D as well as 3D manner (Choi et al. 2016), its low cost, being template-free technique and its drop-on-demand nature, which makes it wastes the least amount of the active material. This attitude makes it the first choice for researchers when asking for an eco-friendly fabrication technique (Singh et al. 2010; Hutchings and Martin 2012).
This work aimed to investigate the feasibility of modifying two biopolymers (chitosan and CMC) by their interaction with graphene to formulate potential biocomposite formulations for bio-electronic applications. The study was based mainly on a computational molecular model calculated via semiemprical quantum mechanical calculations. Then, it was supported by an experimental setup including printing a simple electrode of each biocomposite ink via InkJet printing technique and measuring their sheet resistance as well as charge mobility via Hall Effect measurement.
2 Calculation details
Structures were built up and their geometries were optimized using semiemprical quantum mechanical calculations at PM6 method (Stewart 2007) utilizing SCIGRESS 3.0 software that is executed at Spectroscopy Department, Physics Division, National Research Centre, NRC (Stewart 2009). Some physical and electronic features were extracted, such as total energy (E), total dipole moment (TDM), HOMO/LUMO bandgap energy (\(\varDelta\)E). Then, these optimized structures were utilized to calculate quantitative structure-activity relationship (QSAR) descriptors, such as charge, final heat of formation (FF), ionization potential (IP), Log P, molar refractivity (MR), molecular weight (Mwt) and polarizability (P).
3 Experimental section
3.1 Materials
Graphite (Fisher; general purpose grade, MW: 12.01 g/mol.), Sodium Citrate (Fisher, Anhydrous, Powder, 99-100.5%), Chitosan (ACROS ORGANIC; MW: 100,000-300,000 g/mol, (C6H11NO4)n), CMC (Sodium Salt, ACROS ORGANIC; MW: 250,000 g/mol), Ethanol (Fisher; absolute, 99.8% HPLC grade), Ultrapure water (Resistivity of > 18 MΩ.cm). All received chemicals were used without additional purification.
3.2 Graphene/Biopolymer ink formulation
The formulation steps of graphene-biopolymer ink composites have recently published in Bayoumy et al. 2023. In brief, the graphite powder was mechanically exfoliated in first via ball mill technique in order to get mechanically exfoliated graphene powder. The milling process was conducted in the presence of sodium citrate as a milling agent. Secondly, the resulting powder was rinsed numerous times with ultrapure water to get rid of any unnecessary ions (such as Na ions). Then, graphene was dispersed in a mixture of water and ethanol and probe sonicated for two hours to boost its dispersion. Individually, solutions of Cs and CMC were prepared and then mixed with graphene dispersion with 1:3 v/v ratio.
3.3 Microdrop InkJet printing of G-Cs and G-CMC biocomposites
The printing process was conducted via a microdrop InkJet printer (Autodrop Professional System; Microdrop Technologies GmbH, Norderstedt, Germany). One simple electrode was printed for each prepared sample in order to compare their electrical properties and investigate the effect of modifying Cs and CMC biopolymers with graphene structure.
3.4 Sheet resistance and charge mobility measurements
The sheet resistance of the printed electrodes of the G-Cs and G-CMC composites was measured utilizing four-point probe technique via Ossila Four-Point Probe System at 2D and 3D Printing Lab, Graphene Center of Excellence for Energy and Electronic Applications (GCEE), Egypt-Japan University of Science and Technology (E-JUST). Besides, the charge mobility in the printed samples was measured via Hall-effect using Ecopia 7000 in Energy Materials Lab, E-JUST.
4 Results and discussion
4.1 Building Model Molecules
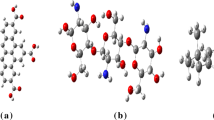
A modified graphene monolayer structure with two hydroxyl and two carboxyl groups (G) was built up. A monomer structure of Cs and CMC biopolymers were constructed. Cs structure has four functional groups (three OH and one NH2 groups), while CMC contains four OH groups and one terminal sodium atom. Figure 1(a, b and c) demonstrates the model molecules of the three proposed candidates. Then, G is chosen to modify the structure of these biopolymers in order to enhance their electrical features for bio-electronic applications. Therefore, four interaction probabilities were suggested for each composite structure. For example, chitosan could interact with G through physical interactions between its OH group and both OH and COOH of G. Other two possibilities could occur between Cs amine group and OH and COOH ones of G, as illustrated in Fig. 1(d, e, f and g). On the same manner, CMC with its OH and terminal Na active sites could interact with the OH and COOH ones of G, as shown in Fig. 1(h, i, j and k). Designing the computational model based on single layer of graphene and monomers of the biopolymer candidates always proves its feasibility to represent somehow the experimental work (Abdel-Karim et al. 2021; Bayoumy et al. 2020b; El-Naggar et al. 2021). This is frequently conducted in order to make the calculations much easier and represent an indicator for the planned work either via higher theoretical models or even experimentally.
Model molecules of (a) graphene (G), (b) chitosan (Cs), (c) CMC, (d) G(COOH)-Cs(NH2), (e) G(COOH)-Cs(OH), (f) G(OH)-Cs(NH2), (g) G(OH)-Cs(OH), (h) G(COOH)-CMC(OH), (i) G(COOH)-CMC(Na), (j) G(OH)-CMC(OH), (k) G(OH)-CMC(Na) calculated at PM6 level. [C in grey, H in white, O in red, Na in violet and N in blue]
4.2 Calculated physical parameters
The chemical structures and their proposed interactions were geometrically optimized at PM6 level of theory. Some electronic and physical parameters were extracted from the optimized files, such as total energy (E), TDM and HOMO/LUMO bandgap energy (∆E) as listed in Table 1.
The listed parameters in Table 1 include total energy, dipole moment and bandgap energy that offer valuable information about the stability, reactivity and electrical conductivity of the investigated structures and their proposed interactions; respectively. Here, a comparison was made between two formulations of graphene composites with biopolymers (Cs and CMC) in order to investigate the most suitable one for bio-electronic applications in biological entities. Regarding stability of the studied structures, the calculated energy can present considerable indication of such important side. Resulting energy of G, Cs and CMC are − 248944.799, -70857.321 and − 104404.98 kcal/mol; respectively referring to highly stable structures. The calculated energy of the proposed eight interactions reflect that the interaction site plays no significant role in the resulting energy values where all the computed energy data for the G-Cs composites are nearly the same. Likewise, all the resulting data are almost the same for all the G-CMC possibilities. The calculated energies for the G-Cs composites are about − 319,795 kcal/mol and they are roughly − 353,609 kcal/mol for the G-CMC ones. It is obvious that the resulting energy for the G-CMC interactions are quite lower than those for the G-Cs ones which indicates quite higher stability for the former group.
Turning to reactivity, TDM is always utilized as one of the considerable physical parameters for reactivity of chemical structures. Modification of graphene enhances the chemical reactivity of the resulting graphene structure with 5.006 Debye. TDM results of chitosan and CMC are 2.875 and 6.861 Debye; respectively ensuring that CMC monomer has much higher reactivity than chitosan one. This can be attributed to the presence of an extra OH group and a terminal alkali metal (Na atom) in CMC than chitosan structure. Therefore, the G-CMC composites have higher TDM results than those for G-Cs ones. TDM data of the G-Cs interactions range from 4.886 to 6.254 Debye. The most reactive interaction between G and Cs occurs between the most reactive functional groups of both structures (NH2 of Cs and COOH of G) which ensures their ability to rise the reactivity of the formulated structure. However, TDM values of the G-CMC composites starts from 5.59 and ends at 12.963 Debye for G(OH)-CMC(OH) interaction probability. These high results are reasonable since both G and CMC show high reactivity in their individual forms. On contrary to the calculated energy, the proposed active site for interaction has a significant part in determining the TDM and hence the reactivity of the resulting structures.
The calculated HOMO/LUMO bandgap energy reflects the required energy for transferring an electron from the valence band to the conduction one transferring a chemical substance from the ground state to the excited one to be more electrically conductive. It is always considered to describe one of the electronic features of the studied chemical structures on a theoretical basis. Chitosan and CMC biopolymers have values of 10.865 and 9.615 eV, respectively. The resulting bandgap values of these structures ensure their low electrical conductivity; hence graphene was chosen, for its unique electrical features, to enhance such poor behaviour. As previously expected, modification of Cs and CMC with graphene has a significant positive impact on their electrical features where their bandgap decreases to more than the half of their original results (~ 4.4 eV). G(COOH)-CMC(OH) interaction probability has the lowest calculated bandgap value indicating a much easier way for electron transfer from valence to conduction band, hence a structure with the highest conductivity with respect to others. Its calculated bandgap is 4.358 eV. Hence, their interactions with graphene boost their potential for emerging in bio-electronic applications. Since the resulting bandgap data are quite similar to each other, a further experimental study is required to differentiate between the two biocomposites on a real infield scale. Therefore, an experimental section was extended in the following sections.
4.3 Calculated QSAR descriptors
Quantitative structure-activity relationship (QSAR) parameters are usually computed as one of the promising computational methods in assessing various chemical entities having biological applications. This can be attributed to that they are characterized by being simple and easy to be accessible for researchers to predict biological activity of structures under investigation. That is why they always success in being the main focus of several latest articles (Bayoumy et al. 2020a; El-Sayed et al. 2018; Ma et al. 2019; Muthukumaran and Rajiniraja 2019; Omar et al. 2022). Table 2 presents some of the calculated QSAR descriptors, such as total charge (C), final heat of formation (FF), ionization potential (IP), Log P, molar refractivity (MR), molecular weight (Mwt) and polarizability (P) for structures under study at PM6 level.
The calculated total charge of all the proposed chemical structures is zero since they are all in the ground state. The second considered QSAR descriptor is final heat of formation (FF) which is the amount of heat either released or absorbed as a result of chemical structure formation (Elhaes et al. 2014). FF of G, Cs and CMC are calculated to be 8.205, -231.267 and − 399.309 kcal/mol. The computed FF of all the proposed interactions is of negative sign referring to exothermal reactions which are spontaneous and thermodynamically favourable. Similar to the the calculated total energy, FF is not affected significantly by the active site of interaction in either G-Cs or G-CMC composites. FF values of the G-Cs range from − 227.413 to -236.336 kcal/mol, while they start from − 416.822 to -453.521 kcal/mol for the G-CMC ones. Releasing more energy for the G-CMC composites than those for the other group, ensuring the ease of their formation relative to the latter group. After that, ionization potential (IP) is presented as the amount of energy needed for ejecting an electron from the outermost shell of a chemical structure and its conversion from a stable structure to a reactive one. IP data are − 7.222, -10.047 and − 9.454 eV for G, Cs and CMC; respectively. G addition to either Cs or CMC has no substantial impact on its IP. Table 2 confirms that all the calculated IP values are nearly equal to that of G ranging from − 7.04 to -7.444 eV. These results are like that of bandgap energies listed in Table 1. Regarding the behaviour of chemical structures in either aqueous or organic solvents, Log P or partition coefficient descriptor is considered as one of the most potential parameters. It is the ratio between the substance amount dissolved in organic solvent to that in aqueous one. Hence, its negative result reflects hydrophilic structure and vice versa. High positive Log P value for G structure indicates its high hydrophobic nature (10.593) while negative ones for the selected biopolymers reflect their hydrophilicity (-2.108 for Cs and − 2.076 for CMC). Addition of G to either Cs or CMC decreases its hydrophobicity to a small extent with 8.485 for the G-Cs and 8.517 for G-CMC. Furthermore, the site of interaction has no role on the calculated Log P values. Molar reflectivity (MR) is always considered among QSAR parameters. It is 235.031 for G structure and 37.581 and 55.651 for Cs and CMC; respectively. The calculated MR values equal 272.612 for all G-Cs composites and 290.683 for G-CMC ones. Then, molecular weight (Mwt) values are listed in Table 2. It is 786.739 a.u. for G, 179.171 a.u. for Cs and 288.227 a.u. for CMC. They are 965.91 and 1074.965 a.u. for the G-Cs and G-CMC; respectively. Finally, polarizability (P) is considered to reflect the ease of a substance to be polarized as a result of its exposure to an external field. It equals 134.666 A3 for G, 10.167 and 15.585 A3 for Cs and CMC; respectively. It ranges from 147.2 to 148.334 A3 for the G-Cs structures and from 146.86 to 154.997 A3 for G-CMC ones.
4.4 Measured electrodes resistance and charge carrier mobility
The formulated G-biopolymer ink formulations were utilized in an experimental setup via a microdrop InkJet printer. Printing parameters were optimized in order to print continuous and homogenous patterns as the illustrated example in figure S1. It was used to print a sixty-layer simple electrode of both biocomposite inks in order to have a simple electrode of both. Then, some electrical features were measured to evaluate the formulated biocomposites. Sheet resistance of both G-Cs and G-CMC printed electrodes was measured via four-point probe technique. In addition, Hall Effect measurement was utilized to determine the charge mobility in both electrodes. Figure 2 demonstrates the resulting sheet resistance and charge mobility data of both G-Cs and G-CMC electrodes.
Sheet resistance is one of the electrical measurements that can be utilized as an indicator for the electrical conductivity of the investigated samples. It refers to the impedance facing the electrons to flow through them. Likewise, charge mobility is another indicator for the ability of electrons to stream and the ease of their movement. Figure 2 illustrates that the measured sheet resistance of the G-Cs printed electrode is about five times higher than that of the G-CMC one. It is 6.8 ± 0.0624 kΩ/Sq. for the G-Cs while it is only 1.457 ± 0.0124 kΩ/Sq. for the other. Such result was confirmed by the measured Hall Effect test which showed that the charge mobility in the G-CMC electrode equals 24.8 cm2/V.s which is much greater than that in G-Cs one (1.3 cm2/V.s). This may refer to that CMC could emerge between graphene layers to a much more extent than Cs does reducing the graphene inter-sheet separation reducing G-CMC electrode resistance for electrons flow enhancing its electrical features (Rahman et al. 2020). These experimental results confirm the resulting theoretical data of the proposed model. Both parts agree that the G-CMC biocomposite has much more electrical conductivity than G-Cs one. Such results propose that the G-CMC biocomposite can be considered as a potential candidate for bio-electronic applications.
5 Conclusion
In conclusion, molecular modelling concepts were utilized for investigating the interaction of chitosan and carboxymethyl cellulose biopolymers with a monolayer of graphene. Geometry optimization calculations were carried out using PM6 method. Results illustrate that all interactions are stable, but the interaction site plays no role in determining the resulting energy values. The calculated energies for the G-Cs interactions are quite higher than those for the G-CMC ones indicating quite lower stability. The calculated QSAR parameters show that the addition of these biopolymers to the graphene structure lowers its hydrophobicity. On contrary to the calculated energy, the proposed active site for interaction has a significant part in determining the TDM, and hence reactivity of the resulting structures. Modification of Cs and CMC with graphene has a significant positive impact on enhancing their electrical features. The resulting bandgap of the proposed structures is lower than half of their original values. However, the chosen level of theory could not differentiate truthfully between the two proposed groups regarding their bandgap energies. Therefore, an experimental trial was carried out by printing a sixty-layer simple electrode of both biocomposite inks via a microdrop InkJet printer. Then, sheet resistance and charge mobility measurements were conducted. Results illustrate that the sheet resistance of the G-Cs printed electrode is about five times higher than that of G-CMC one (However, the resulting values are still quite high which need further enhancement). Such result was confirmed by the measured Hall Effect measurement which showed that the charge mobility in the G-CMC electrode is much greater compared to that in G-Cs one. These experimental results confirm the resulting theoretical data of the proposed model. Both parts agree that the G-CMC biocomposite has much more electrical conductivity proposing it as a potential candidate for bio-electronic applications.
Data availability
The data will be available upon request, contact Medhat A. Ibrahim medahmed6@yahoo.com.
References
Abdel-Karim, A., Elhaes, H., El-Kalliny, A.S., Badawy, M.I., Ibrahim, M., Gad-Allah, T.A.: Probing protein rejection behaviour of blended PES-based flat-sheet ultrafiltration membranes: A density functional theory (DFT) study. Spectrochim. Acta A Mol Biomol. Spectrosc. 238, 118399 (2020). https://doi.org/10.1016/j.saa.2020.118399
Abdel-Karim, A., Ismail, S.H., Bayoumy, A.M., Ibrahim, M., Mohamed, G.G.: Antifouling PES/Cu@Fe3O4 mixed matrix membranes: Quantitative structure–activity relationship (QSAR) modeling and wastewater treatment potentiality. Chem. Eng. J. 407, 126501 (2021). https://doi.org/10.1016/j.cej.2020.126501
Agnihotri, S.A., Mallikarjuna, N.N., Aminabhavi, T.M.: Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control Release. 100, 5–28 (2004). https://doi.org/10.1016/j.jconrel.2004.08.010
Ali, I., Asim, M., Khan, T.A.: Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manage. 113, 170–183 (2012). https://doi.org/10.1016/j.jenvman.2012.08.028
Aljawish, A., Muniglia, L., Klouj, A., Jasniewski, J., Scher, J., Desobry, S.: Characterization of films based on enzymatically modified chitosan derivatives with phenol compounds. Food Hydrocoll. 60, 551–558 (2016). https://doi.org/10.1016/j.foodhyd.2016.04.032
Basavaraja, C., Kim, J.K., Thinh, P.X., Huh, D.S.: Characterization and DC electrical conductivity of the composite films containing polyaniline-carboxymethyl cellulose. Polym. Compos. 33, 1541–1548 (2012). https://doi.org/10.1002/pc.22289
Bayoumy, A.M., Youssif, G., Elgohary, E.A., Husien, S., Deen, S.E., Albeltagy, H., Abdelnaby, N.M., Elhaes, D.R., Ibrahim, H.: Impact of solvation on the geometrical parameters of some amino acids. Biointerface Res. Appl. Chem. 8, 2, 567–570 (2019). https://doi.org/10.33263/LIANBS82.567570
Bayoumy, A.M., Elhaes, H., Osman, O., Kholmurodov, K.T., Hussein, T., Ibrahim, M.A.: Effect of nano metal oxides on heme molecule: Molecular and biomolecular approaches. Biointerface Res. Appl. Chem. 10, 1, 4837–4845 (2020a). https://doi.org/10.33263/briac101.837845
Bayoumy, A.M., Omar, A., El-Sayed, E.S.M., Ibrahim, M.: Removal of pharmaceuticals from aquatic environment using modified biomaterials. Biointerface Res. Appl. Chem. 10, 5986–5993 (2020b). https://doi.org/10.33263/briac104.986993
Bayoumy, A.M., Ibrahim, M., Osman, A., Abdelmoneim, A.: InkJet-printed Supercapacitor Electrodes of Graphene-Carboxymethyl Cellulose Biocomposite Ink. In Press, Key Eng. Mater. The 13th International Conference on Advanced Materials Research (ICAMR 2023), Singapore
Bulat, F.A., Toro-Labbé, A., Brinck, T., Murray, J.S., Politzer, P.: Quantitative analysis of molecular surfaces: Areas, volumes, electrostatic potentials and average local ionization energies. J. Mol. Model. 16, 1679–1691 (2010). https://doi.org/10.1007/s00894-010-0692-x
Camargo, J.R., Silva, T.A., Rivas, G.A., Janegitz, B.C.: Novel eco-friendly water-based conductive ink for the preparation of disposable screen-printed electrodes for sensing and biosensing applications. Electrochim. Acta. 409, 139968 (2022). https://doi.org/10.1016/j.electacta.2022.139968
Cancelliere, R., Albano, D., Brugnoli, B., Buonasera, K., Leo, G., Margonelli, A., Rea, G.: Electrochemical and morphological layer-by-layer characterization of electrode interfaces during a label-free impedimetric immunosensor build-up: The case of ochratoxin A. Appl. Surf. Sci. 567, 150791 (2021). https://doi.org/10.1016/j.apsusc.2021.150791
Chen, H., Fan, M.: Chitosan/carboxymethyl cellulose polyelectrolyte complex scaffolds for pulp cells regeneration. J. Bioact Compat. Polym. 22, 5, 475–491 (2007). https://doi.org/10.1177/0883911507081329
Chen, P., Xie, F., Tang, F., McNally, T.: Structure and properties of thermomechanically processed chitosan/carboxymethyl cellulose/graphene oxide polyelectrolyte complexed bionanocomposites. Int. J. Biol. Macromol. 158, 420–429 (2020). https://doi.org/10.1016/j.ijbiomac.2020.04.259
Choi, K.H., Yoo, J.T., Lee, C.K., Lee, S.Y.: All-inkjet-printed, solid-state flexible supercapacitors on paper. Energy Environ. Sci. 9, 2812–2821 (2016). https://doi.org/10.1039/C6EE00966B
Di Tinno, A., Cancelliere, R., Mantegazza, P., Cataldo, A., Paddubskaya, A., Ferrigno, L., Kuzhir, P., Maksimenko, S., Shuba, M., Maffucci, A., et al.: Sensitive detection of Industrial Pollutants using modified Electrochemical Platforms. Nanomater. 12, 1779 (2022). https://doi.org/10.3390/nano12101779
El Knidri, H., Belaabed, R., Addaou, A., Laajeb, A., Lahsini, A.: Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 120, 1181–1189 (2018). https://doi.org/10.1016/j.ijbiomac.2018.08.139
El-Naggar, M.E., Abdel-Karim, A., Radwan, E.K., Sharmoukh, W., Wassel, A.R., Bayoumy, A.M., Ibrahim, M.: Experimental and theoretical investigations on fouling resistant cellulose acetate/SiO2 NPs/PEDOT ultrafiltration nanocomposite membranes. J. Clean. Prod. 324, 129288 (2021). https://doi.org/10.1016/j.jclepro.2021.129288
El-Sayed, E.M., Omar, A., Bayoumy, A.M., Ibrahim, M.: Chitosan Ibuprofen Interaction: Modeling Approach. Sens. Lett. 16, 347–355 (2018). https://doi.org/10.1166/sl.2018.3956
El-Sayed, N., El-bakary, M., Ibrahim, M., Elgamal, M.: Molecular modeling analysis of chitosan-dopamine blend with iron oxide nanoparticles for tissue engineering applications. Biointerface Res. Appl. Chem. 11, 12483–12494 (2021). https://doi.org/10.33263/briac115.1248312494
Elhaes, H., Saleh, N.A., Omar, A., Ibrahim, M.: Molecular spectroscopic study of fulleropyrrolidine carbodithioic acid. J. Comput. Theor. Nanosci. 11, 2136–2140 (2014). https://doi.org/10.1166/jctn.2014.3618
Ezzat, H.A., Hegazy, M.A., Nada, N.A., Ibrahim, M.A.: Effect of nano metal oxides on the electronic properties of cellulose, chitosan and sodium alginate. Biointerface Res. Appl. Chem. 9, 4143–4149 (2019). https://doi.org/10.33263/briac94.143149
Fahim, A.M., Shalaby, M.A., Ibrahim, M.A.: Microwave-assisted synthesis of novel 5-aminouracil-based compound with DFT calculations. J. Mol. Struct. 1194, 211–226 (2019). https://doi.org/10.1016/j.molstruc.2019.04.078
Ferreira, F.V., Mariano, M., Lepesqueur, L.S.S., Pinheiro, I.F., Santos, L.G., Burga-Sanchez, J., Souza, D.H.S., Koga-Ito, C.Y., et al.: Silver nanoparticles coated with dodecanethiol used as fillers in non-cytotoxic and antifungal PBAT surface based on nanocomposites. Mater. Sci. Eng. C. 98, 800–807 (2019). https://doi.org/10.1016/j.msec.2019.01.044
Gamil, M., Tabata, O., Nakamura, K., El-Bab, A.M.R.F., El-Moneim, A.A.: Investigation of a new high sensitive micro-electromechanical strain gauge sensor based on graphene piezoresistivity. Key Eng. Mater. 605, 207–210 (2014). https://doi.org/10.4028/www.scientific.net/kem.605.207
Hadad, C., González-Domínguez, J.M., Armelloni, S., Mattinzoli, D., Ikehata, M., Istif, A., Ostric, A., Cellesi, F., Alferi, C.M., Messa, P.: Graphene quantum dots: From efficient preparation to safe renal excretion. Nano Res. 14, 3, 674–683 (2021). https://doi.org/10.1007/s12274-020-3096-y
Hadwiger, L.A.: Multiple effects of chitosan on plant systems: Solid science or hype. Plant. Sci. 208, 42–49 (2013). https://doi.org/10.1016/j.plantsci.2013.03.007
Hassan, S., Suzuki, M., El-Moneim, A.A.: Synthesis of MnO2–chitosan nanocomposite by one-step electrodeposition for electrochemical energy storage application. J. Power Sources. 246, 68–73 (2014a). https://doi.org/10.1016/j.jpowsour.2013.06.085
Hassan, S., Suzuki, M., El-Moneim, A.A.: Facile synthesis of MnO2/graphene electrode by two-steps electrodeposition for energy storage application. Int. J. Electrochem. Sci. 9, 12, 8340–8354 (2014b)
Hutchings, I.M., Martin, G.D.: Inkjet Technology for Digital Fabrication. John Wiley & Sons (2012)
Ibrahim, M., Mahmoud, A.A.: Computational notes on the reactivity of some functional groups. J. Comput. Theor. Nanosci. 6, 1523–1526 (2009). https://doi.org/10.1166/jctn.2009.1205
Ibrahim, M., Mahmoud, A.A., Osman, O., Abd El-Aal, M., Eid, M.: Molecular spectroscopic analyses of gelatin. spectrochim Acta Part A. 81, 1, 724–729 (2011). https://doi.org/10.1016/j.saa.2011.07.012
Ibrahim, A., Elhaes, H., Meng, F., Ibrahim, M.: Effect of hydration on the physical properties of glucose. Biointerface Res. Appl. Chem. 9, 4, 4114–4118 (2019). https://doi.org/10.33263/briac94.114118
Jarmila, V., Eva, V.: Chitosan derivatives with antimicrobial, antitumour and antioxidant activities-a review. Curr. Pharm. Des. 17, 3596–3607 (2011). https://doi.org/10.2174/138161211798194468
Kang, X., Wang, J., Wu, H., Aksay, I.A., Liu, J., Lin, Y.: Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 25, 901–905 (2009). https://doi.org/10.1016/j.bios.2009.09.004
Kim, D.G., Jeong, Y.I., Choi, C., Roh, S.H., Kang, S.K., Jang, M.K., Nah, J.W.: Retinol-encapsulated low molecular water-soluble chitosan nanoparticles. Int. J. Pharm. 319, 130–138 (2006). https://doi.org/10.1016/J.ijpharm.2006.03.040
Koneru, A., Dharmalingam, K., Anandalakshmi, R.: Cellulose based nanocomposite hydrogel films consisting of sodium carboxymethylcellulose–grapefruit seed extract nanoparticles for potential wound healing applications. Int. J. Biol. Macromol. 148, 833–842 (2020). https://doi.org/10.1016/j.ijbiomac.2020.01.018
Lee, B.R., Oh, E.S.: Effect of molecular weight and degree of substitution of a sodium-carboxymethyl cellulose binder on Li4Ti5O12 anodic performance. J. Phys. Chem. C. 117, 4404–4409 (2013). https://doi.org/10.1021/jp311678p
Liu, P., Chen, M., Xiong, C., Cao, X., Wang, H.: Flexible and highly sensitive graphene/carboxymethyl cellulose films for bending sensing. J. Mater. Sci. : Mater. Electron. 31, 14118–14127 (2020). https://doi.org/10.1007/s10854-020-03966-8/tables/2
Ma, W., Wang, Y., Chu, D., Yan, H.: 4D-QSAR and MIA-QSAR study on the Bruton’s tyrosine kinase (btk) inhibitors. J. Mol. Graph Model. 92, 357–362 (2019). https://doi.org/10.1016/j.jmgm.2019.08.009
Maruyama, S., Miyauchi, Y., Edamura, T., lgarashi, Y., Chiashi, S., Murakami, Y.: Synthesis of single-walled carbon nanotubes with narrow diameter-distribution from fullerene. Chem. Phys. Lett. 375, 553–559 (2003). https://doi.org/10.1016/S0009-2614(03)00907-2
Matinfar, M., Mesgar, A.S., Mohammadi, Z.: Evaluation of physicochemical, mechanical and biological properties of chitosan/carboxymethyl cellulose reinforced with multiphasic calcium phosphate whisker-like fibers for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 100, 341–353 (2019). https://doi.org/10.1016/j.msec.2019.03.015
Mohan, T., Štiglic, D., Beaumont, A., Konnerth, M., Gürer, J., Makuc, F., Maver, D., Gradišnik, U., Plavec, L., Kargl, J., Kleinschek, R.S.: Generic method for designing self-standing and dual porous 3d bioscaffolds from cellulosic nanomaterials for tissue engineering applications. ACS Appl. Bio Mater. 3, 1197–1209 (2020). https://doi.org/10.1021/acsabm.9b01099
Morsy, M., Ibrahim, M., Yuan, Z., Meng, F.: Graphene Foam decorated with ZnO as a Humidity Sensor. IEEE Sens. J. 20, 1721–1729 (2020). https://doi.org/10.1109/jsen.2019.2948983
Muthukumaran, P., Rajiniraja, M.: Aug-MIA-QSAR based strategy in bioactivity prediction of a series of flavonoid derivatives as HIV-1 inhibitors. J. Theor. Biol. 469, 18–24 (2019). https://doi.org/10.1016/j.jtbi.2019.02.019
Mydlova, L., Sahraoui, B., Waszkowska, K., El Karout, H., Makowska-Janusik, M., Migalska-Zalas, A.J.M.: Computational and experimental study of nonlinear optical susceptibilities of composite materials based on PVK polymer matrix and benzonitrile derivatives. Mater. 15, 6, 2073 (2022). https://doi.org/10.3390/ma15062073
Nitschke, M., Marangon, C.A.: Microbial surfactants in nanotechnology: Recent trends and applications. Crit. Rev. Biotechnol. 42, 2, 294–310 (2022). https://doi.org/10.1080/07388551.2021.1933890
Omar, A., Bayoumy, A.M., Aly, A.A.: Functionalized Graphene Oxide with Chitosan for dopamine biosensing. J. Funct. Biomater. 13 (2022). https://doi.org/10.3390/jfb13020048
Phillips, M.C., Mousa, S.A.: Clinical application of nano-targeting for enhancing chemotherapeutic efficacy and safety in cancer management. Nanomedicine. 17, 6, 405–421 (2022). https://doi.org/10.2217/nnm-2021-0361
Pinto, R.M.R., Nemala, S.S., Faraji, M., Capasso, A., Vinayakumar, K.B.: Inkjet-Printing of Carbon Nano Onions for Sensor Applications in Flexible Printed Electronics. IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Vienna, Austria 1–4 (2022). https://doi.org/10.1109/FLEPS53764.2022.9781548
Rahman, S.U., Bilal, S., ul Haq Ali Shah, A.: Synthesis and characterization of polyaniline-chitosan patches with enhanced stability in physiological conditions. Polymers. 12, 12, 2870 (2020). https://doi.org/10.3390/polym12122870
Rashed, A.E., El-Moneim, A.A.: Two steps synthesis approach of MnO2/graphene nanoplates/graphite composite electrode for supercapacitor application. Mater. Today Energy. 3, 24–31 (2017). https://doi.org/10.1016/j.mtener.2017.02.004
Refaat, A., Elhaes, H., Ammar, N.S., Ibrahim, H.S., Ibrahim, M.: Green Route for the removal of pb from aquatic environment. Comb. Chem. High. Throughput Screen. 23, 587–598 (2020). https://doi.org/10.2174/1386207323666200127123349
Rinaudo, M.: Chitin and chitosan: Properties and applications. Prog Polym. Sci. 31, 603–632 (2006). https://doi.org/10.1016/j.progpolymsci.2006.06.001
Rivero, S., García, M.A., Pinotti, A.: Correlations between structural, barrier, thermal and mechanical properties of plasticized gelatin films. Innov. Food Sci. Emerg. Technol. 11, 369–375 (2010). https://doi.org/10.1016/j.ifset.2009.07.005
Sabnis, S., Block, L.H.: Chitosan as an enabling excipient for drug delivery systems. I. Molecular modifications. Int. J. Biol. Macromol. 27, 181–186 (2000). https://doi.org/10.1016/s0141-8130(00)00118-5
Sabry, N.M., Tolba, S.T.M., Abdel-Gawad, F.K., Bassem, S.M., Nassar, H., El-Taweel, G.E., Ibrahim, M.A.: On the molecular modelling analyses of the interaction between nano zinc oxide and bacteria. Biointerface Res. Appl. Chem. 8, 3, 3294–3297 (2018).
Sadeghi, S., Nourmohammadi, J., Ghaee, A., Soleimani, N.: Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 147, 1239–1247 (2020). https://doi.org/10.1016/j.ijbiomac.2019.09.251
Sajedi-Moghaddam, A., Rahmanian, E., Naseri, N.: Inkjet-Printing Technology for Supercapacitor Application: Current state and perspectives. ACS Appl. Mater. Interfaces. 12, 31, 34487–34504 (2020). https://doi.org/10.1021/acsami.0c07689
Sakki, B., Taboukhat, S., Messaadia, L., Guergouri, M., Bouraiou, A., Nasri, R., Figa, V., Bouchouit, K., Sahraoui, B.J.T.E.P.J.D.: DFT analysis and third-harmonic generation properties of one series of push–pull benzylidenemalononitrile derivatives. Eur. Phys. J. D. 76, 6, 1–11 (2022). https://doi.org/10.1140/epjd/s10053-022-00424-4
Saladino, M.L., Markowska, M., Carmone, C., Cancemi, P., Alduina, R., Presentato, A., Scaffaro, R., Biały, D., Hasiak, M., Hreniak, D., Wawrzyńska, M.: Graphene oxide carboxymethylcellulose nanocomposite for dressing materials. Mater. 13, 1980 (2020). https://doi.org/10.3390/ma13081980
Saleh, N.A., Elhaes, H., Ibrahim, M.: Design and development of some viral protease inhibitors by qsar and molecular modeling studies. Viral proteases and their inhibitors. 1st Edition, Elsevier. 25–58 (2017). https://doi.org/10.1016/b978-0-12-809712-0.00002-2
Sarkar, C., Anuvrat, K., Garai, S., Sahu, S.K., Chakraborty, J.: One pot method to synthesize three-dimensional porous hydroxyapatite nanocomposite for bone tissue engineering. J. Porous Mater. 27, 225–235 (2020). https://doi.org/10.1007/s10934-019-00805-y/figures/8
Sharmila, G., Muthukumaran, C., Kirthika, S., Keerthana, S., Kumar, N.M., Jeyanthi, J.: Fabrication and characterization of Spinacia oleracea extract incorporated alginate/carboxymethyl cellulose microporous scaffold for bone tissue engineering. Int. J. Biol. Macromol. 156, 430–437 (2020). https://doi.org/10.1016/j.ijbiomac.2020.04.059
Singh, A., Amiji, M.M.: Application of nanotechnology in medical diagnosis and imaging. Curr. Opin. Biotechnol. 74, 241–246 (2022). https://doi.org/10.1016/j.copbio.2021.12.011
Singh, M., Haverinen, H.M., Dhagat, P., Jabbour, G.E.: Inkjet Printing—Process and its applications. Adv. Mater. 22, 6, 673–685 (2010). https://doi.org/10.1002/adma.200901141
Singh, B.N., Panda, N.N., Mund, R., Pramanik, K.: Carboxymethyl cellulose enables silk fibroin nanofibrous scaffold with enhanced biomimetic potential for bone tissue engineering application. Carbohydr. Polym. 151, 335–347 (2016). https://doi.org/10.1016/j.carbpol.2016.05.088
Sohrabi, H., Arbabzadeh, O., Khaaki, P., Khataee, A., Majidi, M.R., Orooji, Y.: Patulin and Trichothecene: Characteristics, occurrence, toxic effects and detection capabilities via clinical, analytical and nanostructured electrochemical sensing/biosensing assays in foodstuffs. Crit. Rev. Food Sci. Nutr. 62, 20, 5540–5568 (2022). https://doi.org/10.1080/10408398.2021.1887077
Stewart, J.J.P.: Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 13, 1173–1213 (2007). https://doi.org/10.1007/s00894-007-0233-4/figures/10
Stewart, J.J.P.: SCIGRESS, Version 2.9.0, Fujitsu Limited, Sunnyvale, Calif, USA, (2009)
Suginta, W., Khunkaewla, P., Schulte, A.: Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev. 113, 5458–5479 (2013). https://doi.org/10.1021/cr300325r
Tănase, E.E., Râpă, M., Popa, O.: Biopolymers based on renewable resources-a review. Sci. Bull. Ser. F Biotechnol. 18, 188–195 (2014)
Thiruvengadam, M., Rajakumar, G., Chung, I.M.: Nanotechnology: current uses and future applications in the food industry. 3 Biotech. 8, 74 (2018). https://doi.org/10.1007/s13205-018-1104-7
Verma, N., Pramanik, K., Singh, A.K., Biswas, A.: Design of magnesium oxide nanoparticle incorporated carboxy methyl cellulose/poly vinyl alcohol composite film with novel composition for skin tissue engineering. Technol. 37, 706–716 (2021). https://doi.org/10.1080/10667857.2021.1873634
Waite, S.L., Karton, A., Chan, B., Page, A.J.: Thermochemical stabilities of giant fullerenes using density functional tight binding theory and isodesmic-type reactions. J. Comput. Chem. 42, 4, 222–230 (2021). https://doi.org/10.1002/jcc.26449
Wei, M., Zhang, F., Wang, W., Alexandridis, P., Zhou, C., Wu, G.: 3D direct writing fabrication of electrodes for electrochemical storage devices. J. Power Sources. 354, 134–147 (2017). https://doi.org/10.1016/j.jpowsour.2017.04.042
Zhang, Y.J., Gao, B., Liu, X.W.: Topical and effective hemostatic medicines in the battlefield. Int. J. Clin. Exp. Med. 8, 1, 10–19 (2015).
Zheng, S., Chen, H., Zhang, T., Yao, Y., Chen, Y., Zhang, S., Bai, B.: Gene-modified BMSCs encapsulated with carboxymethyl cellulose facilitate osteogenesis in vitro and in vivo. J. Biomater. Appl. 35, 814–822 (2021). https://doi.org/10.1177/0885328220948030
Zhu, C., Liu, T., Qian, F., Han, T.Y.-J., Duoss, E.B., Kuntz, J.D., Spadaccini, C.M., Worsley, M.A., Li, Y.: Supercapacitors based on three-dimensional hierarchical Graphene Aerogels with periodic macropores. Nano Lett. 16, 6, 3448–3456 (2016). https://doi.org/10.1021/acs.nanolett.5b04965
Acknowledgements
The authors show their gratitude for the Missions Sector-Higher Education Ministry-MOHE, Egypt, for providing a Ph.D. scholarship fund, and for Science and Technology Development Fund (STDF) for funding this research work through Grant No. 31306 entitled “Graphene Center for Energy and Electronic Applications”.
Funding
This research was supported financially by Science and Technology Fund (STDF), Egypt, Grant No. 31306 entitled “Graphene Center for Energy and Electronic Applications”.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A. B.; Build up the model molecules, conduct the calculations and experimental part, write the manuscript and analyse the data. M. I., Assign the problem, supervise the conducted calculations, contribute in writing and analysing data and supervise the work. A.O.;: Contribute to the problem assignment, revise the manuscript and supervise the work. A. A.; Assign the problem, supervise the conducted experimental work, contribute to analysing data; receive the fund and supervise the work. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This work is not applicable for both human and/or animal studies.
Competing interests
I declare that the authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bayoumy, A.M., A. Ibrahim, M., Osman, A. et al. Interaction of biopolymers with graphene for bio-electronic applications. Opt Quant Electron 55, 622 (2023). https://doi.org/10.1007/s11082-023-04827-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-023-04827-4