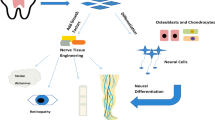
Abstract
Neurological disorders are the leading cause of disability and the world's second leading cause of death. Despite the availability of significant knowledge to reduce the burden of some neurological disorders, various studies are exploring more effective treatment options. While the human body can repair and regenerate damaged tissue through stem cell recruitment, nerve regeneration in case of injury is minimal due to the restriction on the location of nerve stem cells. Recently, different types of stem cells extracted from various tissues have been used in combination with natural stimuli to treat neurologic disorders in neuronal tissue engineering. Flavonoids are polyphenolic compounds that can induce the differentiation of stem cells into neurons and stimulate stem cell proliferation, migration, and survival. They can also increase the secretion of nutritional factors from stem cells. In addition to the effects that flavonoids can have on stem cells, they can also have beneficial therapeutic effects on the nervous system alone. Therefore, the simultaneous use of these compounds and stem cells can multiply the therapeutic effect. In this review, we first introduce flavonoid compounds and provide background information on stem cells. We then compile available reports on the effects of flavonoids on stem cells for the treatment of neurological disorders.




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper.
Abbreviations
- AMSC:
-
Adipose mesenchymal stem cell
- Aß42:
-
ß-Amyloid 1–42
- AHR:
-
Aryl hydrocarbon receptor
- APP:
-
Amyloid precursor protein
- BDNF:
-
Amyloid precursor protein
- BMSC:
-
Bone marrow Mesenchymal stem cell
- CNTF:
-
Ciliary neurotrophic factor
- CREB:
-
CAMP-response element binding protein
- DCX:
-
Doublecortin
- 3,2'-DHF:
-
3,2'-Dihydroxyflavone
- 7,8-DHF:
-
7,8-Dihydroxyflavone
- EGCG:
-
Epigallocatechin gallate
- EGR-1:
-
Early growth response protein 1
- Erk 1/2:
-
Extracellular signal-regulated kinase1/2
- GAP-43:
-
Growth-associated protein 43 kDa; also known as neuromodulin
- GDNF:
-
Glial cell line-derived neurotrophic factor
- GFAP:
-
Glial fibrillary acidic protein
- hAMSC:
-
Human Adipose mesenchymal stem cell
- ICA:
-
Icariin
- ICAII:
-
IcarisideII
- iPSC:
-
Induced pluripotent stem cells
- MEK:
-
Mitogen-activated protein kinase kinase
- MESC:
-
Mouse Embryonic stem cells
- NeuN:
-
Neuronal nuclear protein
- TH:
-
Tyrosine hydroxylase
- TRX:
-
Troxerutin
- NGF:
-
Nerve growth factor
- NSC:
-
Neural stem cell
- NPC:
-
Neural progenitor cell
- PKB also known as Akt:
-
Protein kinase B
- PI3K:
-
Phosphoinositide 3-kinase
- PKCd:
-
Protein Kinase C Delta
- PPAR-β:
-
Peroxisome proliferator-activated receptor β
- PR:
-
Puerarin
- Q3GA:
-
Quercetin-3-O-glucuronide
- STAT3:
-
Signal transducer and activator of transcription 3
- SGZ:
-
Subgranular region
- SVZ:
-
Subventricular zone
- TrkB:
-
Tropomyosin receptor kinase B
- TUJ 1:
-
Neuron-specific class III beta-tubulin
References
(2017) Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16(11):877–897
Lindvall O, Kokaia Z (2010) Stem cells in human neurodegenerative disorders–time for clinical translation? J Clin Invest 120(1):29–40
Lunn JS et al (2011) Stem cell technology for neurodegenerative diseases. Ann Neurol 70(3):353–361
(2019) Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(5):459–480
Alessandrini M et al (2019) Stem cell therapy for neurological disorders. S Afr Med J 109(8b):70–77
Grochowski C, Radzikowska E, Maciejewski R (2018) Neural stem cell therapy—brief review. Clin Neurol Neurosurg 173:8–14
Martínez-Morales P et al (2013) Progress in stem cell therapy for major human neurological disorders. Stem Cell Rev Rep 9(5):685–699
Mathesius U (2018) Flavonoid functions in plants and their interactions with other organisms. MDPI
Kumar, S. and A.K. Pandey, Chemistry and biological activities of flavonoids: an overview. The scientific world journal, 2013. 2013.
Panche, A.N., A.D. Diwan, and S.R. Chandra, Flavonoids: an overview. Journal of nutritional science, 2016. 5.
Yang X et al (2015) Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends Food Sci Technol 44(1):93–104
Ahmed IF, MagedSaad A-K (2017) Isoflavonoids. In: Goncalo CJ (ed) Flavonoids. IntechOpen, Rijeka
Kumar P, Ahamad T, Mishra DP, Khan MF (2020) Plant neoflavonoids: chemical structures and biological functions. In: Swamy M (ed) Plant-derived bioactives. Springer, Singapore, pp 35–57
Rice-Evans CA, Packer L (2003) Flavonoids in health and disease. CRC Press, Boca Raton
Havsteen B (1983) Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol 32(7):1141–1148
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J 2013:162750–162750
Baier A, Szyszka R (2020) Compounds from natural sources as protein kinase inhibitors. Biomolecules 10(11):1546
Abotaleb M et al (2018) Flavonoids in cancer and apoptosis. Cancers 11:E28. https://doi.org/10.3390/cancers11010028
Kurutas EB (2016) The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J 15(1):71
Cui J et al (2017) Bone marrow mesenchymal stem cell transplantation increases GAP-43 expression via ERK1/2 and PI3K/Akt pathways in intracerebral hemorrhage. Cell Physiol Biochem 42(1):137–144
Cazarolli LH et al (2008) Flavonoids: prospective drug candidates. Mini Rev Med Chem 8(13):1429–1440
Romano B et al (2013) Novel insights into the pharmacology of flavonoids. Phytother Res 27(11):1588–1596
Williams RJ, Spencer JP, Rice-Evans C (2004) Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med 36(7):838–849
Spencer JP (2008) Flavonoids: modulators of brain function? Br J Nutr 99:60–77
Mandel S, Youdim MB (2004) Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic Biol Med 37(3):304–317
Vafeiadou K, Vauzour D, Spencer J (2007) Neuroinflammation and its modulation by flavonoids. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders) 7(3):211–224
Basu P, Basu A (2020) In vitro and in vivo effects of flavonoids on peripheral neuropathic pain. Molecules 25(5):1171
Innos J, Hickey MA (2021) Using rotenone to model Parkinson’s disease in mice: a review of the role of pharmacokinetics. Chem Res Toxicol 34(5):1223–1239
Ramalingam M et al (2022) Autophagy Signaling by Neural-Induced Human Adipose Tissue-Derived Stem Cell-Conditioned Medium during Rotenone-Induced Toxicity in SH-SY5Y Cells. Int J Mol Sci 23(8):4193
Inden M et al (2013) Therapeutic effects of human mesenchymal and hematopoietic stem cells on rotenone-treated parkinsonian mice. J Neurosci Res 91(1):62–72
Ramalingam, M., S. Jang, and H.S. Jeong, Neural-Induced Human Adipose Tissue-Derived Stem Cells Conditioned Medium Ameliorates Rotenone-Induced Toxicity in SH-SY5Y Cells. Int J Mol Sci, 2021. 22(5).
Abdel-Rahman M et al (2018) Therapeutic efficacy of olfactory stem cells in rotenone induced Parkinsonism in adult male albino rats. Biomed Pharmacother 103:1178–1186
Ramalingam M, Jang S, Jeong HS (2021) Therapeutic effects of conditioned medium of neural differentiated human bone marrow-derived stem cells on rotenone-induced alpha-synuclein aggregation and apoptosis. Stem Cells Int 2021:6658271
Ishido M, Suzuki J (2010) Inhibition by rotenone of mesencephalic neural stem-cell migration in a neurosphere assay in vitro. Toxicol In Vitro 24(2):552–557
Alessandrini, M., et al., Stem cell therapy for neurological disorders. South African Medical Journal, 2019. 109(8 Supplement 1): p. S71-S78.
Bongso A, Richards M (2004) History and perspective of stem cell research. Best Pract Res Clin Obstet Gynaecol 18(6):827–842
Ilic D, Polak JM (2011) Stem cells in regenerative medicine: introduction. Br Med Bull 98(1):117–126
Smith A (2006) A glossary for stem-cell biology. Nature 441(7097):1060–1060
Kolios G, Moodley Y (2013) Introduction to stem cells and regenerative medicine. Respiration 85(1):3–10
Rossant J (2008) Stem cells and early lineage development. Cell 132(4):527–531
Brown C et al (2019) Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med 13(9):1738–1755
Kumar A et al (2017) Current perspective of stem cell therapy in neurodegenerative and metabolic diseases. Mol Neurobiol 54(9):7276–7296
Inden M et al (2016) Therapeutic effects of mesenchymal stem cells for Parkinson’s disease. Ann Neurodegener Dis 1(1):1002–1009
Gugliandolo A, Bramanti P, Mazzon E (2017) Mesenchymal stem cell therapy in Parkinson’s disease animal models. Curr Res Transl Med 65(2):51–60
Lie DC et al (2002) The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci 22(15):6639–6649
Amariglio N et al (2009) Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 6(2):e1000029
Shih C-C et al (2007) Human embryonic stem cells are prone to generate primitive, undifferentiated tumors in engrafted human fetal tissues in severe combined immunodeficient mice. Stem Cells Dev 16(6):893–902
Hong SG, Dunbar CE, Winkler T (2013) Assessing the risks of genotoxicity in the therapeutic development of induced pluripotent stem cells. Mol Ther 21(2):272–281
Lazennec G, Jorgensen C (2008) Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells 26(6):1387–1394
Mohib K, Allan D, Wang L (2010) Human embryonic stem cell-extracts inhibit the differentiation and function of monocyte-derived dendritic cells. Stem Cell Rev Rep 6(4):611–621
Herberts CA, Kwa MS, Hermsen HP (2011) Risk factors in the development of stem cell therapy. J Transl Med 9(1):1–14
Zou J et al (2019) Mechanisms shaping the role of ERK1/2 in cellular senescence (Review). Mol Med Rep 19(2):759–770
Albert-Gascó H et al (2020) MAP/ERK signaling in developing cognitive and emotional function and its effect on pathological and neurodegenerative processes. Int J Mol Sci 21(12):4471
Kong T et al (2019) Role of the extracellular signal-regulated kinase 1/2 signaling pathway in ischemia-reperfusion injury. Front Physiol 10:1038
Xu F et al (2020) RETRACTED ARTICLE: roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci 10(1):54
Holborn J, et al (2022) Interference of neuronal TrkB signaling by the cannabis-derived flavonoids cannflavins A and B. bioRxiv. p. 2022.02.03.478734
Li W-Y et al (2020) Combinatory transplantation of mesenchymal stem cells with flavonoid small molecule in acellular nerve graft promotes sciatic nerve regeneration. J Tissue Eng 11:2041731420980136–2041731420980136
Ge P, Guo Y, Shen J (2019) IcarisideII facilitates the differentiation of ADSCs to SCs via let-7i/STAT3 axis to preserve erectile function. Biol Res 52(1):54
Zheng T et al (2018) Icariside II promotes the differentiation of adipose tissue-derived stem cells to Schwann cells to preserve erectile function after cavernous nerve injury. Mol Cells 41(6):553–561
Zheng T et al (2020) Icariside II facilitates the differentiation of ADSCs to schwann cells and restores erectile dysfunction through regulation of miR-33/GDNF axis. Biomed Pharmacother 125:109888
Liu D et al (2018) Icariin and mesenchymal stem cells synergistically promote angiogenesis and neurogenesis after cerebral ischemia via PI3K and ERK1/2 pathways. Biomed Pharmacother 108:663–669
Fu X et al (2018) Stimulatory effect of icariin on the proliferation of neural stem cells from rat hippocampus. BMC Complement Altern Med 18(1):34–34
Lu Q et al (2020) Icariin sustains the proliferation and differentiation of Aβ(25–35)-treated hippocampal neural stem cells via the BDNF-TrkB-ERK/Akt signaling pathway. Neurol Res 42(11):936–945
Kuang W et al (2021) Icariside II promotes the differentiation of human amniotic mesenchymal stem cells into dopaminergic neuron-like cells. In Vitro Cell Dev Biol 57(4):457–467
Li R et al (2013) Puerarin attenuates neuronal degeneration in the substantia nigra of 6-OHDA-lesioned rats through regulating BDNF expression and activating the Nrf2/ARE signaling pathway. Brain Res 1523:1–9
Zhu G et al (2014) Neuroprotective Effects of Puerarin on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson’s Disease Model in Mice. Phytother Res 28(2):179–186
Cheng Y-F et al (2009) Involvement of ubiquitin proteasome system in protective mechanisms of Puerarin to MPP+-elicited apoptosis. Neurosci Res 63(1):52–58
Shiying L et al (2018) Puerarin promoted proliferation and differentiation of dopamine-producing cells in Parkinson’s animal models. Biomed Pharmacother 106:1236–1242
Itoh T et al (2012) (-)-Epigallocatechin-3-gallate increases the number of neural stem cells around the damaged area after rat traumatic brain injury. J Neural Transm (Vienna) 119(8):877–890
Zhang Y et al (2016) Effects of epigallocatechin-3-gallate on proliferation and differentiation of mouse cochlear neural stem cells: Involvement of PI3K/Akt signaling pathway. Eur J Pharm Sci 88:267–273
Hsieh DJ-Y et al (2020) Epigallocatechin-3-gallate preconditioned Adipose-derived Stem Cells confer Neuroprotection in aging rat brain. Int J Med Sci 17(13):1916–1926
Babri S et al (2012) Protective effects of troxerutin on β-amyloid (1–42)-induced impairments of spatial learning and memory in rats. Neurophysiology 44(5):387–393
Masood MI et al (2020) Troxerutin flavonoid has neuroprotective properties and increases neurite outgrowth and migration of neural stem cells from the subventricular zone. PLoS ONE 15(8):e0237025–e0237025
Farajdokht F et al (2017) Troxerutin protects hippocampal neurons against amyloid beta-induced oxidative stress and apoptosis. EXCLI J 16:1081–1089
Baluchnejadmojarad T et al (2017) Troxerutin exerts neuroprotection in 6-hydroxydopamine lesion rat model of Parkinson’s disease: possible involvement of PI3K/ERβ signaling. Eur J Pharmacol 801:72–78
Lu J et al (2013) Troxerutin counteracts domoic acid-induced memory deficits in mice by inhibiting CCAAT/enhancer binding protein β-mediated inflammatory response and oxidative stress. J Immunol 190(7):3466–3479
Karimipour M et al (2019) Quercetin promotes learning and memory performance concomitantly with neural stem/progenitor cell proliferation and neurogenesis in the adult rat dentate gyrus. Int J Dev Neurosci 74:18–26
Zhang L et al (2011) Effect of quercetin on neural stem cell proliferation in the subventricular zone of rats after focal cerebral ischemia-reperfusion injury. J S Med Univ 31(7):1200–1203
Baral S et al (2017) Quercetin-3-O-glucuronide promotes the proliferation and migration of neural stem cells. Neurobiol Aging 52:39–52
Bianchi ME, Mezzapelle R (2020) The chemokine receptor CXCR4 in cell proliferation and tissue regeneration. Front Immunol 11:2109
Li M et al (2011) Neuronal differentiation of C17.2 neural stem cells induced by a natural flavonoid, baicalin. Chembiochem 12(3):449–56
He Z et al (2018) Neurite development and neurotoxicity. In: Slikker W, Paule MG, Wang C (eds) Handbook of developmental neurotoxicology (Second Edition). Academic Press, Washington, DC, pp 23–32
Zuo W et al (2017) Baicalin promotes the viability of Schwann cells in vitro by regulating neurotrophic factors. Exp Ther Med 14(1):507–514
Zhao J et al (2018) Baicalin and ginsenoside Rb1 promote the proliferation and differentiation of neural stem cells in Alzheimer’s disease model rats. Brain Res 1678:187–194
Maćkowiak M et al (2004) Neurogenesis in the adult brain. Pol J Pharmacol 56(6):673–687
Zhou W-B et al (2019) Luteolin induces hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome. Neural Regen Res 14(4):613–620
Lim JS et al (2010) Wogonin induces differentiation and neurite outgrowth of neural precursor cells. Biochem Biophys Res Commun 402(1):42–47
Liu RT et al (2010) Promotion of rat brain-derived progenitor cell neurogenesis by liquiritigenin treatment: underlying mechanisms. Neurosci Lett 481(3):139–143
Cheng A, Hou Y, Mattson MP (2010) Mitochondria and neuroplasticity. ASN Neuro 2(5):AN20100019
Ou L et al (2011) Design, synthesis and 3D-QSAR study of cytotoxic flavonoid derivatives. Mol Diversity 15(3):665–675
Allegra A et al (2019) Relationship between mitofusin 2 and cancer. In: Donev R (ed) Advances in protein chemistry and structural biology. Academic Press, Washington, DC, pp 209–236
Mei Y-Q et al (2016) A flavonoid compound promotes neuronal differentiation of embryonic stem cells via PPAR-β modulating mitochondrial energy metabolism. PLoS ONE 11(6):0157747–0157747
Wang D-Y et al (2011) Promoting effects of isobavachin on neurogenesis of mouse embryonic stem cells were associated with protein prenylation. Acta Pharmacol Sin 32(4):425–432
Wang Z et al (2009) Enhanced co-expression of β-tubulin III and choline acetyltransferase in neurons from mouse embryonic stem cells promoted by icaritin in an estrogen receptor-independent manner. Chemico-Biol Interact 179(2):375–385
Han D et al (2015) 3,2’-Dihydroxyflavone-treated pluripotent stem cells show enhanced proliferation, pluripotency marker expression, and neuroprotective properties. Cell Transplant 24(8):1511–1532
Reiners JJ Jr et al (1998) PD98059 is an equipotent antagonist of the aryl hydrocarbon receptor and inhibitor of mitogen-activated protein kinase kinase. Mol Pharmacol 53(3):438–445
Nguyen Thi PA et al (2016) PD98059 protects brain against cells death resulting from ROS/ERK activation in a cardiac arrest rat model. Oxid Med Cell Longev 3723762–3723762
Shieh DB et al (2010) Effects of genistein on beta-catenin signaling and subcellular distribution of actin-binding proteins in human umbilical CD105-positive stromal cells. J Cell Physiol 2(2):423–434
Funding
This work was supported by Mashhad University of Medical Sciences, Iran.
Author information
Authors and Affiliations
Contributions
MSL wrote the draft by collecting information and designing images. FK helped prepare the final version by checking the information and editing the initial version. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the present work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lotfi, M.S., Kalalinia, F. Flavonoids in Combination with Stem Cells for the Treatment of Neurological Disorders. Neurochem Res 48, 3270–3282 (2023). https://doi.org/10.1007/s11064-023-03986-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03986-w