Abstract
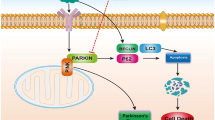
Lead (Pb) is considered to be a major environmental pollutant and occupational health hazard worldwide which may lead to neuroinflammation. However, an effective treatment for Pb-induced neuroinflammation remains elusive. The aim of this study was to investigate the mechanisms of Pb-induced neuroinflammation, and the therapeutic effect of sodium para-aminosalicylic acid (PAS-Na, a non-steroidal anti-inflammatory drug) in rat cerebral cortex. The results indicated that Pb exposure induced pathological damage in cerebral cortex, accompanied by increased levels of inflammatory factors tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β). Moreover, Pb decreased the expression of silencing information regulator 2 related enzyme 1 (SIRT1) and brain-derived neurotrophic factor (BDNF), and increased the levels of high mobile group box 1 (HMGB1) expression and p65 nuclear factor-κB (NF-κB) phosphorylation. PAS-Na treatment ameliorated Pb-induced histopathological changes in rat cerebral cortex. Moreover, PAS-Na reduced the Pb-induced increase of TNF-α and IL-1β levels concomitant with a significant increase in SIRT1 and BDNF levels, and a decrease in HMGB1 and the phosphorylation of p65 NF-κB expression. Thus, PAS-Na may exert anti-inflammatory effects by mediating the SIRT1/HMGB1/NF-κB pathway and BDNF expression. In conclusion, in this novel study PAS-Na was shown to possess an anti-inflammatory effect on cortical neuroinflammation, establishing its efficacy as a potential treatment for Pb exposures.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Mitra A, Chatterjee S, Kataki S, Rastogi RP, Gupta DK (2021) Bacterial tolerance strategies against lead toxicity and their relevance in bioremediation application. Environ Sci Pollut Res Int 28(12):14271–14284. https://doi.org/10.1007/s11356-021-12583-9
Mitra P, Sharma S, Purohit P, Sharma P (2017) Clinical and molecular aspects of lead toxicity: an update. Crit Rev Clin Lab Sci 54(7–8):506–528. https://doi.org/10.1080/10408363.2017.1408562
Hon KL, Fung CK, Leung AK (2017) Childhood lead poisoning: an overview. Hong Kong Med J 23(6):616–621. https://doi.org/10.12809/hkmj176214
Schwartz BS, Stewart WF, Bolla KI, Simon PD, Bandeen-Roche K, Gordon PB, Links JM, Todd AC (2000) Past adult lead exposure is associated with longitudinal decline in cognitive function. Neurology 55(8):1144–1150. https://doi.org/10.1212/wnl.55.8.1144
Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R (2005) Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect 113(7):894–899. https://doi.org/10.1289/ehp.7688
Rocha A, Trujillo KA (2019) Neurotoxicity of low-level lead exposure: history, mechanisms of action, and behavioral effects in humans and preclinical models. Neurotoxicology 73:58–80. https://doi.org/10.1016/j.neuro.2019.02.021
Cecil KM, Brubaker CJ, Adler CM, Dietrich KN, Altaye M, Egelhoff JC, Wessel S, Elangovan I, Hornung R, Jarvis K, Lanphear BP (2008) Decreased brain volume in adults with childhood lead exposure. PLoS Med 5(5):112. https://doi.org/10.1371/journal.pmed.0050112
Reuben A, Elliott ML, Abraham WC, Broadbent J, Houts RM, Ireland D, Knodt AR, Poulton R, Ramrakha S, Hariri AR, Caspi A, Moffitt TE (2020) Association of childhood lead exposure with MRI measurements of structural brain integrity in midlife. JAMA 324(19):1970–1979. https://doi.org/10.1001/jama.2020.19998
Marshall AT, Betts S, Kan EC, McConnell R, Lanphear BP, Sowell ER (2020) Association of lead-exposure risk and family income with childhood brain outcomes. Nat Med 26(1):91–97. https://doi.org/10.1038/s41591-019-0713-y
Leão LKR, Bittencourt LO, Oliveira ACA, Nascimento PC, Ferreira MKM, Miranda GHN, Ferreira RO, Eiró-Quirino L, Puty B, Dionizio A, Cartágenes SC, Freire MAM, Buzalaf MAR, Crespo-Lopez ME, Maia CSF, Lima RR (2021) Lead-induced motor dysfunction is associated with oxidative stress, proteome modulation, and neurodegeneration in motor cortex of rats. Oxid Med Cell Longev. https://doi.org/10.1155/2021/5595047
Ji X, Wang B, Paudel YN, Li Z, Zhang S, Mou L, Liu K, Jin M (2021) Protective effect of chlorogenic acid and its analogues on lead-induced developmental neurotoxicity through modulating oxidative stress and autophagy. Front Mol Biosci 8:655549. https://doi.org/10.3389/fmolb.2021.655549
Liu F, Wang Z, Wei Y, Liu R, Jiang C, Gong C, Liu Y, Yan B (2021) The leading role of adsorbed lead in Pm(2.5)-induced hippocampal neuronal apoptosis and synaptic damage. J Hazard Mater 416:125867. https://doi.org/10.1016/j.jhazmat.2021.125867
Hernández-Coro A, Sánchez-Hernández BE, Montes S, Martínez-Lazcano JC, González-Guevara E, Pérez-Severiano F (2021) Alterations in gene expression due to chronic lead exposure induce behavioral changes. Neurosci Biobehav Rev 126:361–367. https://doi.org/10.1016/j.neubiorev.2021.03.031
Lu LL, Zhang YW, Li ZC, Fang YY, Wang LL, Zhao YS, Li SJ, Ou SY, Aschner M, Jiang YM (2021) Therapeutic effects of sodium para-aminosalicylic acid on cognitive deficits and activated Erk1/2-P90(Rsk)/Nf-Κb inflammatory pathway in Pb-exposed rats. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02874-0
Hou Y, Wei Y, Lautrup S, Yang B, Wang Y, Cordonnier S, Mattson MP, Croteau DL, Bohr VA (2021) Nad(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer’s disease via CGAS-sting. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2011226118
Ghosh S, Wu MD, Shaftel SS, Kyrkanides S, LaFerla FM, Olschowka JA, O’Banion MK (2013) Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J Neurosci 33(11):5053–5064. https://doi.org/10.1523/jneurosci.4361-12.2013
Machoń-Grecka A, Dobrakowski M, Boroń M, Lisowska G, Kasperczyk A, Kasperczyk S (2017) The influence of occupational chronic lead exposure on the levels of selected pro-inflammatory cytokines and angiogenic factors. Hum Exp Toxicol 36(5):467–473. https://doi.org/10.1177/0960327117703688
Di Lorenzo L, Vacca A, Corfiati M, Lovreglio P, Soleo L (2007) Evaluation of tumor necrosis factor-alpha and granulocyte colony-stimulating factor serum levels in lead-exposed smoker workers. Int J Immunopathol Pharmacol 20(2):239–247. https://doi.org/10.1177/039463200702000204
Li N, Liu F, Song L, Zhang P, Qiao M, Zhao Q, Li W (2014) The effects of early life Pb exposure on the expression of Il1-Β, Tnf-Α and Aβ in cerebral cortex of mouse pups. J Trace Elem Med Biol 28(1):100–104. https://doi.org/10.1016/j.jtemb.2013.07.003
Chen C, Zhou M, Ge Y, Wang X (2020) Sirt1 and aging related signaling pathways. Mech Ageing Dev 187:111215. https://doi.org/10.1016/j.mad.2020.111215
Jiao F, Gong Z (2020) The beneficial roles of Sirt1 in neuroinflammation-related diseases. Oxid Med Cell Longev 2020:6782872. https://doi.org/10.1155/2020/6782872
Chen X, Chen C, Fan S, Wu S, Yang F, Fang Z, Fu H, Li Y (2018) Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through Sirt1-mediated deacetylation of the Hmgb1/Nf-Κb pathway following experimental traumatic brain injury. J Neuroinflamm 15(1):116. https://doi.org/10.1186/s12974-018-1151-3
Chen F, Zhou CC, Yang Y, Liu JW, Yan CH (2019) Gm1 ameliorates lead-induced cognitive deficits and brain damage through activating the Sirt1/Creb/Bdnf pathway in the developing male rat hippocampus. Biol Trace Elem Res 190(2):425–436. https://doi.org/10.1007/s12011-018-1569-6
Hossain S, Bhowmick S, Jahan S, Rozario L, Sarkar M, Islam S, Basunia MA, Rahman A, Choudhury BK, Shahjalal H (2016) Maternal lead exposure decreases the levels of brain development and cognition-related proteins with concomitant upsurges of oxidative stress, inflammatory response and apoptosis in the offspring rats. Neurotoxicology 56:150–158. https://doi.org/10.1016/j.neuro.2016.07.013
Peng DJ, Li J, Deng Y, Zhu X, Zhao L, Zhang Y, Li Z, Ou S, Li S, Jiang Y (2020) Sodium para-aminosalicylic acid inhibits manganese-induced Nlrp3 inflammasome-dependent pyroptosis by inhibiting Nf-Κb pathway activation and oxidative stress. J Neuroinflammation 17(1):343. https://doi.org/10.1186/s12974-020-02018-6
Li SJ, Qin WX, Peng DJ, Yuan ZX, He SN, Luo YN, Aschner M, Jiang YM, Liang DY, Xie BY, Xu F (2018) Sodium P-aminosalicylic acid inhibits sub-chronic manganese-induced neuroinflammation in rats by modulating Mapk and Cox-2. Neurotoxicology 64:219–229. https://doi.org/10.1016/j.neuro.2017.06.012
Ky SQ, Deng HS, Xie PY, Hu W (1992) A report of two cases of chronic serious manganese poisoning treated with sodium para-aminosalicylic acid. Br J Ind Med 49(1):66–69. https://doi.org/10.1136/oem.49.1.66
Jiang YM, Mo XA, Du FQ, Fu X, Zhu XY, Gao HY, Xie JL, Liao FL, Pira E, Zheng W (2006) Effective treatment of manganese-induced occupational parkinsonism with P-aminosalicylic acid: a case of 17-year follow-up study. J Occup Environ Med 48(6):644–649. https://doi.org/10.1097/01.jom.0000204114.01893.3e
Deng Y, Peng D, Yang C, Zhao L, Li J, Lu L, Zhu X, Li S, Aschner M, Jiang Y (2021) Preventive treatment with sodium para-aminosalicylic acid inhibits manganese-induced apoptosis and inflammation via the Mapk pathway in rat thalamus. Drug Chem Toxicol. https://doi.org/10.1080/01480545.2021.2008127
Li J, Deng Y, Peng D, Zhao L, Fang Y, Zhu X, Li S, Aschner M, Ou S, Jiang Y (2021) Sodium P-aminosalicylic acid attenuates manganese-induced neuroinflammation in Bv2 microglia by modulating Nf-Κb pathway. Biol Trace Elem Res 199(12):4688–4699. https://doi.org/10.1007/s12011-021-02581-w
Deng YF, Ou SY, Jiang YM, Chen HB, Dang X, Lu S, Wang K, Jiang YH, Li G, Lu JP (2009) Efects of sodium para-aminosalicylic acid on hippocampal ultramicro-structure of subchronic lead-exposed rats. J Toxicol 23(03):213–216. https://doi.org/10.16421/j.cnki.1002-3127.2009.03.011 (in Chinese)
Li Z-c, Wang L-l, Zhao Y-s, Peng D-j, Chen J, Jiang S-y, Zhao L, Aschner M, Li S-j, Jiang Y-m (2022) Sodium para-aminosalicylic acid ameliorates lead-induced hippocampal neuronal apoptosis by suppressing the activation of the Ip3r-Ca2+-Ask1-P38 signaling pathway. Ecotoxicol Environ Saf 241:113829. https://doi.org/10.1016/j.ecoenv.2022.113829
He SN, Qin WX, Lu YH, Li K, Luo YN, Yuan ZX, Jiang XL, Mo YH, Li WJ, Jiang YM (2017) Effects of sodium para-aminosalicylic acid on apoptosis of Pc12 cells induced by lead-exposure. Chin J Pharmacol Toxicol 31(02):159–164. https://doi.org/10.3867/j.issn.1000-3002.2017.02.06 (in Chinese)
Roppongi RT, Champagne-Jorgensen KP, Siddiqui TJ (2017) Low-density primary hippocampal neuron culture. J Vis Exp. https://doi.org/10.3791/55000
Caito S, Aschner M (2015) Neurotoxicity of metals. Handb Clin Neurol 131:169–189. https://doi.org/10.1016/b978-0-444-62627-1.00011-1
Chen A, Dietrich KN, Ware JH, Radcliffe J, Rogan WJ (2005) Iq and blood lead from 2 to 7 years of age: are the effects in older children the residual of high blood lead concentrations in 2-year-olds? Environ Health Perspect 113(5):597–601. https://doi.org/10.1289/ehp.7625
Zhao J, Zhang Q, Zhang B, Xu T, Yin D, Gu W, Bai J (2020) Developmental exposure to lead at environmentally relevant concentrations impaired neurobehavior and Nmdar-dependent Bdnf signaling in zebrafish larvae. Environ Pollut 257:113627. https://doi.org/10.1016/j.envpol.2019.113627
Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH (2010) A novel pathway regulates memory and plasticity via Sirt1 and Mir-134. Nature 466(7310):1105–1109. https://doi.org/10.1038/nature09271
Wang T, Zhang J, Xu Y (2020) Epigenetic basis of lead-induced neurological disorders. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph17134878
Atuadu V, Benneth BA, Oyem J, Esom E, Mba C, Nebo K, Ezemeka G, Anibeze C (2020) Adansonia digitata L. leaf extract attenuates lead-induced cortical histoarchitectural changes and oxidative stress in the prefrontal cortex of adult male wistar rats. Drug Metab Pers Ther. https://doi.org/10.1515/dmdi-2020-0116
Chibowska K, Korbecki J, Gutowska I, Metryka E, Tarnowski M, Goschorska M, Barczak K, Chlubek D, Baranowska-Bosiacka I (2020) Pre- and neonatal exposure to lead (Pb) induces neuroinflammation in the forebrain cortex, hippocampus and cerebellum of rat pups. Int J Mol Sci. https://doi.org/10.3390/ijms21031083
Leng F, Edison P (2021) Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol 17(3):157–172. https://doi.org/10.1038/s41582-020-00435-y
Chibowska K, Baranowska-Bosiacka I, Falkowska A, Gutowska I, Goschorska M, Chlubek D (2016) Effect of lead (Pb) on inflammatory processes in the brain. Int J Mol Sci. https://doi.org/10.3390/ijms17122140
Liu MC, Liu XQ, Wang W, Shen XF, Che HL, Guo YY, Zhao MG, Chen JY, Luo WJ (2012) Involvement of microglia activation in the lead induced long-term potentiation impairment. PLoS ONE 7(8):e43924. https://doi.org/10.1371/journal.pone.0043924
Xu L, He D, Bai Y (2016) Microglia-mediated inflammation and neurodegenerative disease. Mol Neurobiol 53(10):6709–6715. https://doi.org/10.1007/s12035-015-9593-4
Pan S, Wu Y, Pei L, Li S, Song L, Xia H, Wang Y, Yu Y, Yang X, Shu H, Zhang J, Yuan S, Shang Y (2018) Bml-111 reduces neuroinflammation and cognitive impairment in mice with sepsis via the Sirt1/Nf-Κb signaling pathway. Front Cell Neurosci 12:267. https://doi.org/10.3389/fncel.2018.00267
Arioz BI, Tastan B, Tarakcioglu E, Tufekci KU, Olcum M, Ersoy N, Bagriyanik A, Genc K, Genc S (2019) Melatonin attenuates Lps-induced acute depressive-like behaviors and microglial Nlrp3 inflammasome activation through the Sirt1/Nrf2 pathway. Front Immunol 10:1511. https://doi.org/10.3389/fimmu.2019.01511
Li D, Liang H, Li Y, Zhang J, Qiao L, Luo H (2021) Allicin alleviates lead-induced bone loss by preventing oxidative stress and osteoclastogenesis via Sirt1/Foxo1 pathway in mice. Biol Trace Elem Res 199(1):237–243. https://doi.org/10.1007/s12011-020-02136-5
Zhao Y, Mao A, Zhang R, Guan S, Lu J (2022) Sirt1/Mtor pathway-mediated autophagy dysregulation promotes Pb-induced hepatic lipid accumulation in Hepg2 cells. Environ Toxicol 37(3):549–563. https://doi.org/10.1002/tox.23420
Okuma Y, Liu K, Wake H, Zhang J, Maruo T, Date I, Yoshino T, Ohtsuka A, Otani N, Tomura S, Shima K, Yamamoto Y, Yamamoto H, Takahashi HK, Mori S, Nishibori M (2012) Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann Neurol 72(3):373–384. https://doi.org/10.1002/ana.23602
Nakamura Y, Fukuta A, Miyashita K, Zhang FF, Wang D, Liu K, Wake H, Hisaoka-Nakashima K, Nishibori M, Morioka N (2021) Perineural high-mobility group box 1 induces mechanical hypersensitivity through activation of spinal microglia: involvement of glutamate-Nmda receptor dependent mechanism in spinal dorsal horn. Biochem Pharmacol 186:114496. https://doi.org/10.1016/j.bcp.2021.114496
Liu X, Lu B, Fu J, Zhu X, Song E, Song Y (2021) Amorphous silica nanoparticles induce inflammation via activation of Nlrp3 inflammasome and Hmgb1/Tlr4/Myd88/Nf-Kb signaling pathway in Huvec cells. J Hazard Mater 404:124050. https://doi.org/10.1016/j.jhazmat.2020.124050
Wang CS, Kavalali ET, Monteggia LM (2022) BDNF signaling in context: from synaptic regulation to psychiatric disorders. Cell 185(1):62–76. https://doi.org/10.1016/j.cell.2021.12.003
Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR (2010) Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling. Toxicol Sci 116(1):249–263. https://doi.org/10.1093/toxsci/kfq111
Lima Giacobbo B, Doorduin J, Klein HC, Dierckx R, Bromberg E, de Vries EFJ (2019) Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol 56(5):3295–3312. https://doi.org/10.1007/s12035-018-1283-6
Patra RC, Swarup D, Dwivedi SK (2001) Antioxidant effects of alpha tocopherol, ascorbic acid and l-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 162(2):81–88. https://doi.org/10.1016/s0300-483x(01)00345-6
Hong L, Jiang W, Zheng W, Zeng S (2011) Hplc analysis of para-aminosalicylic acid and its metabolite in plasma, cerebrospinal fluid and brain tissues. J Pharm Biomed Anal 54(5):1101–1109. https://doi.org/10.1016/j.jpba.2010.11.031
Hong L, Jiang W, Pan H, Jiang Y, Zeng S, Zheng W (2011) Brain regional pharmacokinetics of P-aminosalicylic acid and its N-acetylated metabolite: effectiveness in chelating brain manganese. Drug Metab Dispos 39(10):1904–1909. https://doi.org/10.1124/dmd.111.040915
Zheng W, Jiang YM, Zhang Y, Jiang W, Wang X, Cowan DM (2009) Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague–Dawley rats. Neurotoxicology 30(2):240–248. https://doi.org/10.1016/j.neuro.2008.12.007
Acknowledgements
The authors thank Dr. Yan Li from Guangxi Zhuang Autonomous Region Institute for the Prevention and Treatment of Occupational Disease for her help in the detection of cerebral cortex Pb levels.
Funding
Funding support was provided by grants from the National Natural Science Foundation of China (Grant No. NSFC 81773476).
Author information
Authors and Affiliations
Contributions
YSZ: Investigation, Formal analysis, Writing original draft, Writing—review & editing. JYL: Investigation, Methodology, Formal analysis, Data curation. ZCL: Investigation, Writing original draft, Writing—review & editing. LLW: Methodology, Formal analysis. CLG, JC and SYJ: Methodology, Validation. MA: Language polishing. SYO: Investigation, Supervision. YMJ: Supervision, Project administration, Funding acquisition.
Corresponding authors
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
All animal procedures performed in this study were performed strictly according to the international standards of animal care guidelines and have been approved by the Animal Care and Use Committee of Guangxi Medical University (Approval Number: 201806386).
Consent for Publish
The paper has been approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Ys., Li, Jy., Li, Zc. et al. Sodium Para-aminosalicylic Acid Inhibits Lead-Induced Neuroinflammation in Brain Cortex of Rats by Modulating SIRT1/HMGB1/NF-κB Pathway. Neurochem Res 48, 238–249 (2023). https://doi.org/10.1007/s11064-022-03739-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03739-1