Abstract
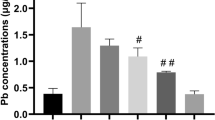
Lead (Pb) is a toxic heavy metal and environmental pollutant that adversely affects the nervous system. However, effective therapeutic drugs for Pb-induced neurotoxicity have yet to be developed. In the present study, we investigated the ameliorative effect of sodium para-aminosalicylic acid (PAS-Na) on Pb-induced neurotoxicity. Male Sprague–Dawley rats were treated with (CH3COO)2 Pb•4H2O (6 mg/kg) for 4 weeks, followed by 3 weeks of PAS-Na (100, 200, and 300 mg/kg). The results showed that subacute Pb exposure significantly decreased rats body-weight gains and increased liver coefficient, and impaired spatial learning and memory. HE staining showed that Pb damaged the structure of the hippocampus. Moreover, Pb activated the ERK1/2-p90RSK/ NF-κB pathway concomitant with increased inflammatory cytokine IL-1β levels in rat hippocampus. PAS-Na reversed the Pb-induced increase in the liver coefficient as well as the learning and memory deficits. In addition, PAS-Na reduced the phosphorylation of ERK1/2, p90RSK and NF-κB p65, decreasing IL-1β levels in hippocampus. Our findings indicated that PAS-Na showed efficacy in reversing Pb-induced rats cognitive deficits and triggered an anti-inflammatory response. Thus, PAS-Na may be a promising therapy for treating Pb-induced neurotoxicity.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Hernberg S (2000) Lead poisoning in a historical perspective. Am J Ind Med 38(3):244–254. https://doi.org/10.1002/1097-0274(200009)38:3%3c244::aid-ajim3%3e3.0.co;2-f
Struzynska L, Dabrowska-Bouta B, Koza K, Sulkowski G (2007) Inflammation-like glial response in lead-exposed immature rat brain. Toxicol Sci 95(1):156–162. https://doi.org/10.1093/toxsci/kfl134
Caito S, Aschner M (2015) Neurotoxicity of metals. Handb Clin Neurol 131:169–189. https://doi.org/10.1016/B978-0-444-62627-1.00011-1
Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R (2005) Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect 113(7):894–899. https://doi.org/10.1289/ehp.7688
Needleman H (2004) Lead poisoning. Annu Rev Med 55:209–222. https://doi.org/10.1146/annurev.med.55.091902.103653
Whitehouse S (2019) A lead-abatement judgment driven by science, history, and the law. Am J Public Health 109(4):544. https://doi.org/10.2105/AJPH.2019.304983
Henn SA, Sussell AL, Li J, Shire JD, Alarcon WA, Tak S (2011) Characterization of lead in US workplaces using data from OSHA’s integrated management information system. Am J Ind Med 54(5):356–365. https://doi.org/10.1002/ajim.20926
Singh PK, Nath R, Ahmad MK, Rawat A, Babu S, Dixit RK (2016) Attenuation of lead neurotoxicity by supplementation of polyunsaturated fatty acid in Wistar rats. Nutr Neurosci 19(9):396–405. https://doi.org/10.1179/1476830515Y.0000000028
Mahmoud YI, Sayed SS (2016) Effects of L-cysteine on lead acetate induced neurotoxicity in albino mice. Biotech Histochem 91(5):327–332. https://doi.org/10.3109/10520295.2016.1164897
Smith MR, Burman P, Sadahiro M, Kidd BA, Dudley JT, Morishita H (2017) Integrative analysis of disease signatures shows inflammation disrupts juvenile experience-dependent cortical plasticity. eNeuro 3(6):0240–16. https://doi.org/10.1523/ENEURO.0240-16.2016
Jing H, Zhang Q, Li S, Gao XJ (2020) Pb exposure triggers MAPK-dependent inflammation by activating oxidative stress and miRNA-155 expression in carp head kidney. Fish Shellfish Immunol 106:219–227. https://doi.org/10.1016/j.fsi.2020.08.015
Myers AP, Corson LB, Rossant J, Baker JC (2004) Characterization of mouse Rsk4 as an inhibitor of fibroblast growth factor-RAS-extracellular signal-regulated kinase signaling. Mol Cell Biol 24(10):4255–4266. https://doi.org/10.1128/MCB.24.10.4255-4266.2004
Zhu X, Lee HG, Raina AK, Perry G, Smith MA (2002) The role of mitogen-activated protein kinase pathways in Alzheimer’s disease. Neurosignals 11(5):270–281. https://doi.org/10.1159/000067426
Aglan HS, Gebremedhn S, Salilew-Wondim D, Neuhof C, Tholen E, Holker M, Schellander K, Tesfaye D (2020) Regulation of Nrf2 and NF-κB during lead toxicity in bovine granulosa cells. Cell Tissue Res 380(3):643–655. https://doi.org/10.1007/s00441-020-03177-x
Haleagrahara N, Jackie T, Chakravarthi S, Rao M, Kulur A (2010) Protective effect of Etlingera elatior (torch ginger) extract on lead acetate–induced hepatotoxicity in rats. J Toxicol Sci 35(5):663–671. https://doi.org/10.2131/jts.35.663
Asanuma M, Miyazaki I, Ogawa N (2004) Neuroprotective effects of nonsteroidal anti-inflammatory drugs on neurodegenerative diseases. Curr Pharm Des 10(6):695–700. https://doi.org/10.2174/1381612043453072
Jiang YM, Mo XA, Du FQ, Fu X, Zhu XY, Gao HY, Xie JL, Liao FL, Pira E, Zheng W (2006) Effective treatment of manganese-induced occupational Parkinsonism with p-aminosalicylic acid: a case of 17-year follow-up study. J Occup Environ Med 48(6):644–649. https://doi.org/10.1097/01.jom.0000204114.01893.3e
Yuan ZX, Chen HB, Li SJ, Huang XW, Mo YH, Luo YN, He SN, Deng XF, Lu GD, Jiang YM (2016) The influence of manganese treatment on the distribution of metal elements in rats and the protection by sodium para-amino salicylic acid. J Trace Elem Med Biol 36:84–89. https://doi.org/10.1016/j.jtemb.2016.04.005
Li SJ, Ou CY, He SN, Huang XW, Luo HL, Meng HY, Lu GD, Jiang YM, Vieira Peres T, Luo YN, Deng XF (2017) Sodium p-aminosalicylic acid reverses sub-chronic manganese-induced impairments of spatial learning and memory abilities in rats, but fails to restore γ-aminobutyric acid levels. Int J Environ Res Public Health 14(4):400. https://doi.org/10.3390/ijerph14040400
Li ZC, Wang F, Li SJ, Zhao L, Li JY, Deng Y, Zhu XJ, Zhang YW, Peng DJ, Jiang YM (2020) Sodium para-aminosalicylic acid reverses changes of glutamate turnover in manganese-exposed rats. Biol Trace Elem Res 197(2):544–554. https://doi.org/10.1007/s12011-019-02001-0
Peng D, Li J, Deng Y, Zhu X, Zhao L, Zhang Y, Li Z, Ou S, Li S, Jiang Y (2020) Sodium para-aminosalicylic acid inhibits manganese-induced NLRP3 inflammasome-dependent pyroptosis by inhibiting NF-κB pathway activation and oxidative stress. J Neuroinflammation 17(1):343. https://doi.org/10.1186/s12974-020-02018-6
Li J, Deng Y, Peng D, Zhao L, Fang Y, Zhu X, Li S, Aschner M, Ou S, Jiang Y (2021) Sodium P-aminosalicylic acid attenuates manganese-induced neuroinflammation in BV2 microglia by modulating NF-κB pathway. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02581-w
Li SJ, Qin WX, Peng DJ, Yuan ZX, He SN, Luo YN, Aschner M, Jiang YM, Liang DY, Xie BY, Xu F (2018) Sodium P-aminosalicylic acid inhibits sub-chronic manganese-induced neuroinflammation in rats by modulating MAPK and COX-2. Neurotoxicology 64:219–229. https://doi.org/10.1016/j.neuro.2017.06.012
Peng DJ, Zhang YW, Li ZC, Li SJ, Cai M, Qin WX, Ou SY, Huang XW, Yuan ZX, Jiang YM (2019) Preventive impacts of PAS-Na on the slow growth and activated inflammatory responses in Mn-exposed rats. J Trace Elem Med Biol 54:134–141. https://doi.org/10.1016/j.jtemb.2019.04.013
Wang F, Wang C, Jiang Y, Deng X, Lu J, Ou S (2014) Protective role of sodium para-amino salicylic acid against manganese-induced hippocampal neurons damage. Environ Toxicol Pharmacol 37(3):1071–1078. https://doi.org/10.1016/j.etap.2014.03.018
Li SJ, Luo YN, Li Y, Chen JW, Mo YH, Yuan ZX, Ou SY, Ou CY, Jiang YM, Deng XF (2016) Sodium para-aminosalicylate protected cultured basal ganglia astrocytes from manganese-induced DNA damages and alteration of amino acid neurotransmitter levels. J Toxicol Sci 41(5):573–581. https://doi.org/10.2131/jts.41.573
Deng XF, Ou SY, Jiang YM, Chen HB, Deng X, Shan Lu, Wang K, Jiang YH, Li G, Lu JP (2009) Effects of sodium para-aminosalicylic acid on hippocampal ultramicro-structure of subchronic lead-exposed rats. J Toxicol 23(3):213–216 (in Chinese)
Luo YN, Li Y, Peng DJ, He SN, Chen JW, Li SJ, Yuan ZX, Mo YH, Huang XW, Jiang YM (2016) The effect of PAS-Na on the learning memory and amino acid neurotransmitter content of lead- induced young rats. J Toxicol 30(6):444–446 (in Chinese)
He SN, Qin WX, Lu YH, Li K, Luo YN, Yuan ZX, Jiang XL, Mo YH, Li WJ, Jiang YM (2016) Effect of sodium para-aminosalicylic acid on apoptosis of PC12 cells induced by lead-exposure. J Toxicol 30(6):444–447 ((in Chinese))
Chen F, Zhou CC, Yang Y, Liu JW, Yan CH (2019) GM1 Ameliorates lead-induced cognitive deficits and brain damage through activating the SIRT1/CREB/BDNF pathway in the developing male rat hippocampus. Biol Trace Elem Res 190(2):425–436. https://doi.org/10.1007/s12011-018-1569-6
Baghurst PA, McMichael AJ, Wigg NR, Vimpani GV, Robertson EF, Roberts RJ, Tong SL (1992) Environmental exposure to lead and children’s intelligence at the age of seven years. The Port Pirie Cohort Study. N Engl J Med 327(18):1279–1284. https://doi.org/10.1056/NEJM199210293271805
Assi MA, Hezmee MN, Haron AW, Sabri MY, Rajion MA (2016) The detrimental effects of lead on human and animal health. Vet World 9(6):660–671. https://doi.org/10.14202/vetworld.2016.660-671
Dominguez S, Flores-Montoya MG, Sobin C (2019) Early chronic exposure to low-level lead alters total hippocampal microglia in pre-adolescent mice. Toxicol Lett 302:75–82. https://doi.org/10.1016/j.toxlet.2018.10.016
Kishi R, Ikeda T, Miyake H, Uchino E, Tsuzuki T, Inoue K (1982) Regional distribution of lead, zinc, iron and copper in suckling and adult rat brains. Brain Res 251(1):180–182. https://doi.org/10.1016/0006-8993(82)91289-6
Liu CM, Sun YZ, Sun JM, Ma JQ (1820) Cheng C (2012) Protective role of quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-κB pathway. Biochim Biophys Acta 10:1693–1703. https://doi.org/10.1016/j.bbagen.2012.06.011
Patrick L (2006) Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern Med Rev 11(1):2–22
Mani MS, Joshi MB, Shetty RR, DSouza VL, Swathi M, Kabekkodu SP, Dsouza HS (2020) Lead exposure induces metabolic reprogramming in rat models. Toxicol Lett 335:11–27. https://doi.org/10.1016/j.toxlet.2020.09.010
Fenga C, Gangemi S, Alibrandi A, Costa C, Micali E (2016) Relationship between lead exposure and mild cognitive impairment. J Prev Med Hyg 57(4):E205–E210
Sakthithasan K, Lévy P, Poupon J, Garnier R (2018) A comparative study of edetate calcium disodium and dimercaptosuccinic acid in the treatment of lead poisoning in adults. Clin Toxicol (Phila) 56(11):1143–1149. https://doi.org/10.1080/15563650.2018.1478424
Hong L, Jiang W, Pan H, Jiang Y, Zeng S, Zheng W (2011) Brain regional pharmacokinetics of p-aminosalicylic acid and its N-acetylated metabolite: effectiveness in chelating brain manganese. Drug Metab Dispos 39(10):1904–1909. https://doi.org/10.1124/dmd.111.040915
Piskorowski RA, Nasrallah K, Diamantopoulou A, Mukai J, Hassan SI, Siegelbaum SA, Gogos JA, Chevaleyre V (2016) Age-dependent specific changes in area CA2 of the hippocampus and social memory deficit in a mouse model of the 22q11.2 Deletion Syndrome. Neuron 89(1):163–176. https://doi.org/10.1016/j.neuron.2015.11.036
Pang CC, Kiecker C, O’Brien JT, Noble W, Chang RC (2019) Ammon’s Horn 2 (CA2) of the hippocampus: a long-known region with a new potential role in neurodegeneration. Neuroscientist 25(2):167–180. https://doi.org/10.1177/1073858418778747
Jiayong Z, Shengchen W, Xiaofang H, Gang S, Shiwen X (2020) The antagonistic effect of selenium on lead-induced necroptosis via MAPK/NF-κB pathway and HSPs activation in the chicken spleen. Ecotoxicol Environ Saf 204:111049. https://doi.org/10.1016/j.ecoenv.2020.111049
Lu H, Guizzetti M, Costa LG (2002) Inorganic lead activates the mitogen-activated protein kinase kinase-mitogen-activated protein kinase-p90(RSK) signaling pathway in human astrocytoma cells via a protein kinase C-dependent mechanism. J Pharmacol Exp Ther 300(3):818–823. https://doi.org/10.1124/jpet.300.3.818
Farkhondeh T, Boskabady MH, Koohi MK, Sadeghi-Hashjin G, Moin M (2013) The effect of lead exposure on selected blood inflammatory biomarkers in guinea pigs. Cardiovasc Hematol Disord Drug Targets 13(1):45–49. https://doi.org/10.2174/1871529x11313010005
Funding
Thanks to the fund support provided by grants from the National Natural Science Foundation of China (NSFC 81773476).
Author information
Authors and Affiliations
Contributions
LLL, YWZ, ZCL and YYF performed equally the majority of experiments and analyzed the data. LLW and YSZ assisted with the establishment of the animal model. SJL and SYO took part in the sample preparation. LLL and ZCL drafted the manuscript. MA critically read and revised the manuscript. YMJ and YWZ designed the study. YMJ led the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal procedures performed in this study were performed strictly according to the international standards of animal care guidelines and have been approved by the Animal Care and Use Committee of Guangxi Medical University.
Consent for publication
The paper has been approved by all authors for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li-li Lu, Yu-wen Zhang, Zhao-cong Li, and Yuan-yuan Fang have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Lu, Ll., Zhang, Yw., Li, Zc. et al. Therapeutic Effects of Sodium Para-Aminosalicylic Acid on Cognitive Deficits and Activated ERK1/2-p90RSK/NF-κB Inflammatory Pathway in Pb-Exposed Rats. Biol Trace Elem Res 200, 2807–2815 (2022). https://doi.org/10.1007/s12011-021-02874-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02874-0