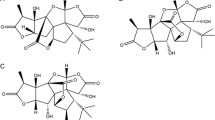
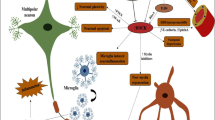
Abstract
Stroke is a sudden neurological disorder that occurs due to impaired blood flow to an area of the brain. Stroke can be caused by the blockage or rupture of a blood vessel in the brain, called ischemic stroke and hemorrhagic stroke, respectively. Stroke is more common in men than women. Atrial fibrillation, hypertension, kidney disease, high cholesterol and lipids, genetic predisposition, inactivity, poor nutrition, diabetes mellitus, family history and smoking are factors that increase the risk of stroke. Restoring blood flow by repositioning blocked arteries using thrombolytic agents or endovascular therapy are the most effective treatments for stroke. However, restoring circulation after thrombolysis can cause fatal edema or intracranial hemorrhage, and worsen brain damage in a process known as ischemia–reperfusion injury. Therefore, there is a pressing need to find and develop more effective treatments for stroke. In the past, the first choice of treatment was based on natural compounds. Natural compounds are able to reduce the symptoms and reduce various diseases including stroke that attract the attention of the pharmaceutical industry. Nowadays, as a result of the numerous studies carried out in the field of herbal medicine, many useful and valuable effects of plants have been identified. The death-associated protein kinase (DAPK) family is one of the vital families of serine/threonine kinases involved in the regulation of some biological functions in human cells. DAPK1 is the most studied kinase within the DAPKs family as it is involved in neuronal and recovery processes. Dysregulation of DAPK1 in the brain is involved in the developing neurological diseases such as stroke. Natural products can function in a variety of ways, including reducing cerebral edema, reducing brain endothelial cell death, and inhibiting TNFα and interleukin-1β (IL-1β) through regulating the DAPK1 signal against stroke. Due to the role of DAPK1 in neurological disorders, the aim of this article was to investigate the role of DAPK1 in stroke and its modulation by natural compounds.
Graphical Abstract



Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
Staessens S, Fitzgerald S, Andersson T, Clarençon F, Denorme F, Gounis M et al (2020) Histological stroke clot analysis after thrombectomy: technical aspects and recommendations. Int J Stroke 15(5):467–476
Franck JA (2020) Rehabilitation of patients with a moderately to severely affected arm-hand in the sub-acute phase after stroke. ProefschriftMaken, Maastricht
Markus H (2008) Stroke: causes and clinical features. Medicine 36(11):586–591
Bots SH, Peters SA, Woodward M (2017) Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health 2(2):e000298
Ali MK, Jaacks LM, Kowalski AJ, Siegel KR, Ezzati M (2015) Noncommunicable diseases: three decades of global data show a mixture of increases and decreases in mortality rates. Health Aff 34(9):1444–1455
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M et al (2015) Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131(4):e29–e322
Lau KK, Li L, Schulz U, Simoni M, Chan KH, Ho SL et al (2017) Total small vessel disease score and risk of recurrent stroke: validation in 2 large cohorts. Neurology 88(24):2260–2267
Ejupi A, Brodie M, Gschwind YJ, Lord SR, Zagler WL, Delbaere K (2016) Kinect-based five-times-sit-to-stand test for clinical and in-home assessment of fall risk in older people. Gerontology 62(1):118–124
Doheny EP, Walsh C, Foran T, Greene BR, Fan CW, Cunningham C et al (2013) Falls classification using tri-axial accelerometers during the five-times-sit-to-stand test. Gait Posture 38(4):1021–1025
Doheny EP, McGrath D, Greene BR, Walsh L, McKeown D, Cunningham C, et al (eds) (2012) Displacement of centre of mass during quiet standing assessed using accelerometry in older fallers and non-fallers. In: 2012 Annual international conference of the IEEE engineering in medicine and biology society. IEEE
Reider N, Gaul C (2016) Fall risk screening in the elderly: a comparison of the minimal chair height standing ability test and 5-repetition sit-to-stand test. Arch Gerontol Geriatr 65:133–139
Canning CG, Ada L, O’Dwyer NJ (2000) Abnormal muscle activation characteristics associated with loss of dexterity after stroke. J Neurol Sci 176(1):45–56
Taub E, Miller NE, Novack TA, Cook EW, Fleming WC, Nepomuceno CS et al (1993) Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil 74(4):347–354
Veerbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Hendriks E, Rietberg M et al (2014) What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE 9(2):e87987
Grotta JC, Hacke W (2015) Stroke neurologist’s perspective on the new endovascular trials. Stroke 46(6):1447–1452
Mullen MT, Pisapia JM, Tilwa S, Messé SR, Stein SC (2012) Systematic review of outcome after ischemic stroke due to anterior circulation occlusion treated with intravenous, intra-arterial, or combined intravenous+ intra-arterial thrombolysis. Stroke 43(9):2350–2355
Chen Y-F (2012) Traditional Chinese herbal medicine and cerebral ischemia. Front Biosci (Elite Ed) 4:809–817
Xiong X-Y, Liu L, Yang Q-W (2018) Refocusing neuroprotection in cerebral reperfusion era: New challenges and strategies. Front Neurol 9:249
Benderska N, Schneider-Stock R (2014) Transcription control of DAPK. Apoptosis 19(2):298–305
Farag AK, Roh EJ (2019) Death-associated protein kinase (DAPK) family modulators: current and future therapeutic outcomes. Med Res Rev 39(1):349–385
Wang W-J, Kuo J-C, Ku W, Lee Y-R, Lin F-C, Chang Y-L et al (2007) The tumor suppressor DAPK is reciprocally regulated by tyrosine kinase Src and phosphatase LAR. Mol Cell 27(5):701–716
Wang S, Shi X, Li H, Pang P, Pei L, Shen H et al (2017) DAPK1 signaling pathways in stroke: from mechanisms to therapies. Mol Neurobiol 54(6):4716–4722
Schumacher AM, Velentza AV, Watterson DM, Wainwright MS (2002) DAPK catalytic activity in the hippocampus increases during the recovery phase in an animal model of brain hypoxic-ischemic injury. Biochim Biophys Acta (BBA) 1600(1–2):128–137
Shamloo M, Soriano L, Wieloch T, Nikolich K, Urfer R, Oksenberg D (2005) Death-associated protein kinase is activated by dephosphorylation in response to cerebral ischemia. J Biol Chem 280(51):42290–42299
Bamford J, Sandercock P, Dennis M, Warlow C, Burn J (1991) Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 337(8756):1521–1526
Donkor ES (2018) Stroke in the century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat 2018:1–10
Béjot Y, Bailly H, Durier J, Giroud M (2016) Epidemiology of stroke in Europe and trends for the 21st century. La Presse Médicale 45(12):e391–e398
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M et al (2016) Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133(4):e38-360
Guzik A, Bushnell C (2017) Stroke epidemiology and risk factor management. Continuum 23(1):15–39
Forsgren L, Beghi E, Oun A, Sillanpää M (2005) The epidemiology of epilepsy in Europe–a systematic review. Eur J Neurol 12(4):245–253
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S et al (2018) Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 137(12):e67–e492
Rennert RC, Wali AR, Steinberg JA, Santiago-Dieppa DR, Olson SE, Pannell JS et al (2019) Epidemiology, natural history, and clinical presentation of large vessel ischemic stroke. Neurosurgery 85(suppl_1):S4–S8
El-Hajj M, Salameh P, Rachidi S, Hosseini H (2016) The epidemiology of stroke in the Middle East. Eur Stroke J 1(3):180–198
Gade P, Manjegowda SB, Nallar SC, Maachani UB, Cross AS, Kalvakolanu DV (2014) Regulation of the death-associated protein kinase 1 expression and autophagy via ATF6 requires apoptosis signal-regulating kinase 1. Mol Cell Biol 34(21):4033–4048
Singh P, Ravanan P, Talwar P (2016) Death associated protein kinase 1 (DAPK1): a regulator of apoptosis and autophagy. Front Mol Neurosci 9:46
Bialik S, Kimchi A (2006) The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem 75:189–210
Nair S, Hagberg H, Krishnamurthy R, Thornton C, Mallard C (2013) Death associated protein kinases: molecular structure and brain injury. Int J Mol Sci 14(7):13858–13872
Shiloh R, Bialik S, Kimchi A (2014) The DAPK family: a structure–function analysis. Apoptosis 19(2):286–297
Kim N, Chen D, Zhou XZ, Lee TH (2019) Death-associated protein kinase 1 phosphorylation in neuronal cell death and neurodegenerative disease. Int J Mol Sci 20(13):3131
Mor I, Carlessi R, Ast T, Feinstein E, Kimchi A (2012) Death-associated protein kinase increases glycolytic rate through binding and activation of pyruvate kinase. Oncogene 31(6):683–693
Song L, Pei L, Hu L, Pan S, Xiong W, Liu M et al (2018) Death-associated protein kinase 1 mediates interleukin-1β production through regulating inlfammasome activation in Bv2 microglial cells and mice. Sci Rep 8(1):1–11
You M-H, Kim BM, Chen C-H, Begley MJ, Cantley LC, Lee TH (2017) Death-associated protein kinase 1 phosphorylates NDRG2 and induces neuronal cell death. Cell Death Differ 24(2):238–250
Fujita Y, Yamashita T (2014) Role of DAPK in neuronal cell death. Apoptosis 19(2):339–345
Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarapandian MM et al (2010) DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell 140(2):222–234
Yamamoto M, Takahashi H, Nakamura T, Hioki T, Nagayama S, Ooashi N et al (1999) Developmental changes in distribution of death-associated protein kinase mRNAs. J Neurosci Res 58(5):674–683
Xu L-z, Li B-q, Jia J-p (2019) DAPK1: a novel pathology and treatment target for Alzheimer’s disease. Mol Neurobiol 56(4):2838–2844
Alsaadi MS (2019) Role of DAPK1 in neuronal cell death, survival and diseases in the nervous system. Int J Dev Neurosci 74:11–17
Tian J-H, Das S, Sheng Z-H (2003) Ca2+-dependent phosphorylation of syntaxin-1A by the death-associated protein (DAP) kinase regulates its interaction with Munc18. J Biol Chem 278(28):26265–26274
Lai TW, Zhang S, Wang YT (2014) Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 115:157–188
Chen CH, Wang WJ, Kuo JC, Tsai HC, Lin JR, Chang ZF et al (2005) Bidirectional signals transduced by DAPK–ERK interaction promote the apoptotic effect of DAPK. EMBO J 24(2):294–304
Xiong W, Wu Y, Xian W, Song L, Hu L, Pan S et al (2018) DAPK1-ERK signal mediates oxygen glucose deprivation reperfusion induced apoptosis in mouse N2a cells. J Neurol Sci 387:210–219
Boots EA, Schultz SA, Clark LR, Racine AM, Darst BF, Koscik RL et al (2017) BDNF Val66Met predicts cognitive decline in the Wisconsin Registry for Alzheimer’s Prevention. Neurology 88(22):2098–2106
Lim YY, Hassenstab J, Cruchaga C, Goate A, Fagan AM, Benzinger TL et al (2016) BDNF Val66Met moderates memory impairment, hippocampal function and tau in preclinical autosomal dominant Alzheimer’s disease. Brain 139(10):2766–2777
Buchman AS, Yu L, Boyle PA, Schneider JA, De Jager PL, Bennett DA (2016) Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology 86(8):735–741
Beeri MS, Sonnen J (2016) Brain BDNF expression as a biomarker for cognitive reserve against Alzheimer disease progression. Neurology 86:702
Inan-Eroglu E, Ayaz A (2018) Is aluminum exposure a risk factor for neurological disorders? J Res Med Sci 23:51
Liu S-B, Zhang N, Guo Y-Y, Zhao R, Shi T-Y, Feng S-F et al (2012) G-protein-coupled receptor 30 mediates rapid neuroprotective effects of estrogen via depression of NR2B-containing NMDA receptors. J Neurosci 32(14):4887–4900
Su Y, Deng M-F, Xiong W, Xie A-J, Guo J, Liang Z-H et al (2019) MicroRNA-26a/death-associated protein kinase 1 signaling induces synucleinopathy and dopaminergic neuron degeneration in Parkinson’s disease. Biol Psychiatry 85(9):769–781
Pei L, Wang S, Jin H, Bi L, Wei N, Yan H et al (2015) A novel mechanism of spine damages in stroke via DAPK1 and tau. Cereb Cortex 25(11):4559–4571
Henshall DC, Araki T, Schindler CK, Shinoda S, Lan JQ, Simon RP (2003) Expression of death-associated protein kinase and recruitment to the tumor necrosis factor signaling pathway following brief seizures. J Neurochem 86(5):1260–1270
Henshall DC, Schindler CK, So NK, Lan JQ, Meller R, Simon RP (2004) Death-associated protein kinase expression in human temporal lobe epilepsy. Ann Neurol 55(4):485–494
Wang X, Pei L, Yan H, Wang Z, Wei N, Wang S et al (2014) Intervention of death-associated protein kinase 1–p53 interaction exerts the therapeutic effects against stroke. Stroke 45(10):3089–3091
Pei L, Shang Y, Jin H, Wang S, Wei N, Yan H et al (2014) DAPK1–p53 interaction converges necrotic and apoptotic pathways of ischemic neuronal death. J Neurosci 34(19):6546–6556
Kim B, You M, Chen C, Lee S, Hong Y, Kimchi A et al (2014) Death-associated protein kinase 1 has a critical role in aberrant tau protein regulation and function. Cell Death Dis 5(5):e1237
Kim BM, You M-H, Chen C-H, Suh J, Tanzi RE, Ho LT (2016) Inhibition of death-associated protein kinase 1 attenuates the phosphorylation and amyloidogenic processing of amyloid precursor protein. Hum Mol Genet 25(12):2498–2513
Araki T, Shinoda S, Schindler CK, Quan-Lan J, Meller R, Taki W et al (2004) Expression, interaction, and proteolysis of death-associated protein kinase and p53 within vulnerable and resistant hippocampal subfields following seizures. Hippocampus 14(3):326–336
Won J, Hong Y (2016) Enhancement of neural regeneration and functional recovery via DAPK1 suppression in stroke animal model. FASEB J 30:630
Kang BN, Ahmad AS, Saleem S, Patterson RL, Hester L, Doré S et al (2010) Death-associated protein kinase-mediated cell death modulated by interaction with DANGER. J Neurosci 30(1):93–98
Duan D-X, Chai G-S, Ni Z-F, Hu Y, Luo Y, Cheng X-S et al (2013) Phosphorylation of tau by death-associated protein kinase 1 antagonizes the kinase-induced cell apoptosis. J Alzheimers Dis 37(4):795–808
Wang S, Chen K, Yu J, Wang X, Li Q, Lv F et al (2020) Presynaptic Caytaxin prevents apoptosis via deactivating DAPK1 in the acute phase of cerebral ischemic stroke. Exp Neurol 329:113303
Guo Y, Li H, Ke X, Deng M, Wu Z, Cai Y et al (2019) Degradation of caytaxin causes learning and memory deficits via activation of DAPK1 in aging. Mol Neurobiol 56(5):3368–3379
Nabavi SF, Sureda A, Sanches-Silva A, Pandima Devi K, Ahmed T, Shahid M et al (2019) Novel therapeutic strategies for stroke: the role of autophagy. Crit Rev Clin Lab Sci 56(3):182–199
Noori T, Dehpour AR, Sureda A, Sobarzo-Sanchez E, Shirooie S (2021) Role of natural products for the treatment of Alzheimer’s disease. Eur J Pharmacol 2021:173974
Li X, Zhang D, Bai Y, Xiao J, Jiao H, He R (2019) Ginaton improves neurological function in ischemic stroke rats via inducing autophagy and maintaining mitochondrial homeostasis. Neuropsychiatry Dis Treat 15:1813
Yan BC, Wang J, Rui Y, Cao J, Xu P, Jiang D et al (2019) Neuroprotective effects of gabapentin against cerebral ischemia reperfusion-induced neuronal autophagic injury via regulation of the PI3K/Akt/mTOR signaling pathways. J Neuropathol Exp Neurol 78(2):157–171
Cai C-C, Zhu J-H, Ye L-X, Dai Y-Y, Fang M-C, Hu Y-Y et al (2019) Glycine protects against hypoxic-ischemic brain injury by regulating mitochondria-mediated autophagy via the AMPK pathway. Oxid Med Cell Longev 2019:1–29
Shi G, Shi J, Liu K, Liu N, Wang Y, Fu Z et al (2013) Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia 61(4):504–512
Mo Y, Sun Y-Y, Liu K-Y (2020) Autophagy and inflammation in ischemic stroke. Neural Regen Res 15(8):1388–1396
Levin-Salomon V, Bialik S, Kimchi A (2014) DAP-kinase and autophagy. Apoptosis 19(2):346–356
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8(9):741–752
Kiyono K, Suzuki HI, Matsuyama H, Morishita Y, Komuro A, Kano MR et al (2009) Autophagy is activated by TGF-β and potentiates TGF-β–mediated growth inhibition in human hepatocellular carcinoma cells. Can Res 69(23):8844–8852
Jang C-W, Chen C-H, Chen C-C, Chen J-Y, Su Y-H, Chen R-H (2002) TGF-β induces apoptosis through Smad-mediated expression of DAP-kinase. Nat Cell Biol 4(1):51–58
Cohen O, Feinstein E, Kimchi A (1997) DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J 16(5):998–1008
Cohen O, Inbal B, Kissil JL, Raveh T, Berissi H, Spivak-Kroizaman T et al (1999) DAP-kinase participates in TNF-α–and Fas-induced apoptosis and its function requires the death domain. J Cell Biol 146(1):141–148
Bialik S, Kimchi A (2010) Lethal weapons: DAP-kinase, autophagy and cell death: DAP-kinase regulates autophagy. Curr Opin Cell Biol 22(2):199–205
Kuo J, Wang W, Yao C, Wu PR, Chen RH (2006) The tumor suppressor daPK inhibits cell motility by blocking the integrin-mediated polarity pathway. J Cell Biol 172:619–631
Atanasov AG, Zotchev SB, Dirsch VM, Orhan IE, Banach M, Rollinger JM et al (2021) Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov 20(3):200–216
Nikhil K, Sharan S, Palla SR, Sondhi SM, Peddinti RK, Roy P (2015) Understanding the mode of action of a pterostilbene derivative as anti-inflammatory agent. Int Immunopharmacol 28(1):10–21
Kim JH, Won J, Hong Y (2017) Suppression of DAPK1 benefits to ischemia brain injury via mechanism (s) of ER stress mediated apoptotic pathway. FASEB J 31:876.1
Che X, Yan H, Sun H, Dongol S, Wang Y, Lv Q et al (2016) Grifolin induces autophagic cell death by inhibiting the Akt/mTOR/S6K pathway in human ovarian cancer cells. Oncol Rep 36(2):1041–1047
Jing S, Ying P, Hu X, Yu Z, Sun J, Ding Y et al (2017) Protective effect of grifolin against brain injury in an acute cerebral ischemia rat model. Trop J Pharm Res 16(6):1299–1305
Wu Z, Li Y (2017) Grifolin exhibits anti-cancer activity by inhibiting the development and invasion of gastric tumor cells. Oncotarget 8(13):21454
Kavanagh F, Hervey A, Robbins WJ (1950) Antibiotic Substances from Basidiomycetes: V. Poria Corticola, Poria Tenuis and an Unidentified Basidiomycete. Proc Natl Acad Sci USA 36(1):1
Zeb M, Lee CH (2021) Medicinal properties and bioactive compounds from wild mushrooms native to North America. Molecules 26(2):251
Ye M, Luo X, Li L, Shi Y, Tan M, Weng X et al (2007) Grifolin, a potential antitumor natural product from the mushroom Albatrellus confluens, induces cell-cycle arrest in G1 phase via the ERK1/2 pathway. Cancer Lett 258(2):199–207
Jin S, Pang R-P, Shen J-N, Huang G, Wang J, Zhou J-G (2007) Grifolin induces apoptosis via inhibition of PI3K/AKT signalling pathway in human osteosarcoma cells. Apoptosis 12(7):1317–1326
Luo X, Yu X, Liu S, Deng Q, Liu X, Peng S et al (2015) The role of targeting kinase activity by natural products in cancer chemoprevention and chemotherapy. Oncol Rep 34(2):547–554
Luo XJ, Li LL, Deng QP, Yu XF, Yang LF, Luo FJ et al (2011) Grifolin, a potent antitumour natural product upregulates death-associated protein kinase 1 DAPK1 via p53 in nasopharyngeal carcinoma cells. Eur J Cancer (Oxford, England: 1990) 47(2):316–325
Chen D, Zhou XZ, Lee TH (2019) Death-associated protein kinase 1 as a promising drug target in cancer and Alzheimer’s disease. Recent Pat Anticancer Drug Discov 14(2):144–157
Yanqin Y, Jing T, Wei C, Nan L (2017) Grifolin attenuates white matter lesion in oxygen/glucose deprivation. Transl Neurosci 8:102–110
Wang Z-M, Zhao D, Nie Z-L, Zhao H, Zhou B, Gao W et al (2014) Flavonol intake and stroke risk: a meta-analysis of cohort studies. Nutrition 30(5):518–523
Tang Z, Li M, Zhang X, Hou W (2016) Dietary flavonoid intake and the risk of stroke: a dose-response meta-analysis of prospective cohort studies. BMJ Open 6(6):e008680
Bondonno NP, Bondonno CP, Blekkenhorst LC, Considine MJ, Maghzal G, Stocker R et al (2018) Flavonoid-rich apple improves endothelial function in individuals at risk for cardiovascular disease: a randomized controlled clinical trial. Mol Nutr Food Res 62(3):1700674
Machha A, Mustafa MR (2005) Chronic treatment with flavonoids prevents endothelial dysfunction in spontaneously hypertensive rat aorta. J Cardiovasc Pharmacol 46(1):36–40
Cassidy A, Bertoia M, Chiuve S, Flint A, Forman J, Rimm EB (2016) Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am J Clin Nutr 104(3):587–594
Williamson G, Kay CD, Crozier A (2018) The bioavailability, transport, and bioactivity of dietary flavonoids: a review from a historical perspective. Comp Rev Food Sci Food Saf 17(5):1054–1112
Khamchai S, Chumboatong W, Hata J, Tocharus C, Suksamrarn A, Tocharus J (2020) Morin protects the blood–brain barrier integrity against cerebral ischemia reperfusion through anti-inflammatory actions in rats. Sci Rep 10(1):13379
Yokoyama T, Kosaka Y, Mizuguchi M (2015) Structural insight into the interactions between death-associated protein kinase 1 and natural flavonoids. J Med Chem 58(18):7400–7408
Chen Y, Li Y, Xu H, Li G, Ma Y, Pang YJ (2017) Morin mitigates oxidative stress, apoptosis and inflammation in cerebral ischemic rats. Afr J Tradit Complement Altern Med 14(2):348–355
Lee J-K, Kwak H-J, Piao M-S, Jang J-W, Kim S-H, Kim H-S (2011) Quercetin reduces the elevated matrix metalloproteinases-9 level and improves functional outcome after cerebral focal ischemia in rats. Acta Neurochir 153(6):1321–1329
Park D-J, Shah F-A, Koh P-O (2018) Quercetin attenuates neuronal cells damage in a middle cerebral artery occlusion animal model. J Vet Med Sci 80:676
Lee JH, Rho SB, Chun T (2005) Programmed cell death 6 (PDCD6) protein interacts with death-associated protein kinase 1 (DAPk1): additive effect on apoptosis via caspase-3 dependent pathway. Biotechnol Lett 27(14):1011–1015
Lin X, Lin C-H, Zhao T, Zuo D, Ye Z, Liu L et al (2017) Quercetin protects against heat stroke-induced myocardial injury in male rats: antioxidative and antiinflammatory mechanisms. Chem Biol Interact 265:47–54
Xia S-F, Xie Z-X, Qiao Y, Li L-R, Cheng X-R, Tang X et al (2015) Differential effects of quercetin on hippocampus-dependent learning and memory in mice fed with different diets related with oxidative stress. Physiol Behav 138:325–331
Sabogal-Guáqueta AM, Munoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodriguez M, Osorio E, Cardona-Gómez GP (2015) The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 93:134–145
Kerimi A, Williamson G (2018) Differential impact of flavonoids on redox modulation, bioenergetics, and cell signaling in normal and tumor cells: a comprehensive review. Antioxid Redox Signal 29(16):1633–1659
Qi P, Li J, Gao S, Yuan Y, Sun Y, Liu N et al (2020) Network pharmacology-based and experimental identification of the effects of quercetin on Alzheimer’s disease. Front Aging Neurosci 12:793012–793012
Tsai Y-M, Chien C-F, Lin L-C, Tsai T-H (2011) Curcumin and its nano-formulation: the kinetics of tissue distribution and blood–brain barrier penetration. Int J Pharm 416(1):331–338
Hagl S, Kocher A, Schiborr C, Kolesova N, Frank J, Eckert GP (2015) Curcumin micelles improve mitochondrial function in neuronal PC12 cells and brains of NMRI mice–Impact on bioavailability. Neurochem Int 89:234–242
Liu L, Zhang W, Wang L, Li Y, Tan B, Lu X et al (2014) Curcumin prevents cerebral ischemia reperfusion injury via increase of mitochondrial biogenesis. Neurochem Res 39(7):1322–1331
Lopresti AL, Maes M, Maker GL, Hood SD, Drummond PD (2014) Curcumin for the treatment of major depression: a randomised, double-blind, placebo controlled study. J Affect Disord 167:368–375
Liu S, Cao Y, Qu M, Zhang Z, Feng L, Ye Z et al (2016) Curcumin protects against stroke and increases levels of Notch intracellular domain. Neurol Res 38(6):553–559
Altinay S, Cabalar M, Isler C, Yildirim F, Celik DS, Zengi O et al (2017) Is chronic curcumin supplementation neuroprotective against ischemia for antioxidant activity, neurological deficit, or neuronal apoptosis in an experimental stroke model. Turk Neurosurg 27(4):537–545
Lan C, Chen X, Zhang Y, Wang W, Wang WE, Liu Y et al (2018) Curcumin prevents strokes in stroke-prone spontaneously hypertensive rats by improving vascular endothelial function. BMC Cardiovasc Disord 18(1):1–10
Ahmad N, Umar S, Ashafaq M, Akhtar M, Iqbal Z, Samim M et al (2013) A comparative study of PNIPAM nanoparticles of curcumin, demethoxycurcumin, and bisdemethoxycurcumin and their effects on oxidative stress markers in experimental stroke. Protoplasma 250(6):1327–1338
Kalani A, Kamat PK, Kalani K, Tyagi N (2015) Epigenetic impact of curcumin on stroke prevention. Metab Brain Dis 30(2):427–435
Miao Y, Zhao S, Gao Y, Wang R, Wu Q, Wu H et al (2016) Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: the possible role of Sirt1 signaling. Brain Res Bull 121:9–15
Li Y, Li J, Li S, Li Y, Wang X, Liu B et al (2015) Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol Appl Pharmacol 286(1):53–63
Wiciński M, Socha M, Walczak M, Wódkiewicz E, Malinowski B, Rewerski S et al (2018) Beneficial effects of resveratrol administration: focus on potential biochemical mechanisms in cardiovascular conditions. Nutrients 10(11):1813
Bastianetto S, Ménard C, Quirion R (2015) Neuroprotective action of resveratrol. Biochim et Biophys Acta (BBA) 1852(6):1195–1201
Clark D, Tuor UI, Thompson R, Institoris A, Kulynych A, Zhang X et al (2012) Protection against recurrent stroke with resveratrol: endothelial protection. PLoS ONE 7(10):e47792
Girbovan C, Plamondon H (2015) Resveratrol downregulates type-1 glutamate transporter expression and microglia activation in the hippocampus following cerebral ischemia reperfusion in rats. Brain Res 1608:203–214
Shin JA, Lee H, Lim Y-K, Koh Y, Choi JH, Park E-M (2010) Therapeutic effects of resveratrol during acute periods following experimental ischemic stroke. J Neuroimmunol 227(1–2):93–100
Simão F, Matté A, Pagnussat AS, Netto CA, Salbego CG (2012) Resveratrol preconditioning modulates inflammatory response in the rat hippocampus following global cerebral ischemia. Neurochem Int 61(5):659–665
Song J, Cheon SY, Jung W, Lee WT, Lee JE (2014) Resveratrol induces the expression of interleukin-10 and brain-derived neurotrophic factor in BV2 microglia under hypoxia. Int J Mol Sci 15(9):15512–15529
Mokni M, Hamlaoui S, Karkouch I, Amri M, Marzouki L, Limam F et al (2013) Resveratrol provides cardioprotection after ischemia/reperfusion injury via modulation of antioxidant enzyme activities. Iran J Pharm Res IJPR 12(4):867
Ghazavi H, Shirzad S, Forouzanfar F, Negah SS, Rad MR, Vafaee F (2020) The role of resveratrol as a natural modulator in glia activation in experimental models of stroke. Avicenna J Phytomed 10(6):557
Yokoyama T, Suzuki R, Mizuguchi M (2021) Crystal structure of death-associated protein kinase 1 in complex with the dietary compound resveratrol. IUCr J 8(1):131–138
Jafari S, Saeidnia S, Abdollahi M (2014) Role of natural phenolic compounds in cancer chemoprevention via regulation of the cell cycle. Curr Pharm Biotechnol 15(4):409–421
Zhang J-C, Xu H, Yuan Y, Chen J-Y, Zhang Y-J, Lin Y et al (2017) Delayed treatment with green tea polyphenol EGCG promotes neurogenesis after ischemic stroke in adult mice. Mol Neurobiol 54(5):3652–3664
Pervin M, Unno K, Nakagawa A, Takahashi Y, Iguchi K, Yamamoto H et al (2017) Blood brain barrier permeability of (-)-epigallocatechin gallate, its proliferation-enhancing activity of human neuroblastoma SH-SY5Y cells, and its preventive effect on age-related cognitive dysfunction in mice. Biochem Biophys Rep 9:180–186
Chen D, Kanthasamy AG, Reddy MB (2015) EGCG protects against 6-OHDA-induced neurotoxicity in a cell culture model. Parkinson’s Dis 2015:1–10
Walker JM, Klakotskaia D, Ajit D, Weisman GA, Wood WG, Sun GY et al (2015) Beneficial effects of dietary EGCG and voluntary exercise on behavior in an Alzheimer’s disease mouse model. J Alzheimers Dis 44(2):561–572
Zeng L, Holly JM, Perks CM (2014) Effects of physiological levels of the green tea extract epigallocatechin-3-gallate on breast cancer cells. Front Endocrinol 5:61
Yao C, Zhang J, Liu G, Chen F, Lin Y (2014) Neuroprotection by (-)-epigallocatechin-3-gallate in a rat model of stroke is mediated through inhibition of endoplasmic reticulum stress. Mol Med Rep 9(1):69–72
Zhang F, Li N, Jiang L, Chen L, Huang M (2015) Neuroprotective effects of (−)-epigallocatechin-3-gallate against focal cerebral ischemia/reperfusion injury in rats through attenuation of inflammation. Neurochem Res 40(8):1691–1698
Bai Q, Lyu Z, Yang X, Pan Z, Lou J, Dong T (2017) Epigallocatechin-3-gallate promotes angiogenesis via up-regulation of Nfr2 signaling pathway in a mouse model of ischemic stroke. Behav Brain Res 321:79–86
Park D-J, Kang J-B, Koh P-O (2020) Epigallocatechin gallate alleviates neuronal cell damage against focal cerebral ischemia in rats. J Vet Med Sci 82(5):639–645
Park D-J, Kang J-B, Koh P-O (2021) Identification of regulated proteins by epigallocatechin gallate treatment in an ischemic cerebral cortex animal model: a proteomics approach. J Vet Med Sci 83:916
Lee WJ, Shim J-Y, Zhu BT (2005) Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol 68(4):1018–1030
Khan MA, Hussain A, Sundaram MK, Alalami U, Gunasekera D, Ramesh L et al (2015) (-)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol Rep 33(4):1976–1984
Takahashi H, Suzuki Y, Mohamed JS, Gotoh T, Pereira SL, Alway SE (2017) Epigallocatechin-3-gallate increases autophagy signaling in resting and unloaded plantaris muscles but selectively suppresses autophagy protein abundance in reloaded muscles of aged rats. Exp Gerontol 92:56–66
Blagosklonny MV (2019) Rapamycin for longevity: opinion article. Aging (Albany NY) 11(19):8048
Liu Y, Feng M, Chen H, Yang G, Qiu J, Zhao F et al (2020) Mechanistic target of rapamycin in the tumor microenvironment and its potential as a therapeutic target for pancreatic cancer. Cancer Lett 485:1–13
Kaeberlein M, Galvan V (2019) Rapamycin and Alzheimer’s disease: time for a clinical trial? Sci Transl Med 11(476):eaar4289
Yin L, Ye S, Chen Z, Zeng Y (2012) Rapamycin preconditioning attenuates transient focal cerebral ischemia/reperfusion injury in mice. Int J Neurosci 122(12):748–756
Jing C-H, Wang L, Liu P-P, Wu C, Ruan D, Chen G (2012) Autophagy activation is associated with neuroprotection against apoptosis via a mitochondrial pathway in a rat model of subarachnoid hemorrhage. Neuroscience 213:144–153
Sheng R, Zhang L-S, Han R, Liu X-Q, Gao B, Qin Z-H (2010) Autophagy activation is associated with neuroprotection in a rat model of focal cerebral ischemic preconditioning. Autophagy 6(4):482–494
Praet J, Guglielmetti C, Berneman Z, Van der Linden A, Ponsaerts P (2014) Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neurosci Biobehav Rev 47:485–505
Chi OZ, Mellender SJ, Barsoum S, Liu X, Damito S, Weiss HR (2016) Effects of rapamycin pretreatment on blood-brain barrier disruption in cerebral ischemia-reperfusion. Neurosci Lett 620:132–136
Kirino T, Tamura A, Sano K (1984) Delayed neuronal death in the rat hippocampus following transient forebrain ischemia. Acta Neuropathol 64(2):139–147
Guo W, Feng G, Miao Y, Liu G, Xu C (2014) Rapamycin alleviates brain edema after focal cerebral ischemia reperfusion in rats. Immunopharmacol Immunotoxicol 36(3):211–223
Wu M, Zhang H, Kai J, Zhu F, Dong J, Xu Z et al (2018) Rapamycin prevents cerebral stroke by modulating apoptosis and autophagy in penumbra in rats. Ann Clin Transl Neurol 5(2):138–146
Lin A-L, Jahrling JB, Zhang W, DeRosa N, Bakshi V, Romero P et al (2017) Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre-symptomatic Alzheimer’s disease. J Cereb Blood Flow Metab 37(1):217–226
Wang Z, Wang X, Cheng F, Wen X, Feng S, Yu F et al (2021) Rapamycin inhibits glioma cells growth and promotes autophagy by miR-26a-5p/DAPK1 axis. Cancer Manag Res 13:2691
Wei Z, Du Q, Li P, Liu H, Xia M, Chen Y et al (2021) Death-associated protein kinase 1 (DAPK1) controls CD8+ T cell activation, trafficking, and antitumor activity. FASEB J 35(1):e21138
Acknowledgements
None.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
SS and TN contributed to the conception of the manuscript. SS, TN, AS and AD drafted the manuscript. All of the authors revised the manuscript and gave the final approval.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noori, T., Shirooie, S., Sureda, A. et al. Regulation of DAPK1 by Natural Products: An Important Target in Treatment of Stroke. Neurochem Res 47, 2142–2157 (2022). https://doi.org/10.1007/s11064-022-03628-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03628-7