Abstract
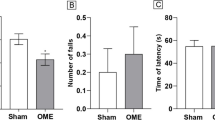
Methylglyoxal (MGO) is a highly reactive dicarbonyl molecule that promotes the formation of advanced glycation end products (AGEs), which are believed to play a key role in a number of pathologies, such as diabetes, Alzheimer’s disease, and inflammation. Here, Swiss mice were treated with MGO by intraperitoneal injection to investigate its effects on motor activity, mood, and cognition. Acute MGO treatment heavily decreased locomotor activity in the open field test at higher doses (80–200 mg/kg), an effect not observed at lower doses (10–50 mg/kg). Several alterations were observed 4 h after a single MGO injection (10–50 mg/kg): (a) plasma MGO levels were increased, (b) memory was impaired (object location task), (c) anxiolytic behavior was observed in the open field and marble burying test, and (d) depressive-like behavior was evidenced as evaluated by the tail suspension test. Biochemical alterations in the glutathione and glyoxalase systems were not observed 4 h after MGO treatment. Mice were also treated daily with MGO at 0, 10, 25 and 50 mg/kg for 11 days. From the 5th to the 11th day, several behavioral end points were evaluated, resulting in: (a) absence of motor impairment as evaluated in the open field, horizontal bars and pole test, (b) depressive-like behavior observed in the tail suspension test, and (c) cognitive impairments detected on working, short- and long-term memory when mice were tested in the Y-maze spontaneous alternation, object location and recognition tests, and step-down inhibitory avoidance task. An interesting finding was a marked decrease in dopamine levels in the prefrontal cortex of mice treated with 50 mg/kg MGO for 11 days, along with a ~ 25% decrease in the Glo1 content. The MGO-induced dopamine depletion in the prefrontal cortex may be related to the observed memory deficits and depressive-like behavior, an interesting topic to be further studied as a potentially novel route for MGO toxicity.








Similar content being viewed by others
References
Biessels GJ, Staekenborg S, Brunner E et al (2006) Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 5:64–74. https://doi.org/10.1016/S1474-4422(05)70284-2
Bora E, Akdede BB, Alptekin K (2017) The relationship between cognitive impairment in schizophrenia and metabolic syndrome: a systematic review and meta-analysis. Psychol Med 47:1030–1040. https://doi.org/10.1017/S0033291716003366
Allaman I, Bélanger M, Magistretti PJ (2015) Methylglyoxal, the dark side of glycolysis. Front Neurosci 9:23. https://doi.org/10.3389/fnins.2015.00023
Choudhary D, Chandra D, Kale RK (1997) Influence of methylglyoxal on antioxidant enzymes and oxidative damage. Toxicol Lett 93:141–152
Dasuri K, Zhang L, Keller JN (2013) Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic Biol Med 62:170–185. https://doi.org/10.1016/j.freeradbiomed.2012.09.016
Kalapos MP (2008) Methylglyoxal and glucose metabolism: a historical perspective and future avenues for research. Drug Metabol Drug Interact 23:69–91
Thornalley PJ (2005) Dicarbonyl intermediates in the maillard reaction. Ann N Y Acad Sci 1043:111–117. https://doi.org/10.1196/annals.1333.014
Thornalley PJ (1996) Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification—a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol 27:565–573
Kalapos MP (1999) Methylglyoxal in living organisms: chemistry, biochemistry, toxicology and biological implications. Toxicol Lett 110:145–175
Lyles GA, Chalmers J (1992) The metabolism of aminoacetone to methylglyoxal by semicarbazide-sensitive amine oxidase in human umbilical artery. Biochem Pharmacol 43:1409–1414
Shibamoto T (2006) Analytical methods for trace levels of reactive carbonyl compounds formed in lipid peroxidation systems. J Pharm Biomed Anal 41:12–25. https://doi.org/10.1016/j.jpba.2006.01.047
Koop DR, Casazza JP (1985) Identification of ethanol-inducible P-450 isozyme 3a as the acetone and acetol monooxygenase of rabbit microsomes. J Biol Chem 260:13607–13612
Pompliano DL, Peyman A, Knowles JR (1990) Stabilization of a reaction intermediate as a catalytic device: definition of the functional role of the flexible loop in triosephosphate isomerase. Biochemistry 29:3186–3194
Thornalley PJ, Langborg A, Minhas HS (1999) Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J 344(Pt 1):109–116
Sousa Silva M, Gomes RA, Ferreira AEN et al (2013) The glyoxalase pathway: the first hundred years… and beyond. Biochem J 453:1–15. https://doi.org/10.1042/BJ20121743
Thornalley PJ (1993) The glyoxalase system in health and disease. Mol Aspects Med 14:287–371
Rabbani N, Thornalley PJ (2014) The critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy. Diabetes 63:50–52. https://doi.org/10.2337/db13-1606
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820. https://doi.org/10.1038/414813a
Hofmann MA, Drury S, Fu C et al (1999) RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97:889–901
Matafome P, Sena C, Seiça R (2013) Methylglyoxal, obesity, and diabetes. Endocrine 43:472–484. https://doi.org/10.1007/s12020-012-9795-8
Tian C, Alomar F, Moore CJ et al (2014) Reactive carbonyl species and their roles in sarcoplasmic reticulum Ca2+ cycling defect in the diabetic heart. Heart Fail Rev 19:101–112. https://doi.org/10.1007/s10741-013-9384-9
Distler MG, Palmer AA (2012) Role of glyoxalase 1 (Glo1) and methylglyoxal (MG) in behavior: recent advances and mechanistic insights. Front Genet 3:250. https://doi.org/10.3389/fgene.2012.00250
McMurray KMJ, Distler MG, Sidhu PS et al (2014) Glo1 inhibitors for neuropsychiatric and anti-epileptic drug development. Biochem Soc Trans 42:461–467. https://doi.org/10.1042/BST20140027
Geng X, Ma J, Zhang F, Xu C (2014) Glyoxalase I in tumor cell proliferation and survival and as a potential target for anticancer therapy. Oncol Res Treat 37:570–574. https://doi.org/10.1159/000367800
Koivisto A, Chapman H, Jalava N et al (2014) TRPA1: a transducer and amplifier of pain and inflammation. Basic Clin Pharmacol Toxicol 114:50–55. https://doi.org/10.1111/bcpt.12138
Angeloni C, Zambonin L, Hrelia S (2014) Role of methylglyoxal in Alzheimer’s disease. Biomed Res Int 2014:238485. https://doi.org/10.1155/2014/238485
Hovatta I, Tennant RS, Helton R et al (2005) Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature 438:662–666. https://doi.org/10.1038/nature04250
Jang S, Kwon DM, Kwon K, Park C (2017) Generation and characterization of mouse knockout for glyoxalase 1. Biochem Biophys Res Commun 490:460–465. https://doi.org/10.1016/j.bbrc.2017.06.063
Krömer SA, Kessler MS, Milfay D et al (2005) Identification of glyoxalase-I as a protein marker in a mouse model of extremes in trait anxiety. J Neurosci Off J Soc Neurosci 25:4375–4384. https://doi.org/10.1523/JNEUROSCI.0115-05.2005
Distler MG, Plant LD, Sokoloff G et al (2012) Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal. J Clin Invest 122:2306–2315. https://doi.org/10.1172/JCI61319
Bierhaus A, Fleming T, Stoyanov S et al (2012) Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med 18:926–933. https://doi.org/10.1038/nm.2750
Andersson DA, Gentry C, Light E et al (2013) Methylglyoxal evokes pain by stimulating TRPA1. PLoS ONE 8:e77986. https://doi.org/10.1371/journal.pone.0077986
Huang X, Wang F, Chen W et al (2012) Possible link between the cognitive dysfunction associated with diabetes mellitus and the neurotoxicity of methylglyoxal. Brain Res 1469:82–91. https://doi.org/10.1016/j.brainres.2012.06.011
Kong X, Ma M, Huang K et al (2014) Increased plasma levels of the methylglyoxal in patients with newly diagnosed type 2 diabetes 2. J Diabetes 6:535–540. https://doi.org/10.1111/1753-0407.12160
Beeri MS, Moshier E, Schmeidler J et al (2011) Serum concentration of an inflammatory glycotoxin, methylglyoxal, is associated with increased cognitive decline in elderly individuals. Mech Ageing Dev 132:583–587. https://doi.org/10.1016/j.mad.2011.10.007
Srikanth V, Westcott B, Forbes J et al (2013) Methylglyoxal, cognitive function and cerebral atrophy in older people. J Gerontol A 68:68–73. https://doi.org/10.1093/gerona/gls100
Watanabe K, Okada K, Fukabori R et al (2014) Methylglyoxal (MG) and cerebro-renal interaction: does long-term orally administered MG cause cognitive impairment in normal Sprague-Dawley rats? Toxins 6:254–269. https://doi.org/10.3390/toxins6010254
Chun HJ, Lee Y, Kim AH, Lee J (2016) Methylglyoxal causes cell death in neural progenitor cells and impairs adult hippocampal neurogenesis. Neurotox Res 29:419–431. https://doi.org/10.1007/s12640-015-9588-y
Hansen F, Pandolfo P, Galland F et al (2016) Methylglyoxal can mediate behavioral and neurochemical alterations in rat brain. Physiol Behav 164:93–101. https://doi.org/10.1016/j.physbeh.2016.05.046
Wong ML, Licinio J (2001) Research and treatment approaches to depression. Nat Rev Neurosci 2:343–351. https://doi.org/10.1038/35072566
Critchlow V, Liebelt RA, Bar-Sela M et al (1963) Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol 205:807–815. https://doi.org/10.1152/ajplegacy.1963.205.5.807
Kitay JI (1961) Sex differences in adrenal cortical secretion in the rat. Endocrinology 68:818–824. https://doi.org/10.1210/endo-68-5-818
Mevel LJC, Abitbol S, Beraud G, Maniey J (1979) Temporal changes in plasma adrenocorticotropin concentration after repeated neurotropic stress in male and female rats. Endocrinology 105:812–817. https://doi.org/10.1210/endo-105-3-812
Seale JV, Wood SA, Atkinson HC et al (2004) Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. J Neuroendocrinol 16:516–524. https://doi.org/10.1111/j.1365-2826.2004.01195.x
Kalapos MP, Schaff Z, Garzó T et al (1991) Accumulation of phenols in isolated hepatocytes after pretreatment with methylglyoxal. Toxicol Lett 58:181–191
Belzung C (1999) Measuring rodent exploratory behavior. In: Crusio WE, Gerlai RT (eds) Techniques in the behavioral and neural sciences. Elsevier, Amsterdam, pp 738–749
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370. https://doi.org/10.1007/bf00428203
Kedia S, Chattarji S (2014) Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J Neurosci Methods 233:150–154. https://doi.org/10.1016/j.jneumeth.2014.06.012
Albelda N, Joel D (2012) Animal models of obsessive-compulsive disorder: exploring pharmacology and neural substrates. Neurosci Biobehav Rev 36:47–63. https://doi.org/10.1016/j.neubiorev.2011.04.006
Assini FL, Duzzioni M, Takahashi RN (2009) Object location memory in mice: pharmacological validation and further evidence of hippocampal CA1 participation. Behav Brain Res 204:206–211. https://doi.org/10.1016/j.bbr.2009.06.005
Vogel-Ciernia A, Wood MA (2014) Examining object location and object recognition memory in mice. Curr Protoc Neurosci. https://doi.org/10.1002/0471142301.ns0831s69
Tolman EC (1925) Purpose and cognition: the determiners of animal learning. Psychol Rev 32:285–297. https://doi.org/10.1037/h0072784
Dember WN, Fowler H (1958) Spontaneous alternation behavior. Psychol Bull 55:412–428
Kleschevnikov AM, Yu J, Kim J et al (2017) Evidence that increased Kcnj6 gene dose is necessary for deficits in behavior and dentate gyrus synaptic plasticity in the Ts65Dn mouse model of down syndrome. Neurobiol Dis 103:1–10. https://doi.org/10.1016/j.nbd.2017.03.009
Moreira ELG, de Oliveira J, Nunes JC et al (2012) Age-related cognitive decline in hypercholesterolemic LDL receptor knockout mice (LDLr-/-): evidence of antioxidant imbalance and increased acetylcholinesterase activity in the prefrontal cortex. J Alzheimers Dis JAD 32:495–511. https://doi.org/10.3233/JAD-2012-120541
Roesler R, Walz R, Quevedo J et al (1999) Normal inhibitory avoidance learning and anxiety, but increased locomotor activity in mice devoid of PrP(C). Brain Res Mol Brain Res 71:349–353
Ogasawara Y, Tanaka R, Koike S et al (2016) Determination of methylglyoxal in human blood plasma using fluorescence high performance liquid chromatography after derivatization with 1,2-diamino-4,5-methylenedioxybenzene. J Chromatogr B 1029–1030:102–105. https://doi.org/10.1016/j.jchromb.2016.07.019
De Benedetto GE, Fico D, Pennetta A et al (2014) A rapid and simple method for the determination of 3,4-dihydroxyphenylacetic acid, norepinephrine, dopamine, and serotonin in mouse brain homogenate by HPLC with fluorimetric detection. J Pharm Biomed Anal 98:266–270. https://doi.org/10.1016/j.jpba.2014.05.039
Hambsch B, Chen B-G, Brenndörfer J et al (2010) Methylglyoxal-mediated anxiolysis involves increased protein modification and elevated expression of glyoxalase 1 in the brain. J Neurochem 113:1240–1251. https://doi.org/10.1111/j.1471-4159.2010.06693.x
Ghosh M, Talukdar D, Ghosh S et al (2006) In vivo assessment of toxicity and pharmacokinetics of methylglyoxal: augmentation of the curative effect of methylglyoxal on cancer-bearing mice by ascorbic acid and creatine. Toxicol Appl Pharmacol 212:45–58. https://doi.org/10.1016/j.taap.2005.07.003
Kullmann DM, Ruiz A, Rusakov DM et al (2005) Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol 87:33–46. https://doi.org/10.1016/j.pbiomolbio.2004.06.003
Benton CS, Miller BH, Skwerer S et al (2012) Evaluating genetic markers and neurobiochemical analytes for fluoxetine response using a panel of mouse inbred strains. Psychopharmacology 221:297–315. https://doi.org/10.1007/s00213-011-2574-z
Wu Z, Fu Y, Yang Y et al (2018) Gating TrkB switch by methylglyoxal enables GLO1 as a target for depression. bioRxiv. https://doi.org/10.1101/435867
Fujimoto M, Uchida S, Watanuki T et al (2008) Reduced expression of glyoxalase-1 mRNA in mood disorder patients. Neurosci Lett 438:196–199. https://doi.org/10.1016/j.neulet.2008.04.024
Patki G, Solanki N, Atrooz F et al (2013) Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res 1539:73–86. https://doi.org/10.1016/j.brainres.2013.09.033
Yang Y, Yang D, Tang G et al (2013) Proteomics reveals energy and glutathione metabolic dysregulation in the prefrontal cortex of a rat model of depression. Neuroscience 247:191–200. https://doi.org/10.1016/j.neuroscience.2013.05.031
de Moura JC, Noroes MM, de Rachetti VPS et al (2014) The blockade of transient receptor potential ankirin 1 (TRPA1) signalling mediates antidepressant- and anxiolytic-like actions in mice. Br J Pharmacol 171:4289–4299. https://doi.org/10.1111/bph.12786
Jimenez JC, Su K, Goldberg AR et al (2018) Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97:670–683.e6. https://doi.org/10.1016/j.neuron.2018.01.016
Lo TW, Westwood ME, McLellan AC et al (1994) Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J Biol Chem 269:32299–32305
Singh R, Barden A, Mori T, Beilin L (2001) Advanced glycation end-products: a review. Diabetologia 44:129–146. https://doi.org/10.1007/s001250051591
Di Loreto S, Zimmitti V, Sebastiani P et al (2008) Methylglyoxal causes strong weakening of detoxifying capacity and apoptotic cell death in rat hippocampal neurons. Int J Biochem Cell Biol 40:245–257. https://doi.org/10.1016/j.biocel.2007.07.019
Thornalley PJ, Rabbani N (2011) Glyoxalase in tumourigenesis and multidrug resistance. Semin Cell Dev Biol 22:318–325. https://doi.org/10.1016/j.semcdb.2011.02.006
Xue J, Ray R, Singer D et al (2014) The receptor for advanced glycation end products (RAGE) specifically recognizes methylglyoxal-derived AGEs. Biochemistry 53:3327–3335. https://doi.org/10.1021/bi500046t
Schmidt AM, Hori O, Chen JX et al (1995) Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest 96:1395–1403. https://doi.org/10.1172/JCI118175
Lee K-I, Lin H-C, Lee H-T et al (2017) Loss of transient receptor potential ankyrin 1 channel deregulates emotion, learning and memory, cognition, and social behavior in mice. Mol Neurobiol 54:3606–3617. https://doi.org/10.1007/s12035-016-9908-0
Thornalley PJ (2008) Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems–role in ageing and disease. Drug Metabol Drug Interact 23:125–150
Dafre AL, Goldberg J, Wang T et al (2015) Methylglyoxal, the foe and friend of glyoxalase and Trx/TrxR systems in HT22 nerve cells. Free Radic Biol Med 89:8–19. https://doi.org/10.1016/j.freeradbiomed.2015.07.005
Dafre AL, Schmitz AE, Maher P (2017) Methylglyoxal-induced AMPK activation leads to autophagic degradation of thioredoxin 1 and glyoxalase 2 in HT22 nerve cells. Free Radic Biol Med 108:270–279. https://doi.org/10.1016/j.freeradbiomed.2017.03.028
Schmitz AE, de Souza LF, Dos Santos B et al (2017) Methylglyoxal-induced protection response and toxicity: role of glutathione reductase and thioredoxin systems. Neurotox Res 32:340–350. https://doi.org/10.1007/s12640-017-9738-5
van Schouwenburg M, Aarts E, Cools R (2010) Dopaminergic modulation of cognitive control: distinct roles for the prefrontal cortex and the basal ganglia. Curr Pharm Des 16:2026–2032
Cools R (2016) The costs and benefits of brain dopamine for cognitive control. Wiley Interdiscip Rev Cogn Sci 7:317–329. https://doi.org/10.1002/wcs.1401
Otero TM (2017) Brief review of fluid reasoning: conceptualization, neurobasis, and applications. Appl Neuropsychol Child 6:204–211. https://doi.org/10.1080/21622965.2017.1317484
Szent-Györgyi A, McLaughlin JA (1975) Interaction of glyoxal and methylglyoxal with biogenic amines. Proc Natl Acad Sci USA 72:1610–1611. https://doi.org/10.1073/pnas.72.4.1610
Deng Y, Zhang Y, Li Y et al (2012) Occurrence and distribution of salsolinol-like compound, 1-acetyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (ADTIQ) in parkinsonian brains 1996. J Neural Transm Vienna Austria 119:435–441. https://doi.org/10.1007/s00702-011-0724-4
Hipkiss AR (2017) On the relationship between energy metabolism, proteostasis, aging and Parkinson’s disease: possible causative role of methylglyoxal and alleviative potential of carnosine. Aging Dis 8:334–345. https://doi.org/10.14336/AD.2016.1030
Song D-W, Xin N, Xie B-J et al (2014) Formation of a salsolinol-like compound, the neurotoxin, 1-acetyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline, in a cellular model of hyperglycemia and a rat model of diabetes. Int J Mol Med 33:736–742. https://doi.org/10.3892/ijmm.2013.1604
Xie B, Lin F, Peng L et al (2014) Methylglyoxal increases dopamine level and leads to oxidative stress in SH-SY5Y cells. Acta Biochim Biophys Sin 46:950–956. https://doi.org/10.1093/abbs/gmu094
Anzalone A, Lizardi-Ortiz JE, Ramos M et al (2012) Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci 32:9023–9034. https://doi.org/10.1523/JNEUROSCI.0918-12.2012
Ford CP (2014) The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 282:13–22. https://doi.org/10.1016/j.neuroscience.2014.01.025
Cowan N (2008) What are the differences between long-term, short-term, and working memory? Prog Brain Res 169:323–338. https://doi.org/10.1016/S0079-6123(07)00020-9
Kodl CT, Seaquist ER (2008) Cognitive dysfunction and diabetes mellitus. Endocr Rev 29:494–511. https://doi.org/10.1210/er.2007-0034
Kopf D, Frölich L (2009) Risk of incident Alzheimer’s disease in diabetic patients: a systematic review of prospective trials. J Alzheimers Dis JAD 16:677–685. https://doi.org/10.3233/JAD-2009-1011
Miles W, Root H (1922) Psychologic tests applied to diabetic patients. Arch Intern Med 30:767–777
Gray S, Green S, Alt M et al (2017) The structure of working memory in young children and its relation to intelligence. J Mem Lang 92:183–201. https://doi.org/10.1016/j.jml.2016.06.004
Puig MV, Rose J, Schmidt R, Freund N (2014) Dopamine modulation of learning and memory in the prefrontal cortex: insights from studies in primates, rodents, and birds. Front Neural Circuits 8:93. https://doi.org/10.3389/fncir.2014.00093
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet Lond Engl 386:896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Lange KW, Robbins TW, Marsden CD et al (1992) L-dopa withdrawal in Parkinson’s disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology 107:394–404
Acknowledgements
This work was supported by the Brazilian funding agency CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, #462333/2014-0, #306204/2014-2), and ALD is a research fellow (307057/2018-1). MPC received a post-doctoral PNPD, and GRLA and JCS received scholarships from CAPES (Coordination for the Improvement of Higher Education Personnel, Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Szczepanik, J.C., de Almeida, G.R.L., Cunha, M.P. et al. Repeated Methylglyoxal Treatment Depletes Dopamine in the Prefrontal Cortex, and Causes Memory Impairment and Depressive-Like Behavior in Mice. Neurochem Res 45, 354–370 (2020). https://doi.org/10.1007/s11064-019-02921-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02921-2