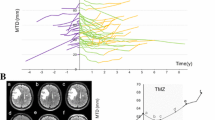
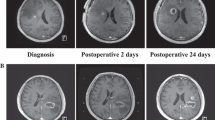
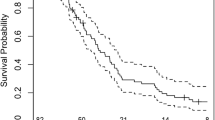
Abstract
Purpose
In the randomized CeTeG/NOA-09 trial, lomustine/temozolomide (CCNU/TMZ) was superior to TMZ therapy regarding overall survival (OS) in MGMT promotor-methylated glioblastoma. Progression-free survival (PFS) and pseudoprogression rates (about 10%) were similar in both arms. Further evaluating this discrepancy, we analyzed patterns of postprogression survival (PPS) and MRI features at first progression according to modified RANO criteria (mRANO).
Methods
We classified the patients of the CeTeG/NOA-09 trial according to long vs. short PPS employing a cut-off of 18 months and compared baseline characteristics and survival times. In patients with available MRIs and confirmed progression, the increase in T1-enhancing, FLAIR hyperintense lesion volume and the change in ADC mean value of contrast-enhancing tumor upon progression were determined.
Results
Patients with long PPS in the CCNU/TMZ arm had a particularly short PFS (5.6 months). PFS in this subgroup was shorter than in the long PPS subgroup of the TMZ arm (11.1 months, p = 0.01). At mRANO-defined progression, patients of the CCNU/TMZ long PPS subgroup had a significantly higher increase of mean ADC values (p = 0.015) and a tendency to a stronger volumetric increase in T1-enhancement (p = 0.22) as compared to long PPS patients of the TMZ arm.
Conclusion
The combination of survival and MRI analyses identified a subgroup of CCNU/TMZ-treated patients with features that sets them apart from other patients in the trial: short first PFS despite long PPS and significant increase in mean ADC values upon mRANO-defined progression. The observed pattern is compatible with the features commonly observed in pseudoprogression suggesting mRANO-undetected pseudoprogressions in the CCNU/TMZ arm of CeTeG/NOA-09.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudoprogression is a well-known and frequently occurring phenomenon in glioblastoma patients and has considerable clinical relevance [1,2,3]. It is defined as MRI changes that mimic tumor progression and eventually resolve or remain stable without change of therapy or can be histologically confirmed as reactive changes without evidence of proliferating tumor. Despite its clinical relevance, it is an insufficiently understood phenomenon and is under further investigation [4, 5].
The exact frequency of pseudoprogression is not clear, current literature describes incidences of 10–30% [6, 7]; transiently increased contrast uptake after radiation even develops in up to 50% [2]. Some authors also describe pseudoprogression as a potential surrogate marker of treatment efficacy, especially in patients with MGMT promotor methylation, although this is still controversial [8,9,10]. MRI perfusion imaging and amino acid positron-emission-tomography (PET) imaging are used in routine clinical practice to detect pseudoprogression [11]. Furthermore, novel imaging approaches, such as amide proton transfer (APT)-weighted MRI showed promising results in the differentiation of therapy-related changes and tumor progression [12,13,14,15]. The apparent diffusion coefficient (ADC) can be derived from diffusion-weighted imaging (DWI) and has been shown to aid distinguishing between true progression and pseudoprogression as higher ADC values in T1-enhancement tissue might indicate the latter one [16,17,18,19].
In the randomized CeTeG/NOA-09 trial [20], combined lomustine (CCNU)/TMZ was superior to TMZ therapy regarding OS in newly diagnosed patients with MGMT-methylated glioblastoma. Despite the OS benefit, PFS and pseudoprogression rates did not differ significantly between treatments. For progression assessment, (modified) RANO criteria were used in this study similar to most clinical trials investigating glioma therapy [21]. Beyond the limit of 12 weeks after the end of radiotherapy, mRANO criteria allow to assume pseudoprogression only if the suspected contrast-enhancing lesion remains stable or decreases in a follow-up MRI within 8 weeks. However, late and prolonged pseudoprogression [22,23,24] that does not show stabilization on the first control MRI may thus go undetected.
In this study, we investigated the hypothesis that undetected pseudoprogressions might be accountable for at least some of the discrepancy for the lack of a PFS-prolonging effect in the CeTeG/NOA-09 trial despite OS prolongation. Hypothesizing that the probability of an undetected pseudoprogression increases in patients who have a particularly short first PFS and a very long postprogression survival (PPS), we analysed patterns of PPS and MRI features including tumor volumetry and ADC analysis at the mRANO-defined progression time point.
Methods
Study design
The prospective, randomized, controlled CeTeG/NOA-09 trial (EudraCT-2009–011252-22, Herrlinger et al. [20]) included 129 patients in the intention-to-treat cohort. Patients were randomized (1:1) to either CCNU/TMZ combination therapy or TMZ standard therapy (Fig. 1). Contrast-enhanced cranial MRI were performed every 12 weeks. We included all patients with disease progression according to mRANO. For patients with a censored PPS below 18 months, subgroup allocation to long vs. short PPS was impossible and these patients were excluded from the analysis. Patients of this trial entered the current MRI-based analyses, if their MRIs were evaluable for T1-enhancement and FLAIR volumetry at the time point of progression and, for comparison, at the last MRI prior to progression. Analyses were performed by an independent neuroradiologist. For determination of progression, the modified RANO criteria [20] were used: up to 12 weeks after completion of radiotherapy, disease progression was considered only for new enhancing lesions outside the radiation field (beyond the 80% isodose) or unequivocal histological demonstration of proliferating tumor. According to previous experience with late pseudoprogression, disease progression 12 to 24 weeks after completion of radiotherapy could only be diagnosed if another MRI showing further progression confirmed it 4–6 weeks afterwards. Figure 1 shows the patient selection process for this analysis in a flowchart. In both arms, patients were further subdivided into those with short PPS (defined as ≤ 18 months) and long PPS (> 18 months). A PPS/PFS ratio was calculated for each patient.
Flowchart of patient identification. Flowchart shows patient selection and identification evaluating patients from the modified intention-to-treat population of the CeTeG/NOA-09 trial. First, subdivision was made by TMZ vs CCNU/TMZ therapy, second subdivision was made by post progression survival with a cut-off of 18 months (defined as survival after progression diagnosis according to mRANO criteria). In this step, patients with unknown PPS (censored PFS and/or censored OS, respectively) were excluded and did not enter any of the following analyses
MRI evaluation
Tumor volume assessment
Manual evaluation of contrast-enhancing and FLAIR hyperintensity volume was performed by using the Medical Imaging Interaction Toolkit software (MITK, Workbench and Toolkit 2016.11, provided by the German Cancer Research Center (DKFZ)). MRI data were performed in 1.5 or 3 T scanners. In this multicenter study cohort, MRIs were conducted in the scanners of the respective centers. The tumor volume was outlined on Gadolinium-enhanced T1 MRI data. Measurement of T1-enhancement volume (solid tumor) and FLAIR volume (solid tumor and edema) were performed separately for each patient and time point for volumetric assessment. For the T1-enhancing volume, the inner necrotic zone has been subtracted (solid tumor volume = T1-enhancement volume – volume of necrosis zone). In supplementary Fig. 1A, a3D reconstruction example of tumor segmentation is shown. The tumor volume, that is defined as “region of interest” (ROI) is shown in red.
ADC analysis
All imaging data were co-registered performed using the “multimodal.rigid.default” registration algorithm in the Medical Imaging Interaction Toolkit [25]. Subsequently, the combined ROI of FLAIR and T1-enhancement volume was used to determine the mean ADC value from both baseline and progression time point in the tumor region (Supplementary Fig. 1B). For comparison between treatment groups, the absolute change of Mean ADC-value from baseline to progression time point was compared between patients with short PPS and long PPS.
Statistical analysis
Statistical analyses have been performed using SPSS (IBM software, Version 27). Analyses of OS, PFS and PPS have been performed according to Kaplan–Meier with a two-sided log-rank test for significance. In contrast to the primary planned confirmatory analysis of CeTeG/NOA-09 trial, which required a log-rank test with stratification by center and recursive partitioning analysis (RPA) class, the analyses in the current report were performed without stratification due to the relatively low number of patients in the subgroups making stratified analyses unapplicable. Median OS, PFS and PPS are reported with a 95% confidence interval (CI).
For comparing PPS/PFS ratios between subgroups we performed a rank sum test (Mann–Whitney-test).Mann–Whitney-test was also performed analyzing tumor/edema volumetry and ADC mean values. Kruskal-Walis-test was performed for comparing median ages and median Karnofsky score between all subgroups. For comparing achieved gross total resections (GTR %) and frequency of second line therapies we used a chi2-test.
For all statistical analyses, p-values of < 0.05 were regarded statistically significant. In the figures, significant results are marked as * = < 0.05, ** = < 0.005. In selected cases, the p-values are shown within the figure; otherwise, they can be found in the figure legend or the manuscript text.
Results
For analysis of post progression survival the modified intention to treat population was used after exclusion of patients that had unknown PPS (n = 90/129 patients). In the TMZ arm, 29 evaluable patients had short PPS and 17 had long PPS (≥ 18 months), in the CCNU/TMZ arm 26 evaluable patients had short PPS and 18 had long PPS (Fig. 1). The median age, rate of gross total resections and the median KPS were similar in the four subgroups (Table 1). Also, there were no significant differences in the use of further line therapies between the long PPS CCNU/TMZ and the long PPS TMZ group (Supplementary Fig. 2).
PFS in the long PPS group of the CCNU/TMZ arm was remarkably shorter as compared to the long PPS group of the TMZ arm (median 5.6 months vs 11.1 months, p = 0.01, Fig. 2A). Going in line with this, the mean PPS/PFS ratio tended to be higher in the long PPS group of the CCNU/TMZ as opposed to the long PPS group of the TMZ arm (7.7 vs. 4.5, p = 0.08, Fig. 2C). The PPS/PFS ratio was similarly low in the short PPS subgroups of TMZ and CCNU/TMZ arms with short PPS (1 vs. 1.1, p = 0.632).
Decreased progression-free survival and higher PPS/PFS ratio in long PPS CCNU/TMZ patients. Kaplan–Meier plots of patients followed TMZ or CCNU/TMZ therapy. Progression free survival (A) is given in patients from the modified intention to treat cohort of CeTeG/NOA-09 trial that had known PPS. Subdivision was made in short post progression survival (< 18 months) and long post progression survival (> 18 months) groups. Median PFS is remarkably low in CCNU/TMZ long PPS group compared to TMZ long PPS group (B), p = 0.01 (log-rank test). (C) Graph shows Mean PPF/PFS ratio of each subgroup
The combination of particularly low PFS with very long OS is prominently seen in the long PPS subgroup of the CCNU/TMZ arm. This raises the question whether at the time point of fulfilling the mRANO progression criteria, the underlying biology (e.g. contribution of pseudoprogression) in this subgroup may be different from the long PPS subgroup of the TMZ arm. We further investigated if these differences are also mirrored in MRI at mRANO-defined progression.
Seventy patients (about 80% of the patients that were included in the survival analysis in this study) were evaluable for imaging analysis and constituted the cohort on which all following MRI analyses are based on. Patients with long PPS in the CCNU/TMZ arm showed a tendency to a stronger increase in T1-enhancement volume (mean delta 4.600,1 mm3 vs. 1.747,9 mm3, p = 0.219, Fig. 3A) but not in FLAIR volume (mean delta 19.749,9 mm3 vs 13.690,8 mm3, p = 0.682, Fig. 3B) at progression as compared to long PPS patients of the TMZ arm. Overall, in the TMZ monotherapy arm, patients with short PPS could be well distinguished from patients with long PPS by their strongly increasing volumes of contrast-enhancement (mean delta 8.055,7 mm3 vs 1.747,9 mm3, p = 0.005) and FLAIR hyperintense lesions (mean delta 31.689,9 mm3 vs 13.690,9 mm3, p = 0.03). In contrast, patients with CCNU/TMZ combination therapy showed no difference in T1-enhancement increase (mean delta 7.227,5 mm3 vs 4.600,1 mm3, p = 0.567) or FLAIR increase (mean delta 25.356,7 mm3 vs. 19.7489,9 mm3, p = 0.914) in short-PPS vs. long-PPS patients. In summary, in the TMZ monotherapy arm, the increase of the contrast-enhancing lesion and FLAIR lesion at mRANO-defined progression was inversely related to OS, whereas in the CCNU/TMZ arm, no such relation was observed. Thus, these parameters do not allow to distinguish patients with long PPS from patients with short PPS in the CCNU/TMZ group at mRANO-defined progression time point.
T1-enhancement and FLAIR volume increase at mRANO defined progression. For each patient in each group T1-enhancement (A) and FLAIR (B) volume increase from baseline time point to mRANO defined progression time point is given (mm3). T1-enhancement and FLAIR volume increase patients in TMZ group differed significantly (p = 0.007 and p = 0.03, respectively) but not in CCNU/TMZ group (p = 0.567 and 0.914, respectively). Comparing long PPS groups, CCNU/TMZ tended to a stronger volumetric increase in T1 enhancement (p = 0.22). Error bars show 95% confidence interval of Means. Statistical analysis was performed using Mann–Whitney-test. p < 0.05 was considered significant and marked as * = < 0.05, ** = < 0.005
Analysis of ADC maps revealed no statistical difference of mean ADC values between treatment groups as a whole at baseline time point (Fig. 4A). However, long PPS patients of the CCNU/TMZ group showed a higher absolute increase in ADC value from baseline (last prior to progression) to mRANO progression time point than long PPS patients of the TMZ group (Fig. 4B, p = 0.017). These data suggest that CCNU/TMZ therapy might lead to changes in tumor structure that can be identified in ADC imaging and may be different from the radiological changes seen in patients after TMZ monotherapy.
ADC analysis of T1-enhancement region of interest (ROI). For each patient in each group Mean ADC value of T1-enhancing tumor ROI was calculated. In (A) the Mean ADC value of baseline tumor (last prior to progression) is shown. There is no significant difference between long PPS TMZ arm and long PPS CCNU/TMZ arm (p = 0.94, Mann–Whitney test). In (B) the absolute change in Mean ADC value of T1-enhancement ROI from baseline to progression time point is shown. The change is significantly higher in long PPS CCNU/TMZ arm, comparing to long PPS TMZ arm (p = 0.017, Mann–Whitney test). Error bars show 95% confidence interval of Means. Statistical analysis was performed using Mann–Whitney-test. p < 0.05 was considered significant and marked as * = < 0.05
Discussion
In the present study based on data from the prospective CeTeG/NOA-09 trial, we are able to identify a subgroup of CCNU/TMZ-treated patients that have a particularly short first PFS despite long PPS and OS. These patients show MRI features (significant increase in mean ADC values; tendency to a stronger increase in contrast enhancement at time point of mRANO-defined progression) that sets them apart from their counterparts with long PPS in the TMZ monotherapy arm.
This observation is remarkable since it is contradictory to the current literature describing a reliable correlation between PFS and OS in glioblastoma and other malignancies [26, 27]. As the PFS is decreased compared to the long PPS group of TMZ, we assumed that tumor changes in MRI of these patient groups might differ biologically from each other. A possible explanation for this difference may be undetected pseudoprogressions that evade correct diagnosis by mRANO criteria and are falsely diagnosed as progressive disease. As we already know, standard MRI and clinical assessment cannot reliably differentiate pseudoprogression and progressive disease. Even histology can be difficult to interpret, as there are no defined criteria for diagnosing progression, pseudoprogression or mixed forms [28]. So far, we are not able to finally proof the hypothesis of undetected pseudoprogressions but we can rule out some alternative hypotheses such as that an imbalanced distribution of further line therapies or other known prognostic factors (Herrlinger et al., 2019 and, for subgroup comparisons see Table 1 and Supplemantary Fig. 2) that may be responsible for the survival differences.
Our image analyses show that the MRI at time of mRANO-defined progression in the long PPS CCNU/TMZ group is particularly characterized by an increase in ADC values, while ADC value of contrast enhancing tumor is stable in TMZ patients. Current literature describes a higher ADC value as compatible with pseudoprogression rather than progression [16,17,18,19]. Thus, our finding might support the hypothesis that pseudoprogressive changes appear to be more frequent and more distinct after CCNU/TMZ therapy. Increase in ADC values could possibly be explained by a variety of biological / histopathological factors, including disintegration of cellular membranes, reduction in cell density and as a result an increase in extracellular space. This pattern is rather observed in pseudoprogression than true progession [29].
The limitations of our analyses are set by the post hoc approach (despite the prospective collection of data in the trial) with MRI data evaluable for volumetric analysis lacking in some of the patients. Further limitations are the small number of patients making detection of small group differences difficult and radiomics approaches impossible, and the lack of histological data. Future imaging analysis in prospective cohorts should also include analysis of MRI perfusion imaging, amino acid positron-emission-tomography (PET) and novel imaging approaches, such as amide proton transfer (APT)-weighted MRI that showed promise results in the differentiation of therapy-related changes and tumor progression [12,13,14,15].
Overall, we conclude that the modified RANO criteria might not be entirely suitable for patients with MGMT-methylated glioblastoma receiving CCNU/TMZ treatment. This is in line with reports that pseudoprogression may be substantially prolonged [30, 31] and thus go undetected by strictly applied mRANO criteria. In the context of CCNU/TMZ therapy, we would therefore rather suggest to perform repeat follow-up examinations (ideally complemented by additional imaging techniques such as MRI perfusion imaging or amino acid PET) instead of prematurely stopping an effective treatment or advancing to further lines of therapy.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
03 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11060-023-04488-z
References
Kruser TJ, Mehta MP, Robins HI (2013) Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother 13(4):389–403. https://doi.org/10.1586/ern.13.7. (PMID: 23545054)
Ellingson BM, Chung C, Pope WB, Boxerman JL, Kaufmann TJ (2017) Pseudoprogression, radionecrosis, inflammation or true tumor progression? challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol 134(3):495–504. https://doi.org/10.1007/s11060-017-2375-2
Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ (2017) Response assessment in neuro-oncology clinical trials. J Clin Oncol 35(21):2439–2449. https://doi.org/10.1200/JCO.2017.72.7511
Jia W, Gao Q, Han A, Zhu H, Yu J (2019) The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med 16(4):655–670
Himes BT, Arnett AL, Merrell KW, Gates MJ, Bhargav AG, Raghunathan A, Brown DA, Burns TC, Parney IF (2020) Glioblastoma recurrence versus treatment effect in a pathology-documented series. Can J Neurol Sci 47(4):525–530. https://doi.org/10.1017/cjn.2020.36
Radbruch A, Fladt J, Kickingereder P, Wiestler B, Nowosielski M, Bäumer P, Schlemmer HP, Wick A, Heiland S, Wick W, Bendszus M (2015) Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro Oncol 17(1):151–159. https://doi.org/10.1093/neuonc/nou129
Ellingson BM, Wen PY, van den Bent MJ, Cloughesy TF (2014) Pros and cons of current brain tumor imaging. Neuro Oncol. https://doi.org/10.1093/neuonc/nou224
Wick W, Chinot OL, Bendszus M, Mason W, Henriksson R, Saran F, Nishikawa R, Revil C, Kerloeguen Y, Cloughesy T (2016) Evaluation of pseudoprogression rates and tumor progression patterns in a phase III trial of bevacizumab plus radiotherapy/temozolomide for newly diagnosed glioblastoma. Neuro Oncol 18(10):1434–1441. https://doi.org/10.1093/neuonc/now
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, Frezza G, Leonardi M, Spagnolli F, Ermani M (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26(13):2192–2197. https://doi.org/10.1200/JCO.2007.14.8163
Gahramanov S, Varallyay C, Tyson RM, Lacy C, Fu R, Netto JP, Nasseri M, White T, Woltjer RL, Gultekin SH, Neuwelt EA (2014) Diagnosis of pseudoprogression using MRI perfusion in patients with glioblastoma multiforme may predict improved survival. CNS Oncol 3(6):389–400. https://doi.org/10.2217/cns.14.42.PMID:25438810;PMCID:PMC4590772
Deuschl C, Kirchner J, Poeppel TD, Schaarschmidt B, Kebir S, El Hindy N, Hense J, Quick HH, Glas M, Herrmann K, Umutlu L, Moenninghoff C, Radbruch A, Forsting M, Schlamann M (2018) 11C-MET PET/MRI for detection of recurrent glioma. Eur J Nucl Med Mol Imaging 45(4):593–601. https://doi.org/10.1007/s00259-017-3916-9. (Epub 2017 Dec 28 PMID: 29282517)
Meissner J-E, Korzowski A, Regnery S, Goerke S, Breitling J, Floca RO et al (2019) Early response assessment of glioma patients to definitive chemoradiotherapy using chemical exchange saturation transfer imaging at 7 T. J Magn Reson Imaging 50(4):1268–1277
Mehrabian H, Myrehaug S, Soliman H, Sahgal A, Stanisz GJ (2018) Evaluation of glioblastoma response to therapy with chemical exchange saturation transfer. Int J Radiation Oncol Biol Phys. 101(3):713–723
Regnery S, Adeberg S, Dreher C, Oberhollenzer J, Meissner J-E, Goerke S et al (2018) Chemical exchange saturation transfer MRI serves as predictor of early progression in glioblastoma patients. Oncotarget 9(47):28772–28783
Sagiyama K, Mashimo T, Togao O, Vemireddy V, Hatanpaa KJ, Maher EA et al (2014) In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc Natl Acad Sci 111(12):4542–4547
Vajapeyam S, Brown D, Billups C, Patay Z, Vezina G, Shiroishi MS, Law M, Baxter P, Onar-Thomas A, Fangusaro JR, Dunkel IJ, Poussaint TY (2020) Advanced ADC histogram, perfusion, and permeability metrics show an association with survival and pseudoprogression in newly diagnosed diffuse intrinsic pontine glioma: a report from the pediatric brain tumor consortium. AJNR Am J Neuroradiol 41(4):718–724. https://doi.org/10.3174/ajnr.A6499
Yu Y, Ma Y, Sun M, Jiang W, Yuan T, Tong D (2020) Meta-analysis of the diagnostic performance of diffusion magnetic resonance imaging with apparent diffusion coefficient measurements for differentiating glioma recurrence from pseudoprogression. Medicine (Baltimore) 99(23):e20270
Reimer C, Deike K, Graf M, Reimer P, Wiestler B, Floca RO, Kickingereder P, Schlemmer HP, Wick W, Bendszus M, Radbruch A (2017) Differentiation of pseudoprogression and real progression in glioblastoma using ADC parametric response maps. PLoS ONE 12(4):e0174620. https://doi.org/10.1371/journal.pone.0174620
Yoo RE, Choi SH, Kim TM, Lee SH, Park CK, Park SH, Kim IH, Yun TJ, Kim JH, Sohn CH (2015) Independent poor prognostic factors for true progression after radiation therapy and concomitant temozolomide in patients with glioblastoma: subependymal enhancement and low ADC value. AJNR Am J Neuroradiol 36(10):1846–1852. https://doi.org/10.3174/ajnr.A4401
Herrlinger U, Tzaridis T, Mack F et al (2019) Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet 393(10172):678–688. https://doi.org/10.1016/S0140-6736(18)31791-4
Ellingson BM, Wen PY, Cloughesy TF (2017) Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics 14(2):307–320
Voss M, Franz K, Steinbach JP, Fokas E, Forster MT, Filipski K, Hattingen E, Wagner M, Breuer S (2019) Contrast enhancing spots as a new pattern of late onset pseudoprogression in glioma patients. J Neurooncol 142(1):161–169. https://doi.org/10.1007/s11060-018-03076-w
Kebir S, Fimmers R, Galldiks N, Schäfer N, Mack F, Schaub C, Stuplich M, Niessen M, Tzaridis T, Simon M, Stoffels G, Langen KJ, Scheffler B, Glas M, Herrlinger U (2016) Late pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-L-Tyrosine PET. Clin Cancer Res 22(9):2190–2196. https://doi.org/10.1158/1078-0432.CCR-15-1334
Galante JR, Rodriguez F, Grossman SA, Strowd RE (2017) Late post-treatment radiographic changes 3 years following chemoradiation for glioma: the importance of histopathology. CNS Oncol 6(3):195–201. https://doi.org/10.2217/cns-2016-0040
Nolden M, Zelzer S, Seitel A, Wald D, Muller M, Franz AM et al (2013) The medical imaging interaction toolkit: challenges and advances : 10 years of open-source development. Int J Comput Assist Radiol Surg 8(4):607–620
Tykocki T, Eltayeb M (2018) Ten-year survival in glioblastoma. A systematic review. J Clin Neurosci 54:7–13. https://doi.org/10.1016/j.jocn.2018.05.002
Liang F, Zhang S, Wang Q, Li W (2018) Evolution of randomized controlled trials and surrogacy of progression-free survival in advanced/metastatic urothelial cancer. Crit Rev Oncol Hematol 130:36–43. https://doi.org/10.1016/j.critrevonc.2018.07.007
Melguizo-Gavilanes I, Bruner JM, Guha-Thakurta N, Hess KR, Puduvalli VK (2015) Characterization of pseudoprogression in patients with glioblastoma: is histology the gold standard? J Neurooncol 123(1):141–150. https://doi.org/10.1007/s11060-015-1774-5
Padhani AR, Liu G, Koh DM et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11(2):102–125. https://doi.org/10.1593/neo.81328
Stuplich M, Hadizadeh DR, Kuchelmeister K, Scorzin J, Filss C, Langen KJ, Schäfer N, Mack F, Schüller H, Simon M, Glas M, Pietsch T, Urbach H, Herrlinger U (2012) Late and prolonged pseudoprogression in glioblastoma after treatment with lomustine and temozolomide. J Clin Oncol 30(21):e180–e183. https://doi.org/10.1200/JCO.2011.40.9565
Werner JM, Weller J, Ceccon G, Schaub C, Tscherpel C, Lohmann P, Bauer EK, Schäfer N, Stoffels G, Baues C, Celik E, Marnitz S, Kabbasch C, Gielen GH, Fink GR, Langen KJ, Herrlinger U, Galldiks N (2021) Diagnosis of pseudoprogression following lomustine-temozolomide chemoradiation in newly diagnosed glioblastoma patients using FET-PET. Clin Cancer Res 27(13):3704–3713
Funding
Open Access funding enabled and organized by Projekt DEAL. The trial was funded by the German Ministry for Education and Science.
Author information
Authors and Affiliations
Contributions
TZ, CS, DP and UH designed the analysis. TZ performed data analysis and wrote the first draft of the manuscript. CS, DP and UH supervised the work. All authors commented on previous versions and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Helsinki Declaration and its later amendments and the Guidelines for Good Clinical Practice. The study was approved by the Ethics committees of all participating centers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article has been revised: A note has been added that authors Thomas Zeyen and Daniel Paech have equally contributed.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zeyen, T., Paech, D., Weller, J. et al. Undetected pseudoprogressions in the CeTeG/NOA-09 trial: hints from postprogression survival and MRI analyses. J Neurooncol 164, 607–616 (2023). https://doi.org/10.1007/s11060-023-04444-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04444-x