Abstract
Introduction
There is mounting evidence supporting the role of tryptophan metabolism via the kynurenine pathway (KP) in the pathogenesis of primary brain tumors. Under normal physiological conditions, the KP is the major catabolic pathway for the essential amino acid tryptophan. However, in cancer cells, the KP becomes dysregulated, depletes local tryptophan, and contributes to an immunosuppressive tumor microenvironment.
Methods
We examined the protein expression levels (in 73 gliomas and 48 meningiomas) of the KP rate-limiting enzymes indoleamine 2,3-dioxygenase (IDO) 1, IDO2, and tryptophan 2,3-dioxygenase (TDO2), as well as, the aryl hydrocarbon receptor (AhR), a carcinogenic transcription factor activated by KP metabolites. In addition, we utilized commercially available small-molecules to pharmacologically modulate IDO1, IDO2, TDO2, and AhR in patient-derived glioma and meningioma cell lines (n = 9 each).
Results
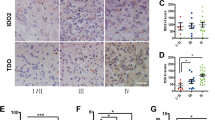
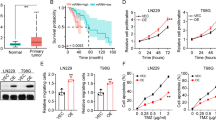
We observed a positive trend between the grade of the tumor and the average immunohistochemical staining score for IDO1, IDO2, and TDO2, with TDO2 displaying the strongest immunostaining. AhR immunostaining was present in all grades of gliomas and meningiomas, with the greatest staining intensity noted in glioblastomas. Immunocytochemical staining showed a positive trend between nuclear localization of AhR and histologic grade in both gliomas and meningiomas, suggesting increased AhR activation with higher tumor grade. Unlike enzyme inhibition, AhR antagonism markedly diminished patient-derived tumor cell viability, regardless of tumor type or grade, following in vitro drug treatments.
Conclusions
Collectively, these results suggest that AhR may offer a novel and robust therapeutic target for a patient population with highly limited treatment options.





Similar content being viewed by others
References
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2017) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol 19:v1–v88. https://doi.org/10.1093/neuonc/nox158
Delgado-Lopez PD, Corrales-Garcia EM, Martino J, Lastra-Aras E, Duenas-Polo MT (2017) Diffuse low-grade glioma: a review on the new molecular classification, natural history and current management strategies. Clin Transl Oncol. https://doi.org/10.1007/s12094-017-1631-4
Mittal S, Szlaczky MC, Barger GR (2008) Low-grade gliomas in adults. Curr Treat Options Neurol 10:271–284
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, for the European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups and the National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996 https://doi.org/10.1056/NEJMoa043330
Norden AD, Drappatz J, Wen PY (2009) Advances in meningioma therapy. Curr Neurol Neurosci Rep 9:231–240
Chamberlain MC (2012) Hydroxyurea for recurrent surgery and radiation refractory high-grade meningioma. J Neurooncol 107:315–321. https://doi.org/10.1007/s11060-011-0741-z
McNeill KA (2016) Epidemiology of brain tumors. Neurol Clin 34:981–998. https://doi.org/10.1016/j.ncl.2016.06.014
Xie Q, Mittal S, Berens ME (2014) Targeting adaptive glioblastoma: an overview of proliferation and invasion. Neuro Oncol 16:1575–1584. https://doi.org/10.1093/neuonc/nou147
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Adams S, Teo C, McDonald KL, Zinger A, Bustamante S, Lim CK, Sundaram G, Braidy N, Brew BJ, Guillemin GJ (2014) Involvement of the kynurenine pathway in human glioma pathophysiology. PLoS ONE 9:e112945. https://doi.org/10.1371/journal.pone.0112945
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M (2011) An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478:197–203. https://doi.org/10.1038/nature10491
Batista CE, Juhász C, Muzik O, Kupsky WJ, Barger G, Chugani HT, Mittal S, Sood S, Chakraborty PK, Chugani DC (2009) Imaging correlates of differential expression of indoleamine 2,3-dioxygenase in human brain tumors. Mol Imaging Biol 11:460–466. https://doi.org/10.1007/s11307-009-0225-0
Guastella AR, Michelhaugh SK, Klinger NV, Kupsky WJ, Polin LA, Muzik O, Juhász C, Mittal S (2016) Tryptophan PET Imaging of the Kynurenine pathway in patient-derived xenograft models of glioblastoma. Mol Imaging. https://doi.org/10.1177/1536012116644881
Talari NK, Panigrahi M, Madigubba S, Challa S, Phanithi PB (2016) Altered tryptophan metabolism in human meningioma. J Neurooncol 130:69–77. https://doi.org/10.1007/s11060-016-2225-7
Peters JC (1991) Tryptophan nutrition and metabolism: an overview. Adv Exp Med Biol 294:345–358
Badawy AA (2017) Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. https://doi.org/10.1177/1178646917691938
Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB (2002) Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med 196:459–468
Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P (2006) The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 176:6752–6761
Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ (2003) Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 9:1269–1274. https://doi.org/10.1038/nm934
Alkonyi B, Mittal S, Zitron I, Chugani DC, Kupsky WJ, Muzik O, Chugani HT, Sood S, Juhász C (2012) Increased tryptophan transport in epileptogenic dysembryoplastic neuroepithelial tumors. J Neurooncol 107:365–372. https://doi.org/10.1007/s11060-011-0750-y
Juhász C, Nahleh Z, Zitron I, Chugani DC, Janabi MZ, Bandyopadhyay S, Ali-Fehmi R, Mangner TJ, Chakraborty PK, Mittal S, Muzik O (2012) Tryptophan metabolism in breast cancers: molecular imaging and immunohistochemistry studies. Nucl Med Biol 39:926–932. https://doi.org/10.1016/j.nucmedbio.2012.01.010
Zitron IM, Kamson DO, Kiousis S, Juhász C, Mittal S (2013) In vivo metabolism of tryptophan in meningiomas is mediated by indoleamine 2,3-dioxygenase 1. Cancer Biol Ther 14:333–339. https://doi.org/10.4161/cbt.23624
Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA (2010) An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 185:3190–3198. https://doi.org/10.4049/jimmunol.0903670
DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH (2010) Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci 115:89–97. https://doi.org/10.1093/toxsci/kfq024
Safe S, Lee SO, Jin UH (2013) Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol Sci 135:1–16. https://doi.org/10.1093/toxsci/kft128
Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M, Ott M, Ochs K, Lutz C, Liu X, Anastasov N, Lehmann I, Hofer T, von Deimling A, Wick W, Platten M (2014) Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget 5:1038–1051
Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F (2008) Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun 375:331–335. https://doi.org/10.1016/j.bbrc.2008.07.156
Stone TW, Darlington LG (2002) Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov 1:609–620. https://doi.org/10.1038/nrd870
Johnson J, Ascierto ML, Mittal S, Newsome D, Kang L, Briggs M, Tanner K, Marincola FM, Berens ME, Vande Woude GF, Xie Q (2015) Genomic profiling of a hepatocyte growth factor-dependent signature for MET-targeted therapy in glioblastoma. J Transl Med 13:306. https://doi.org/10.1186/s12967-015-0667-x
Gramatzki D, Pantazis G, Schittenhelm J, Tabatabai G, Kohle C, Wick W, Schwarz M, Weller M, Tritschler I (2009) Aryl hydrocarbon receptor inhibition downregulates the TGF-beta/Smad pathway in human glioblastoma cells. Oncogene 28:2593–2605. https://doi.org/10.1038/onc.2009.104
Safe S, Cheng Y, Jin UH (2017) The aryl hydrocarbon receptor (AhR) as a drug target for cancer chemotherapy. Curr Opin Toxicol 2:24–29. https://doi.org/10.1016/j.cotox.2017.01.012
Kolluri SK, Jin UH, Safe S (2017) Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch Toxicol 91:2497–2513. https://doi.org/10.1007/s00204-017-1981-2
Michelhaugh SK, Guastella AR, Varadarajan K, Klinger NV, Parajuli P, Ahmad A, Sethi S, Aboukameel A, Kiousis S, Zitron IM, Ebrahim SA, Polin LA, Sarkar FH, Bollig-Fischer A, Mittal S (2015) Development of patient-derived xenograft models from a spontaneously immortal low-grade meningioma cell line, KCI-MENG1. J Transl Med 13:227. https://doi.org/10.1186/s12967-015-0596-8
Fischer AH, Jacobson KA, Rose J, Zeller R (2008) Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. https://doi.org/10.1101/pdb.prot4986
Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2017) SEER cancer statistics review, 1975–2014. National Cancer Institute. Bethesda. https://seer.cancer.gov/csr/1975_2014/
Fotopoulou C, Sehouli J, Pschowski R, S VONH., Domanska G, Braicu EI, Fusch G, Reinke P, Schefold JC (2011) Systemic changes of tryptophan catabolites via the indoleamine-2,3-dioxygenase pathway in primary cervical cancer. Anticancer Res 31:2629–2635
Trott JF, Kim J, Abu Aboud O, Wettersten H, Stewart B, Berryhill G, Uzal F, Hovey RC, Chen CH, Anderson K, Graef A, Sarver AL, Modiano JF, Weiss RH (2016) Inhibiting tryptophan metabolism enhances interferon therapy in kidney cancer. Oncotarget 7:66540–66557. https://doi.org/10.18632/oncotarget.11658
Santhanam S, Alvarado DM, Ciorba MA (2016) Therapeutic targeting of inflammation and tryptophan metabolism in colon and gastrointestinal cancer. Transl Res 167:67–79. https://doi.org/10.1016/j.trsl.2015.07.003
Heng B, Lim CK, Lovejoy DB, Bessede A, Gluch L, Guillemin GJ (2016) Understanding the role of the kynurenine pathway in human breast cancer immunobiology. Oncotarget 7:6506–6520. https://doi.org/10.18632/oncotarget.6467
D’Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, Nemkov TG, D’Alessandro A, Hansen KC, Richer JK (2015) A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res 75:4651–4664. https://doi.org/10.1158/0008-5472.CAN-15-2011
Chuang SC, Fanidi A, Ueland PM, Relton C, Midttun O, Vollset SE, Gunter MJ, Seckl MJ, Travis RC, Wareham N, Trichopoulou A, Lagiou P, Trichopoulos D, Peeters PH, Bueno-de-Mesquita HB, Boeing H, Wientzek A, Kuehn T, Kaaks R, Tumino R, Agnoli C, Palli D, Naccarati A, Aicua EA, Sanchez MJ, Quiros JR, Chirlaque MD, Agudo A, Johansson M, Grankvist K, Boutron-Ruault MC, Clavel-Chapelon F, Fagherazzi G, Weiderpass E, Riboli E, Brennan PJ, Vineis P, Johansson M (2014) Circulating biomarkers of tryptophan and the kynurenine pathway and lung cancer risk. Cancer Epidemiol Biomark Prev 23:461–468. https://doi.org/10.1158/1055-9965.EPI-13-0770
Mandi Y, Vecsei L (2012) The kynurenine system and immunoregulation. J Neural Transm 119:197–209. https://doi.org/10.1007/s00702-011-0681-y
Bosnyák E, Kamson DO, Guastella AR, Varadarajan K, Robinette NL, Kupsky WJ, Muzik O, Michelhaugh SK, Mittal S, Juhász C (2015) Molecular imaging correlates of tryptophan metabolism via the kynurenine pathway in human meningiomas. Neuro Oncol 17:1284–1292. https://doi.org/10.1093/neuonc/nov098
Tsuji N, Fukuda K, Nagata Y, Okada H, Haga A, Hatakeyama S, Yoshida S, Okamoto T, Hosaka M, Sekine K, Ohtaka K, Yamamoto S, Otaka M, Grave E, Itoh H (2014) The activation mechanism of the aryl hydrocarbon receptor (AhR) by molecular chaperone HSP90. FEBS Open Bio 4:796–803. https://doi.org/10.1016/j.fob.2014.09.003
Tsai CF, Hsieh TH, Lee JN, Hsu CY, Wang YC, Lai FJ, Kuo KK, Wu HL, Tsai EM, Kuo PL (2014) Benzyl butyl phthalate induces migration, invasion, and angiogenesis of Huh7 hepatocellular carcinoma cells through nongenomic AhR/G-protein signaling. BMC Cancer 14:556. https://doi.org/10.1186/1471-2407-14-556
Jin UH, Kim SB, Safe S (2015) Omeprazole inhibits pancreatic cancer cell invasion through a nongenomic aryl hydrocarbon receptor pathway. Chem Res Toxicol 28:907–918. https://doi.org/10.1021/tx5005198
Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K (2013) Causes, consequences, and reversal of immune system aging. J Clin Invest 123:958–965. https://doi.org/10.1172/JCI64096
Moyer BJ, Rojas IY, Murray IA, Lee S, Hazlett HF, Perdew GH, Tomlinson CR (2017) Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors activate the aryl hydrocarbon receptor. Toxicol Appl Pharmacol 323:74–80. https://doi.org/10.1016/j.taap.2017.03.012
Acknowledgements
The study was supported, in part, by the following Grants: 1F31CA210682-01A1 (A.R.G.) from the National Cancer Institute; 4T32CA009531-30 (A.R.G.) from the National Cancer Institute; R25GM058905 (A.R.G.) from the National Institute of General Medical Sciences; R01CA123451 (C.J. and S.M.) from the National Cancer Institute; the Fund for Medical Research and Education, Wayne State University School of Medicine (S.M.); a Strategic Research Initiative Grant from Karmanos Cancer Institute (S.M.); and The Office of the Vice President for Research, Wayne State University (S.M.). The Biobanking and Correlative Sciences Core is supported, in part, by the National Institute of Health Center Grant P30CA022453 to the Karmanos Cancer Institute at Wayne State University. We wish to thank the patients who graciously donated their tumor tissue for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
. Immunohistochemical staining of positive controls. Immunostaining for the stated antibody were performed on the following tissues: lymph node (IDO1) and liver (IDO2, TDO2, and AhR). Images are 20× magnification, with scale bar of 100 μm. (TIF 3387 KB)
Supplemental Figure 2
. Immunocytochemical staining of patient-derived cells. Cultured patient-derived glioma and meningioma cells were plated onto 8-well chamber slides. When cells were at a sufficient density, cells were fixed in formalin for 30 min. Immunocytochemical staining was performed as described in the Methods section. Cells were stained for the three rate-limiting enzymes of the kynurenine pathway (indoleamine 2,3-dioxygenase (IDO) 1, IDO2, and tryptophan 2,3-dioxygenase 2 (TDO2)), as well as the aryl hydrocarbon receptor (AhR). IDO1 showed lower staining in gliomas than in meningiomas. IDO2 and TDO2 showed staining in all tumor sections, with TDO2 having the greatest staining intensity. Unlike in the tissue staining, cellular staining of AhR was greatest in the highest grade of each tumor type. Furthermore, cellular staining showed that higher grade tumors also had abundant nuclear staining of AhR. Images are representative of the average staining score for the cell type and antibody used and are 20× magnification, with scale bar of 100 μm. (TIF 31141 KB)
Supplemental Figure 3
. Representative primary antibody immunocytochemical negative controls are shown for each of the tumor types and histological grades. Images are 20x magnification, with scale bar of 100 µm. (TIF 1031 KB)
Supplemental Figure 4
. Drug combination study of high-grade gliomas and meningiomas. Cultured patient-derived grade IV glioblastoma (n = 3) and grade III meningioma cell lines (n = 3) were treated in triplicate for 48 hrs with the listed drug combinations. Drugs used were as follows: IDO1 – epacadostat (20 nM); IDO2 – tenatoprazole (4 µM); and TDO2 – 680C91 (2 µM). After treatment, cell viability was measured via MTT assay. Cell viability is represented as percent control and plotted with a box and whiskers plot using the Tukey method. No combination of the rate-limiting enzyme inhibitors produced a significant loss of cell viability. (TIF 1608 KB)
Rights and permissions
About this article
Cite this article
Guastella, A.R., Michelhaugh, S.K., Klinger, N.V. et al. Investigation of the aryl hydrocarbon receptor and the intrinsic tumoral component of the kynurenine pathway of tryptophan metabolism in primary brain tumors. J Neurooncol 139, 239–249 (2018). https://doi.org/10.1007/s11060-018-2869-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2869-6