Abstract
Background
The widespread use of phthalates as plasticizers has raised public health concerns regarding their adverse effects, including an association with cancer. Although animal investigations have suggested an association between phthalate exposure and hepatocellular carcinoma, the mechanisms are unknown.
Methods
The hepatocellular carcinoma cell line Huh7 was treated with benzyl butyl phthalate (BBP), and then analyzed by total internal reflection fluorescence microscopy, confocal microscopy and double immunogold transmission electron microscopy. Following BBP treatment, mRNA levels were measured by RT-PCR, protein levels were measured using western blot, and vascular endothelial growth factor levels were measured by an enzyme-linked immunosorbent assay. Cell migration and invasion assays were evaluated by transwell, and angiogenesis were performed by a tube formation assay. Nude mice were used to investigate metastasis and angiogenesis in vivo.
Results
BBP affected hepatocellular carcinoma progression through the aryl hydrocarbon receptor (AhR) and that benzyl butyl phthalate (BBP) stimulated AhR at the cell surface, which then interacted with G proteins and triggered a downstream signaling cascade. BBP activated AhR through a nongenomic action involving G-protein signaling rather than the classical genomic AhR action. BBP treatment promoted cell migration and invasion in vitro and metastasis in vivo via the AhR/Gβ/PI3K/Akt/NF-κB pathway. In addition, BBP induced both in vitro and in vivo angiogenesis through the AhR/ERK/VEGF pathway.
Conclusions
These findings suggest a novel nongenomic AhR mechanism involving G-protein signaling induced by phthalates, which contributes to tumor progression of hepatocellular carcinoma.
Similar content being viewed by others
Background
Globally, hepatocellular carcinoma is the sixth most common cancer and the third most common cause of cancer-related deaths [1]. Risk factors for hepatocellular carcinoma include infection with hepatitis B or C viruses, alcohol consumption, smoking, and environmental factors [2]. Oral administration of phthalates to rats results in liver enlargement and cause increased malondialdehyde levels in the liver, indicating that phthalates cause oxidative damage of the liver [3]. Bis(2-ethylhexyl) phthalate (DEHP) acts as a promoter of hepatocellular tumors initiated by N-nitrosodiethylamine [4]. Moreover, lifetime DEHP treatment induces testis and liver cancer in rats [5]. Based on proteomic analysis, the proteins secreted by HepG2 cells that have been treated with benzyl butyl phthalate (BBP) are associated with DNA damage, tumor progression, apoptosis, energy metabolism, and cell structure and motility [6]. The observed roles of BBP in DNA damage and methylation, as well as cell migration, invasion, and proliferation suggest the involvement of BBP in tumor development and progression. These findings support carcinogenesis induced by phthalates, but the mechanism remains largely unknown.
Previous studies have shown that phthalates affect the activation of the aryl hydrocarbon receptor (AhR) [7, 8]. Moreover, phthalates suppressed type I interferon expression in human plasmacytoid dendritic cells via AhR [9]. Our previous study showed that BBP induces necrosis in human granulosa cells via AhR activation followed by downstream CYP1B1 induction [10]. We also showed that phthalates induce proliferation and invasiveness of breast cancer through the AhR/HDAC6/c-Myc signaling pathway [11]. These results suggest that AhR is an important receptor that mediates multiple biological effects of phthalates.
The ligand-activated transcriptional factor AhR regulates the enzymatic functions needed for xenobiotic metabolism. Previous reports revealed two pathways that mediate AhR effects including genomic and non-genomic pathways. The classical genomic function involves AhR nuclear translocation and binding to xenobiotic responsive elements located in the promoters of target genes, CYP1A1 and CYP1B1 [12]. In addition to the classical genomic AhR function, AhR can regulate gene expression through a nongenomic mechanism. The Study of nongenomic signaling is important in the field of toxicology. It is difficult to identify dioxin response element (DRE)-based target genes and many reports suggest that the toxic effect of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) is more compatible with the nongenomic signaling of AhR, rather than the genomic action [13]. Moreover, our previous study reported a phthalate mediated AhR/HDAC6/c-Myc pathway that demonstrated a nongenomic effect of AhR [10]. Several studies have reported that TCDD induces inflammatory responses through a nongenomic AhR function [14–17], although this nongenomic AhR function remains poorly understood.
Here, we found that BBP promotes angiogenesis, migration and invasion in vitro as well as angiogenesis and metastasis in vivo of hepatocellular carcinoma. Because G-protein signaling is involved in the regulation of AhR stability [18], we further investigated the AhR function and its possible relationship to G-protein signaling in hepatocellular carcinoma.
Additionally, we revealed that the mechanism through which phthalates activate the nongenomic AhR pathway is associated with G-protein signaling.
Methods
Chemicals and plasmid
Fluo-4 was purchased from Invitrogen (Carlsbad, CA, USA). BBP, 2-aminoethoxydiphenyl borate (2-APB), and 6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Pd98059 and wortmannin were obtained from Calbiochem-Novabiochem (San Diego, CA, USA).
pEGFP-C1-AhR, a kind gift from Dr. Hsin-yu Lee (Department of Life Science, National Taiwan University), was cloned the AhR gene into pEGFP-C1 (Clontech).
Cell culture
Huh7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, Grand Island, NY, USA), PLC/PRF/5 and HepG2 cells were cultured in minimum essential medium (MEM) (Life Technologies, Grand Island, NY, USA) and supplemented with 10% fetal bovine serum (Gibco, California,CA, USA), 1% penicillin (100 U/mL), streptomycin (10 μg/mL), and amphotericin-B (250 μg/mL) (Sigma-Aldrich Co, St. Louis, MO). Human umbilical vein endothelial cells (HUVEC) were grown in EGM-2 medium (Lonza, Basel, Switzerland). All cells were cultured at 37°C in 5% CO2.
Total internal reflection fluorescennce microscopy
For total internal reflection fluorescennce (TIRF) microscopy studies, Huh7 cells were transfected with pEGFP-C1-AhR or pEGFP-C1 as a control using LT1 transfection reagent (Mirus, Madison, WI, USA). After transfection for 24 hours, the cells were harvested and cultured on coverslips for 1 day. Cells were then treated with DMSO as a control or BBP (1 μM) and analyzed by TIRF microscopy (Carl Zeiss, Oberkochen, Germany). GFP intensity was analyzed by Axio Vision Rel. 4.8 software (Carl Zeiss, Oberkochen, Germany).
Calcium imaging
Calcium imaging was performed using the same method as in a previous study [19] with some modifications. For live cell calcium imaging, Cell-R software was used for microscopy (Olympus, Japan). Huh7 cells were seeded on coverslips and cultured for 24 hours. Cells were incubated with 1 μM Fluo-4, a Ca2+-specific dye, at 37°C for 20 minutes in Buffer Salt Saline (BSS) (2 mM CaCl2, 5.5 mM d-glucose, 130 mM NaCl, 5.4 mM KCl, 20 mM HEPES pH = 7.4, 1 mM MgSO4) and then washed three times before measuring the relative fluorescence intensity. Cells were pretreated with various concentrations of 2-APB for 10 minutes, and then loaded with 1 μM Fluo-4 for 20 minutes. After washing, cells were maintained in calcium-free medium (5.5 mM d-glucose, 130 mM NaCl, 5.4 mM KCl, 20 mM HEPES (pH = 7.4), and 3 mM MgSO4) during the experimental periods. The cells were then stimulated by adding BBP (1 μM) after 1 minute. Data were analyzed with Cell-R software.
Confocal microscopy
Huh7 cells were transfected with pEGFP-C1-AhR using LT1 transfection reagent. After overnight transfection, the cells were harvested and cultured on coverslips for 1 day. BBP (1 μM) was added to stimulate the cells before analysis by confocal microscopy. GFP intensity was analyzed by FV10-ASW 3.0 software (Olympus, Japan).
Double immunogold transmission electron microscopy
Ultrathin sections of plastic-embedded cells were pretreated with 5% sodium metaperiodate (10 min) by microwave fixation and processing. The grids were incubated with an aliquot of IgG antibodies against AhR or Gαq/11 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by probing with secondary antimouse IgG gold particles (6 nm) or anti rabbit IgG gold particles (20 nm), respectively. After washing, the sections were blocked by placing the grids on a drop of phosphate-buffered saline (PBS) containing 1% ovalbumin and incubating for 15 minutes. Sections were then stained with uranyl acetate and lead citrate for characterization by transmission electron microscopy (H-700, Hitachi, Japan).
Fluorescence in situ hybridization
After treatment with BBP (1 μM) or DMSO for the control group, cells were fixed by adding fixation solution (in 3.7% formaldehyde/PBS) at room temperature for 10 minutes. The cells were washed with PBS twice and then permeabilized by adding 70% EtOH at 4°C for 1 hour. Cells were then washed in wash buffer (5 mL 20× saline-sodium citrate (SSC), 5 mL formamide, and nuclease-free water to a final volume of 50 mL) for 5 minutes. Hybridization was performed by mixing 100 μL of hybridization solution (1 g dextran sulfate, 20 × SSC, 1 mL formamide for a 10% final concentration and nuclease-free water to a final volume of 10 mL) with a specific AhR probe (Stellaris™ FISH Probes, Biosearch Technology, Novato, CA, USA) and incubating the mixture overnight at 37°C. Cells were then stained with DAPI (1 μg/mL) for 5 minutes. After washing, a drop of mounting solution was applied. The slides were then covered with the cell-attached cover glasses and sealed with nail polish. Imaging was performed by confocal microscopy.
RNA isolation and RT-PCR
Huh7 cells (3 × 105) were seeded in six-well plates, cultured for 24 hours, and then incubated overnight in serum free medium. The cells were then treated with BBP (1 μM) for various times intervals. After stimulation, cells were washed twice with PBS. Total RNA was extracted with TRIzol (Invitrogen). The RNA (2 μg) was applied to a Reverse Transcription System (Promega Biosciences, San Luis Obispo, CA, USA) to synthesize cDNA. The cDNA was then amplified by specific primers. The primer pairs were as follows: AhR, forward 5′-TACTCTGCCGCCCAA ACTGG-3′, reverse 5′-GCTCTGCAACCTCCGATTCC-3′; β-actin, forward 5′-CTCGCTGTCCACCTTCCA-3′, reverse 5′-GCTGTCACCTTCACCGTTC-3′. The PCR conditions were 95°C for 5 min, and then 34 cycles of 95°C for 30 sec, 54°C for 30 sec, and 72°C for 1 min, and a final extension at 72°C for 10 min. PCR products were separated on 2% agarose gels and visualized using ethidium bromide.
siRNA and shRNA transfection
The following siRNAs were used: scrambled siRNA sense: 5′-GAUCAUACGUGCGAUCAGA-3′, antisense: 5′-UCUGAUCGCACGUAUGAUC-3′ ; AhR siRNA (SASI_Hs02_00332181, Sigma). The following shRNAs were obtained from the National RNAi Core Facility at Academic Sinica: control shRNA, 5′-TACAACAGCCACAACGTCTAT-3′; AhR shRNA (1) (TRCN0000021255), AhR shRNA (2) (TRCN00000245285), NF-κB shRNA (1) (TRCN0000006518), and NF-κB shRNA (2) (TRCN0000006520). Cells were transfected with siRNA (10 nM) or shRNA (2 μg) using LT1 transfection reagent.
Immunoblot analysis
Whole cell extracts were prepared in RIPA lysis buffer (Millipore, Temecula, CA, USA) containing 1× protease inhibitor cocktail (Thermo Scientific, Waltham, MA, USA). Protein concentrations were determined using a BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). Equal amounts of protein (50 μg protein) were resolved by sodium dodecyl sulfate-polyacaryamide gel electrophoresis (SDS-PAGE), transferred onto a polyvinylidene difluoride membrane, and blocked with 5% nonfat dry milk for 1 hour at room temperature. After blocking, the membrane was incubated overnight with primary antibodies at 4°C and washed three times with PBST. The horseradish peroxidase conjugated secondary antibodies (Santa Cruz Biotechnology) were incubated for 1 hour at room temperature. The blots were washed three times with PBST and then visualized with an enhanced chemiluminescence kit (Thermo Scientific, Rockford, IL, USA). The primary antibodies were as follows: AhR, Gαq/11, Gβ, PIP2, IP3R, and PI3K from Santa Cruz Biotechnology (1:100); p44/42 MAPK (Erk1/2), Akt, p-Akt (ser473), NFκB, LaminA/C, Histion H3 and α-tubulin from Cell Signaling Technology (1:1000); COX-2 from Abcam (1:1000); β-actin from Sigma (1:1000).
Immunoprecipitation
After preclearing for 30 minutes with protein G agarose (Millipore), antibodies specific for Gαq/11, Gβ, or PI3K (1:100; Santa Cruz Biotechnology) or IgG (2 μg, Sigma-Aldrich Co, St. Louis, MO, USA) were added before overnight incubation at 4°C, followed by precipitation for 2 hours with protein G agarose. The beads were washed three times with RIPA lysis buffer, boiled in sample buffer, and the protein were resolved by 8% SDS-PAGE before performing immunoblot analysis of the indicated proteins.
Transwell migration and invasion assays
Cell migration assays were performed in 24-well inserts (8-μm pore size; BD Biosciences, Franklin Lakes, NJ, USA) and cell invasion assays were performed in 24-well Matrigel™ Invasion inserts (8-μm pore size; BD Biosciences, Franklin Lakes, NJ, USA). Cells (1 × 104) in serum-free DMEM were seeded in the upper chamber of the insert and DMEM containing 10% fetal bovine serum was added to the lower chamber and followed by incubation for 1 day (for migration) or 2 days (for invasion). The medium and cells were then removed from the top chamber using cotton swabs and PBS. The cells were fixed with 4% paraformaldehyde for 30 minutes, stained with a 0.5% crystal violet solution for 2 hours, and counted under a microscopy.
Measurement of vascular endothelial growth factor
Huh7 cells were grown in 12-well plates and treated with BBP for 1 day. After treatment, the cells were incubated in fresh medium for 1 day. The media were then collected and centrifuged at 1,000 rpm for 5 minutes to remove cell debris. Vascular endothelial growth factor (VEGF) levels in the conditioned medium were measured with an enzymed-linked immunosorbent assay (ELISA) kit (R&D System, Minneapolis, MN, USA).
Angiogenesis tube formation assay
HUVEC (2 × 104 cells/mL) were seeded in a 48-well plate pre-coated with Matrigel (BD Biosciences, San Jose, CA, USA). After Huh7 cell-conditioned medium was added to a final volume of 20%, the cells were cultured for 16 hours, stained with calcium-AM (Invitrogen), and visualized under a fluorescence microscope (Olympus). Total tube lengths were measured by MetaMorph software (Leica)
Animals
Male 6-week-old nude mice (BALB/cA-nu nu/nu) were purchased from the National Science Council Animal Center (Taiwan). All animal experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee of Kaohsiung Medical University Hospital (IACUC Approval No: 101060).
In vivotumor xenograft experiments
Huh7 cells were stably transfected with infrared fluorescent protein [20]. Briefly, 293 T cells were transfected with pCMV-ΔR8.91, pMD.G, and pLKO AS3-reporter gene using LT1 transfection reagent for 3 days, and the supernatant (lentivirus-containing medium) was collected the next day. Huh7 cells (2 × 105) were seeded into six-well plates and incubated for 1 day. The lentivirus-containing medium (200 μL) was mixed with 800 μL of DMEM containing 8 μg/mL polybrene and added to each well, and the cells were incubated for 1 day. A stable clone was selected by puromycin treatment (2 μg/mL) for 14 days. The cells were incubated with 25 μM biliverdin overnight and then purified Huh7-IFP cells by flow cytometry. The hepatocellular carcinoma model of direct intrahepatic injection was performed according to a previous study [21] with some modifications. After a small incision was made in nude mice to access the liver, Huh7-IFP cells (1 × 106) suspended in PBS were slowly injected into the upper left lobe of the liver using a 28-gauge needle. A transparent bleb of cells was formed through the liver capsule after injection. To prevent bleeding, a small piece of sterile gauze was placed, and light pressure was applied on the injection site. After implantation, the mice were placed on a heating pad or below a heating lamp until fully active. The mice were randomly divided into two groups (vehicle or BBP treatment), 18 mice of each. After 3 days, BBP (500 mg/kg) was administered by intraperitoneal (i.p.) injection every 2 days. Previous studies have reported that administration of BBP by i.p. at a dose of 800 mg/kg for 24 weeks results in no significant toxic effects [11, 22], which is a higher dose than that used in this study. Tumor growth was detected by injecting biliverdin (250 nM) into the tail vein 30 minutes before imaging. After 1 month, the mice were sacrificed and the organs (liver, lungs, kidneys, and spleen) were removed and viewed with an Ultra Sensitive Molecular Imaging System (Berthold Technology). The numbers of the organs (lung, kidneys, spleen) that expressed fluorescence, considered as metastasis positive organs were determined.
Immunohistochemistry
Liver tissues were fixed with 4% paraformaldehyde, embedded in paraffin, and cut into 4-μm thick sections. The sections were deparaffinized in xylene and rehydrated with a graded series of ethanol/water solutions (100% and 95% ethanol) and then water washes. The sections were treated with 10 mM citrate buffer at 95°C to retrieve antigens and blocked with 5% bovine serum albumin. Primary antibodies against PI3K (1:100; Santa Cruz Biotechnology) and NF-κB (1:200; Cell Signaling) were applied to the sections at 4°C overnight, and then the sections were incubated with secondary antibodies and 3,3’-diaminobenzidine. Intensities of PI3K and NF-κB staining were analyzed by Tissue Quest software (Tissue Genomics)
In vivo Matrigel™ -plug angiogenesis assay
Huh7 cells (3 × 106) were suspended in 150 μL PBS, mixed with 50 μL Matrigel (BD Biosciences), and injected into the flanks of nude mice. BBP (1 μM) was added to the cell suspensions of the treatment groups. After 21 days, the Matrigel plugs were removed. Hemoglobin levels were determined by Drabkin reagent (Sigma), and protein concentrations were normalized to measure blood vessel formation.
Statistical analysis
Statistical significance was established using the Student’s t-test. A p-value of < 0.05 was considered statistically significant.
Results
BBP induced AhR expression
The effect of BBP on AhR mRNA expression was examined by RT-PCR. BBP transiently increased AhR mRNA expression until it reached its highest level at 15 minutes after treatment (Figure 1A). Next, we examined the RNA level using a specific AhR mRNA probe. As a result, AhR mRNA expression was increased at 15 minutes after BBP teratment, which was comparable with the RT-PCR results (Figure 1B). Immunoblot analysis of the effect of BBP on AhR expression showed that BBP stimulated AhR expression in a time-dependent manner (Figure 1C).
BBP activates AhR mRNA and protein expression. (A) Huh7 cells were treated with BBP (1 μM), and mRNA expression of AhR was analyzed by RT-PCR at the indicated time points. The agarose gel image is the expression of AhR mRNA. Each values in the graph was obtained by densitometry and is the mean of three independent experiments. Each value in the graph is the mean ± SD from three independent experiments. The asterisks indicate a significant difference expression relative to the level at 0 minute, as analyzed by Student’s t-test (p < 0.05). (B) Huh7 cells were treated with BBP (1 μM) or DMSO as the control group for 15 minutes, and then AhR mRNA was stained by fluorescence (carboxyfluorescein, FAM) in situ hybridization. Imaging was performed by confocal microscopy. (C) Huh7 cells were treated with BBP (1 μM), and then AhR levels were analyzed by immunoblotting at the indicated time.
BBP activates AhR at the cell membrane, which interacts with G proteins
To investigate whether AhR can be activated at the cell membrane by BBP, Huh7 cells were transfected with pEGFP-C1 as a plasmid control or pEGFP-C1-AhR treated with DMSO as a viechle control or BBP, and then analyzed by TIRF microscopy. AhR-GFP expression peaked at 2 minutes after BBP treatment (Figure 2A). Analysis of AhR movement in Huh7 cells showed that AhR expression near the membrane increased in a time-dependent manner. The experiment was performed by confocal microscopy and analyzed by FlowView 3.0 (Olympus, Japan) (Figure 2B). Stimulation of both Gαq/11and Gβ expression by BBP was analyzed by immunoblotting (Figure 2C). Double immunogold transmission electron microscopy and immunoprecipitation (Figure 2D, 2E) further showed an interaction between AhR and G proteins. The action of AhR was notably nongenomic. To investigate whether the G-protein signaling induced by BBP was AhR dependent, we knocked down AhR using an AhR shRNA. The results showed that BBP-induced Gαq/11 and Gβ expression was suppressed by transfection of the AhR shRNA (Figure 2F).
BBP activates AhR at the cell membrane, which interacts with G proteins. (A) Huh7 cells were transfected with pEGFP-C1-AhR or pEGFP-C1 as a plasmid control. Cells were stimulated by adding DMSO or BBP (1 μM) and then analyzed by real-time TIRF microscopy. Scale bars: 10 μm. The left panel shows Huh7 cells transfected with the pEGFP-C1 plasmid control treated with DMSO (upper) or BBP (lower). The middle panel shows Huh7 cells transfected with pEGFP-C1-AhR and then treated with DMSO (upper) or BBP (lower). The increased intensity of GFP fluorescence indicates AhR expression at the cell membrane, which was induced by BBP. The right panel shows the GFP intensity analyzed by Axio Vision Rel 4.8 software. (B) Huh7 cells were transfected with pEGFP-C1-AhR and then stimulated with BBP (1 μM) before analyzed by real-time confocal microscopy (upper panel), Scale bars: 10 μm. The GFP intensity was analyzed by FV10-ASW 2.1 software (Olympus) (lower panel). (C) Expression of Gαq/11 and Gβ proteins after BBP treatment for the indicated time was detected by immunoblotting. β-actin was used as an internal control. (D) Interaction of AhR with Gαq/11 at the cell membrane after treatment with BBP (1 μM) was imaged by double immunogold electron microscopy. Black arrows indicate Gαq/11, and the white arrows indicate AhR. The localization of G protein and AhR protein are shown. (upper left panel) shows the untreatment group and (Lower left, Right left panel) indicated BBP treatment groups. Scale bars: 500 nm. CM, cell membrane; N, nucleus. (E) Huh7 cells after 30 minutes of treatment with BBP (1 μM) or DMSO as the control. The interaction of AhR with Gαq/11 and Gβ was detected by immunoprecipitation (IP) followed by immunoblot analysis. The IgG were used cell lysates mixed with control and BBP treatment group. Normal rabbit IgG were used as negative control. (F) Huh7 cells were transfected with two different AhR shRNAs as described the Methods or a control shRNA. After treatment with or without BBP (1 μM), AhR, Gαq/11, and Gβ levels were measured by immunoblotting. β-actin was used as an internal control.
Initial activation of COX-2 by AhR/Gαq/11signaling
Downstream signaling triggered by Gαq/11 was measured by immunoblot analysis of Gαq/11, PIP2 and IP3R levels. A decrease in PIP2 and an increase in IP3R indicated cleavage of PIP2 to IP3, followed by activation of IP3R (Figure 3A) [23]. The calcium response to BBP was then analyzed with a live-cell calcium imaging system that showed a sharp signal immediately after BBP addition (Figure 3B). To determine whether the calcium was derived from external or internal stores, calcium-free medium was used in a second round of experiments. As a result, calcium release was quickly stimulated by addition of BBP, presumably from internal stores (Figure 3C). The results were then confirmed using 2-APB, an IP3R inhibitor. 2-APB inhibited the internal release of calcium in a dose-dependent manner (Figure 3D). Expression of COX-2 was activated by BBP and inhibited by 2-APB (Figure 3E, 3F). These results suggest that BBP promotes COX-2 expression via AhR/Gαq/11/calcium signaling.
AhR triggers Gα q/11 /calcium/COX-2 signaling. (A) BBP induced a reduction in the level of PIP2 and activation of IP3R via Gαq/11. After Huh7 cells treated with BBP (1 μM), protein levels were analyzed by immunoblotting at the indicated time points. (B) Real-time imaging of calcium was performed by Cell-R microscopy. The experiment was performed in BSS medium. (C) Elevated intracellular calcium was induced by BBP treatment. The experiment was performed with a calcium free medium. The arrows indicate the time points of BBP (1 μM) addition (1 minute after the experiment started). The fluorescence intensity (Y-axis) indicates the relative calcium levels. (D) Huh7 cells in calcium-free medium were pretreated with various concentrations of 2-APB for 30 minutes before stimulation with BBP (1 μM). Internal calcium release was inhibited by 2-APB in a dose-dependent manner. The relative intensity of fluo-4 indicates the calcium levels: peak/baseline ratio of fluorescence intensity. Calcium-free medium was used for each experimental interval. Each value in the graph is the mean ± SD of six replicate using at least ten cells. (E) COX-2 expression after BBP treatment was measured by immunoblotting. Huh7 cells were treated with BBP (1 μM) for the indicated times before harvesting the cells. β-actin was used as an internal control. (F) Huh7 cells were pretreated with 2-APB (20 μM) for 2 hours and followed by treated with BBP (1 μM). Control groups were treated with DMSO. COX-2 levels were suppressed by 2-APB pretreatment.
Effect of BBP on cell migration and invasion through AhR/Gβ/PI3K/Akt/NF-κB signaling
Gβ protein activates PI3K by direct binding [24]. Immunoprecipitation was performed to examine the effect of BBP treatment on interactions between Gβ protein and PI3K (Figure 4A). The binding of Gβ protein to PI3K was increased after BBP treatment. To further study the downstream pathway triggered by Gβ activation, we analyzed PI3K enhancement and Akt phosphorylation by immunoblotting. PI3K and Akt phosphorylation level were increased after BBP treatment (Figure 4B). We also found translocation of NF-κB into the nucleus (Figure 4C). Treatment with a PI3K inhibitor (wortmannin) reduced Akt phosphorylation (Figure 4D) and inhibited NF-κB translocation into the nucleus (Figure 4E). To further confirm whether PI3K/Akt/ NF-κB activation is specifically stimulated by BBP via AhR, we transfected Huh7 cells with two different AhR shRNAs. The increase of PI3K and p-Akt (Figure 4F), and translocation of NF-κB into the nucleus (Figure 4G) induced by BBP were inhibited by transfection of the shRNAs. PI3K/Akt/NF-κB enhances cell migration and invasion [25]. Thus, we further investigated the mechanisms of cell migration and invasion which are induced by BBP. Huh7 cells were transfected with two different AhR shRNAs, NF-κB shRNA, or control shRNA for 48 hours, followed by preparation of cell lysates. The protein levels of AhR and NF-κB markedly decreased after transfection with AhR and NF-κB shRNAs compared with those after transfection with control shRNA (Figure 5A). To further study the effects of BBP on Huh7 cells migration and invasion, transwell migration and invasion assays were performed. AhR and NF-κB shRNAs inhibited cell migration and invasion induced by BBP (Figure 5B). These results suggest that BBP promotes cell migration and invasion by activation of AhR/Gβ/PI3K/Akt/NF-κB signaling.
AhR triggers G β /PI3K/Akt/NF-κB signaling. (A) The PI3K and Gβ protein interaction was assessed by immunoprecipitation. Huh7 cells were immunoprecipitated with antibodies against Gβ or PI3K. Normal IgG was used as a negative control. (B) Cells were treated with BBP (1 μM) for various durations, and then PI3K and phosphorylation levels of Akt were determined by immunoblotting. (C) Nuclear and cytoplasmic fractions of NF-κB were detected by immunoblotting. Lamin A/C and α-tubulin were used as internal markers for nuclear and cytoplasmic proteins, respectively. Huh7 cells were pretreated with wortmannin (100 nM) and then treated with BBP (1 μM) for 2 hours. (D) The Akt phosphorylation levels were measured and β-actin was used as an internal control. (E) The nuclear and cytoplasmic fractions of NF-κB were analyzed by immunoblotting. Huh7 cells were transfected with two different shRNA and treated with BBP (1 μM) for 2 hours. (F) PI3K, p-Akt, Akt levels were measured by immunoblotting. β-actin was used as an internal control. (G) The nuclear and cytoplasmic fractions of NF-κB were analyzed by immunoblotting. Histone H3 and α-tubulin were used as internal markers for nuclear and cytoplasmic proteins, respectively.
Effects of BBP on cell migration and invasion in vitro and metastasis in vivo. (A) Huh7 cells were transfected with two different AhR (top) and NF-κB (bottom) shRNA as described in the Methods, and then protein levels were detected by immunoblotting. (B) Transwell migration and invasion assays. Huh7 cells transfected with control, AhR, or NF-κB shRNAs were seeded onto inserts, treated with or without BBP, and allowed to migrate for 24 (migration) or 48 (invasion) hours. The numbers of migrating and invading cells were counted under a microscope. Scale bars: 100 μm. (C) Tumor growth of intrahepatically injected Huh7-IFP cells in vehicle control and BBP treatment groups for the indicated times of was detected by non-invasive imaging. (D) Numbers of other organs with metastasis of vehicle control or BBP treatment group (n = 18 for each group) after 4 weeks. (E) Immunohistochemical staining for PI3K and NF-κB in the tumor region of the mouse liver after treatment of BBP for 4 weeks. Magnificantion, 40x. The intensity of PI3K and NF-κB staining was analyzed by Tissue Quest software. Data shown in panels B and E are representative of three independent experiments. Each value is the mean ± SD of three independent experiments. The asterisks indicate a significant difference between vehicle treated group and BBP treated groups, as analyzed by Student’s t-test (*p < 0.05; **p < 0.01).
BBP promotes in vivometastasis
The mouse intrahepatic injection model was established to examine tumor metastasis [26]. Huh7-IFP cells (1 × 106) were suspended in 30 μL PBS and injected into the mouse liver (At 3 days after of implantation, 500 mg/kg BBP was administered by i.p. injection every 2 days. Weekly imaging of the mice confirmed that the tumor sizes increased over time (Figure 5C). The mice were sacrificed after 1 month to compare tumor metastasis between control and BBP treatment groups. The metastasis rates in the lungs, kidneys, and spleen were higher in the BBP treatment group than those in the control group (Figure 5D), suggesting that BBP promotes metastasis. Immunohistochemistry showed that liver PI3K and NF-κB levels were significantly higher in the treatment group than those in the control group (Figure 5E).
BBP promotes angiogenesis in vitro and in vivo
To examine the effect of BBP on angiogenesis, the conditioned medium of Huh7 cells that had been treated with BBP was examined for its ability to induce the formation of capillary-like structures by HUVEC (Figure 6A). The levels of VEGF, which promotes angiogenesis [27], were also measured in the conditioned medium (Figure 6A). To explore the associated mechanisms, Huh7 cells were treated with the ERK inhibitor Pd98059 (Figure 6A) or transfected with AhR siRNA (Figure 6B) before collection of the medium. Analyses of the media showed that both Pd98059 and AhR siRNA inhibited tube formation of HUVEC and reduced VEGF induction after BBP treatment. Moreover, we evaluated the phosphorylation levels of ERK (Figure 6C). To further confirm whether activation of ERK was AhR dependent, we trasfected Huh7 cells with two different AhR shRNAs and the results showed inhibition of the phosphorylation levels of ERK induced by BBP (Figure 6D). The angiogenic effects of BBP were then assessed in an in vivo Matrigel plug angiogenesis assay model. Briefly, Huh7 cells were mixed with Matrigel and injected into the flanks of nude mice. After 3 weeks, the mice were sacrificed, and hemoglobin levels in the plug were measured. Hemoglobin levels in the Matrigel plug treated with BBP were significantly higher than those in the control group (Figure 6E).
Effects of BBP on angiogenesis in vitro and in vivo. Effect of Huh7 cell-conditioned medium on HUVEC tube formation. Huh7 cells were pretreated with or without Pd98059 (A) or transfected with scrambled siRNA or AhR siRNA (B) followed by BBP treatment, and the conditioned medium was then collected from each culture dish. HUVEC were seeded in BioCoated angiogenesis plates, treated with 20% Huh7-conditioned medium, and incubated for 16 hours. HUVEC were imaged after staining with Calcein-AM. Total tube lengths were analyzed by MetaMorph software. Scale bars: 200 μm. VEGF levels in the conditioned media were measured by ELISA. (C) Huh7 cells were treated with BBP for the indicated times. p-ERK and ERK levels were measured by immunoblotting. β-actin used as an internal control. (D) Huh7 cells were transfected with two different Huh7 shRNAs and then treated with BBP (1 μM) for 15 minutes. p-ERK, ERK levels were evaluated by immunoblotting. (E) Huh7 cells were mixed with Matrigel with or without BBP (1 μM), injected into the flanks of nude mice, and allowed to grow for 21 days. Hemoglobin levels in Matrigel plugs, which indicated blood vessel formation, were measured by Drabkin reagent kit 525. Each value is the mean ± SD of three independent experiments. The asterisks indicate a significant difference between control and test groups, as analyzed by Student’s t-test (*p < 0.05; **p < 0.01).
Discussion
In this study, we provide evidence that phthalate promotes hepatocellular carcinoma progression through a nongenomic AhR pathway. Rodent studies of the carcinogenesis of phthalate have yielded substantial data [5]. Some human epidemiological studies have also shown a cancer risk associated with phthalate exposure, including respiratory cancer [28], pancreatic cancer [29], and breast cancer [30]. However, the mechanisms of carcinogenesis have been rarely explored for phthalate. To further investigate such mechanisms, we treated hepatocellular carcinoma cell lines, Huh7, HepG2 and PLC cells with BBP (1 μM). All cell lines showed the activation of AhR after treatment (Additional file 1: Figure S1). We then tested the functions of cells treated with BBP including migration, invasion and angiogenesis. We found that BBP induced migration of Huh7 and PLC cell lines. In addition, BBP induced invasion and angiogenesis of Huh7 cells (Additional file 2: Figure S2). These results may be due to the higher constitutional level of AhR in Huh7 than in that in PLC cells (Additional file 2: Figure S2D). HepG2 cells are not appropriate for further animal studies for non-tumorigenic properties in immunosuppressed mice [31]. Therefore, we further investigated the mechanism induced by BBP in Huh7 cells. We used Huh7 cells in the in vivo study, and clarified the effect of BBP induced metastasis and angiogenesis in vivo.
Our current study showed that BBP treatment induces a nongenomic function through AhR and further clarified that AhR translocates to the membrane and subsequently activates G-protein signaling. Our investigation is consistent with a previous report in the nongenomic AhR mechanism induced by TCDD, showing that TCDD rapidly increases calcium concentrations and induces COX-2 expression in U937 macrophages, mouse MMDD1 macula densa cells, and MCF10 cells [14, 17]. We believe that the nongenomic action explains why cells elict fast inflammatory responses to the environmental pollutant TCDD and the endocrine disrupting agent, phthalate. After BBP treatment, the AhR mRNA levels were unregulated. Certain molecular such as NF-κB, interleukin (IL)-27 and IL-6 were investigated that can regulate AhR expression [32–34]. However, the mechanism of BBP induced AhR mRNA upregulation has remained unclear. Subsequently the nongenomic action triggers the cell signal pathways and affects the cell function. A previous study showed that by interacting with AhR-interacting protein, Gα13 destabilized AhR via the ubiquitin-proteasome pathway [18]. Polycyclic aromatic hydrocarbons (PAH), which is the AhR ligand, induceds expression of AhR target genes through directed bind to G protein coupled receptor and activates G protein/ cAMP/ 1,4,5-trisphosphate (IP3) pathway, which results in calcium induction [35]. The current study further confirmed that AhR positively regulates G proteins, including Gαq/11 and Gβ through nongenomic mechnissams. To our knowledge, the present study is the first investigation to elicit that phthalate activates AhR through a non-genomic mechanism that involving G protein signaling. Importantly, we provide evidence that the tumor progression, including migration, invasion and angiogenesis were regulated by this phthalate-induced signaling pathways.
Immortalized fibroblasts from Ahr −/− mice demonstrate a lower tumorigenic potential because of diminished cell motility and responses to angiogenic factors [36]. Overexpression of AhR promotes the development of malignant cellular phenotypes and also increases their cell proliferation rate, motility, migration, and confers the ability to invade Matrigel in immortalized normal human mammary epithelial cells [37]. Activation of AhR is functionally connected to a signaling cascade that dramatically alters plasticity and increases motility in MCF-7 cells [38].
Angiogenesis contributes to tumor metastasis [39]. It has been reported AhR regulates both in vitro and in vivo angiogenesis. AhR−/− endothelial cells fail to form tube-like structures, and impaired angiogenesis limits tumor xenograft growth in AhR null mice [40]. The current study showed that BBP promoted angiogenesis through nongenomic AhR mechanisms, and VEGF expression was increased by ERK1/2 phosphorylation. This finding is consistent with an earlier study showing that an ER-dependent mechanism involving activation of MEK, p38 kinase, and PI3K pathways by xenoestrogens up-regulates VEGF expression in breast cancer cells [41]. However, our current study is the first to show that BBP increases both in vitro and in vivo angiogenesis of liver cancer.
Previous reports have revealed that phthalates promote cancer cell migration, invasion and epithelial-mesenchymal transition, which may explain the cancer progression observed in both ER-dependent and AhR-dependent pathways [10, 42–44]. Several reports have shown that AhR regulates cell migration, invasion and plasticity, all of which contribute to tumor progression [10, 36, 45–47]. Our study showed that BBP promoted cell migration and invasion through the AhR/Gβ/PI3K/Akt/NF-κB pathway in hepatocellular carcinoma cells. These indicate phthalate stimulates various cell signaling pathways of cancer cell migration and invasion. Our data showed metastasis can be promoted by phthatlate in vitro and in an animal model in vivo. This is a phenomenon need to be addressed as metastasis is the key for the prognosis of cancer treatment.
Overexpression of COX-2 occurs in many pre-malignant, malignant, and metastatic cancers, including hepatocellular carcinoma. COX-2 regulates carcinogenesis by regulating angiogenesis [48], suppressing the immune response, and by inhibiting apoptosis, and tumor cell invasion, and metastasis [49]. Our data suggest that BBP may contribute to angiogenesis, tumor cell invasion and metastasis by increasing COX-2 expression.
Conclusions
Taking these results together, we propose a signaling pathway for BBP stimulated hepatocellular carcinoma progression (Figure 7). BBP induces membrane translocation of AhR and the initial activation of COX-2 by AhR/Gαq/11 signaling. In addition, BBP promotes angiogenesis via the AhR/ERK/VEGF pathway and cell migration and invasion through AhR/Gβ/PI3K/Akt/NF-κB signaling.
In conclusion, we revealed a nongenomic AhR mechanism that may account for the modulated progression of liver cancer after phthalate exposure. Our results imply that BBP has a promoting impact on hepatocellular carcinoma, and that phthalate should be avoided in theses patients.
Abbreviations
- (BBP):
-
Benzyl butyl phthalaye
- (AhR):
-
Aryl hydrocarbon receptor
- (DEHP):
-
Bis(2-ethylhexyl) phthalate
- (TCDD):
-
2,3,7,8-tetrachlorodibenzo-p-dioxin
- (2-APB):
-
2-aminoethoxydiphenyl borate
- (DAPI):
-
6-diamidino-2-phenylindole
- (PBS):
-
Phosphate-buffered saline
- (SSC):
-
Saline-sodium citrate
- (i.p):
-
Intraperitoneal
- (DRE):
-
Dioxin response element.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM: Estimates of worldwide burden of cancer in 2008: GLOBOCAN. Int J Cancer. 2010, 127 (12): 2893-2917.
Chuang SC, La Vecchia C, Boffetta P: Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009, 286 (1): 9-14.
Seo KW, Kim KB, Kim YJ, Choi JY, Lee KT, Choi KS: Comparison of oxidative stress and changes of xenobiotic metabolizing enzymes induced by phthalates in rats. Food Chem Toxicol. 2004, 42 (1): 107-114.
Ward JM, Diwan BA, Ohshima M, Hu H, Schuller HM, Rice JM: Tumor-initiating and promoting activities of di(2-ethylhexyl) phthalate in vivo and in vitro. Environ Health Perspect. 1986, 65: 279-291.
Voss C, Zerban H, Bannasch P, Berger MR: Lifelong exposure to di-(2-ethylhexyl)-phthalate induces tumors in liver and testes of Sprague–Dawley rats. Toxicology. 2005, 206 (3): 359-371.
Choi S, Park SY, Kwak D, Phark S, Lee M, Lim JY, Jung WW, Sul D: Proteomic analysis of proteins secreted by HepG2 cells treated with butyl benzyl phthalate. J Toxicol Environ Health A. 2010, 73 (21–22): 1570-1585.
Krüger T, Long M, Bonefeld-Jørgensen EC: Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology. 2008, 246 (2–3): 112-123.
Mankidy R, Wiseman S, Ma H, Giesy JP: Biological impact of phthalates. Toxicol Lett. 2013, 217 (1): 50-58.
Kuo CH, Hsieh CC, Kuo HF, Huang MY, Yang SN, Chen LC, Huang SK, Hung CH: Phthalates suppress type I interferon in human plasmacytoid dendritic cells via epigenetic regulation. Allergy. 2013, 68: 870-879.
Chen HS, Chiang PH, Wang YC, Kao MC, Shieh TH, Tsai CF, Tsai EM: Benzyl butyl phthalate induces necrosis by AhR mediation of CYP1B1 expression in human granulosa cells. Reprod Toxicol. 2011, 33 (1): 67-75.
Hsieh TH, Tsai CF, Hsu CY, Kuo PL, Lee JN, Chai CY, Wang SC, Tsai EM: Phthalates induce proliferation and invasiveness of estrogen receptor-negative breast cancer through the AhR/HDAC6/c-Myc signaling pathway. FASEB J. 2012, 26 (2): 778-787.
Guyot E, Coumoul X, Chassé JF, Khallouki F, Savouret JF, Poirot M, Barouki R: Identification of a new stilbene-derived inducer of paraoxonase 1 and ligand of the Aryl hydrocarbon Receptor. Biochem Pharmacol. 2012, 83 (5): 627-632.
Matsumura F: The significance of the nongenomic pathway in mediating inflammatory signaling of the dioxin-activated Ah receptor to cause toxic effects. Biochem Pharmacol. 2009, 77 (4): 608-626.
Sciullo EM, Dong B, Vogel CF, Matsumura F: Characterization of the pattern of the nongenomic signaling pathway through which TCDD-induces early inflammatory responses in U937 human macrophages. Chemosphere. 2009, 74 (11): 1531-1537.
Dong B, Matsumura F: Roles of cytosolic phospholipase A2 and Src kinase in the early action of 2,3,7,8-tetrachlorodibenzo-p-dioxin through a nongenomic pathway in MCF10A cells. Mol Pharmacol. 2008, 74 (1): 255-263.
Li W, Matsumura F: Significance of the nongenomic, inflammatory pathway in mediating the toxic action of TCDD to induce rapid and long-term cellular responses in 3 T3-L1 adipocytes. Biochemistry. 2008, 47 (52): 13997-14008.
Dong B, Nishimura N, Vogel CF, Tohyama C, Matsumura F: TCDD-induced cyclooxygenase-2 expression is mediated by the nongenomic pathway in mouse MMDD1 macula densa cells and kidneys. Biochem Pharmacol. 2010, 79 (3): 487-497.
Nakata A, Urano D, Fujii-Kuriyama Y, Mizuno N, Tago K, Itoh H: G-protein signalling negatively regulates the stability of aryl hydrocarbon receptor. EMBO Rep. 2009, 10 (6): 622-628.
Hsu WL1, Tsai MH, Lin MW, Chiu YC, Lu JH, Chang CH, Yu HS, Yoshioka T: Differential effects of arsenic on calcium signaling in primary keratinocytes and malignant (HSC-1) cells. Cell Calcium. 2012, 52 (2): 161-169.
Shu X, Royant A, Lin MZ, Aguilera TA, Lev-Ram V, Steinbach PA, Tsien RY: Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science. 2009, 324 (5928): 804-807.
Yao X1, Hu JF, Daniels M, Yien H, Lu H, Sharan H, Zhou X, Zeng Z, Li T, Yang Y, Hoffman AR: A novel orthotopic tumor model to study growth factors and oncogenesis in hepatocarcinogenesis. Clin Cancer Res. 2003, 9 (7): 2719-2726.
Singletary K, MacDonald C, Wallig M: The plasticizer benzyl butyl phthalate (BBP) inhibits 7, 12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary DNA adduct formation and tumorigenesis. Carcinogenesis. 1997, 18 (8): 1669-1673.
Liu D, Liman ER: Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003, 100 (25): 151601-155165.
Rommel C, Camps M, Ji H: PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond?. Nat Rev Immunol. 2007, 7 (3): 1912-01.
Yan X, Shen H, Jiang H, Hu D, Zhang C, Wang J, Wu X: External Qi of Yan Xin Q gong induces apoptosis and inhibits migration and invasion of estrogen-independent breast cancer cells through suppression of Akt/NF-kB signaling. Cell Physiol Biochem. 2010, 25 (2–3): 263-270.
Ge NL, Elferink CJ: A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. J Biol Chem. 1998, 273 (35): 22708-22713.
Zhang L, Wang JN, Tang JM, Kong X, Yang JY, Zheng F, Guo LY, Huang YZ, Zhang L, Tian L, Cao SF, Tuo CH, Guo HL, Chen SY: VEGF is essential for the growth and migration of human hepatocellular carcinoma cells. Mol Biol Rep. 2012, 39 (5): 5085-5093.
Hagmar L, Akesson B, Nielsen J, Andersson C, Lindén K, Attewell R, Möller T: Mortality and cancer morbidity in workers exposed to low levels of vinyl chloride monomer at a polyvinyl chloride processing plant. Am J Ind Med. 1990, 17 (5): 553-565.
Selenskas S, Teta MJ, Vitale JN: Pancreatic cancer among workers processing synthetic resins. Am J Ind Med. 1995, 28 (3): 385-398.
López-Carrillo L, Hernández-Ramírez RU, Calafat AM, Torres-Sánchez L, Galván-Portillo M, Needham LL, Ruiz-Ramos R, Cebrián ME: Exposure to phthalates and breast cancer risk in northern Mexico. Environ Health Perspect. 2010, 118 (4): 5394-44.
Wang Z, Bai Z, Zhang H, Huan T, Li J, Xiumin D, Zhang J: Growth potential of human hepatocarcinoma cells in the liver of neonatal immunocompetent mice and its relation to immunological tolerance. Prog Nat Sci. 2009, 19: 705-712.
Apetoh L1, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK: The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010, 11 (9): 854-861.
Monticelli S, Sallusto F: Negative regulators take center stage. Nat Immunol. 2012, 13 (8): 7197-20.
Champion S, Sauzet C, Bremond P, Benbrahim K, Abraldes J, Seree E, Barra Y, Villard PH: Activation of the NF κ B Pathway Enhances AhR Expression in Intestinal Caco-2 Cells. ISRN Toxicol. 2013, doi:10.1155/2013/792452
Mayati A, Levoin N, Paris H, N'diaye M, Courtois A, Uriac P, Lagadic-Gossmann D, Fardel O, Le Ferrec E: Induction of intracellular calcium concentration by environmental benzo(a)pyrene involves a β2-adrenergic receptor/adenylyl cyclase/epac-1/inositol 1,4,5-trisphosphate pathway in endothelial cells. J Biol Chem. 2012, 287 (6): 4041-4052.
Mulero-Navarro S, Pozo-Guisado E, Pérez-Mancera PA, Alvarez-Barrientos A, Catalina-Fernández I, Hernández-Nieto E, Sáenz-Santamaria J, Martínez N, Rojas JM, Sánchez-García I, Fernández-Salguero PM: Immortalized mouse mammary fibroblasts lacking dioxin receptor have impaired tumorigenicity in a subcutaneous mouse xenograft model. J Biol Chem. 2005, 280 (31): 28731-28741.
Brooks J, Eltom SE: Malignant transformation of mammary epithelial cells by ectopic overexpression of the aryl hydrocarbon receptor. Curr Cancer Drug Targets. 2011, 11 (5): 654-669.
Diry M, Tomkiewicz C, Koehle C, Coumoul X, Bock KW, Barouki R, Transy C: Activation of the dioxin/aryl hydrocarbon receptor (AhR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene. 2006, 25 (40): 5570-5574.
Kirsch M, Schackert G, Black PM: Metastasis and angiogenesis. Cancer Treat Res. 2004, 117: 285-304.
Roman AC, Carvajal-Gonzalez JM, Rico-Leo EM, Fernandez-Salguero PM: Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J Biol Chem. 2009, 284 (37): 251352-255148.
Buteau-Lozano H, Velasco G, Cristofari M, Balaguer P, Perrot-Applanat M: Xenoestrogens modulate vascular endothelial growth factor secretion in breast cancer cells through an estrogen receptor-dependent mechanism. J Endocrinol. 2008, 196 (2): 399-412.
Hsieh TH, Tsai CF, Hsu CY, Kuo PL, Lee JN, Chai CY, Hou MF, Chang CC, Long CY, Ko YC, Tsai EM: Phthalates stimulate the epithelial to mesenchymal transition through an HDAC6-dependent mechanism in human breast epithelial stem cells. Toxicol Sci. 2012, 128 (2): 3653-3676.
Zhu H, Zheng J, Xiao X, Zheng S, Dong K, Liu J, Wang Y: Environmental endocrine disruptors promote invasion and metastasis of SK-N-SH human neuroblastoma cells. Oncol Rep. 2010, 23 (1): 129-139.
Yao PL, Lin Y, Richburg JH: Mono-(2-ethylhexyl) phthalate (MEHP) promotes invasion and migration of human testicular embryonal carcinoma cells. Biol Reprod. 2012, 86 (5): 1601-1670.
Peng TL, Chen J, Mao W, Song X, Chen MH: Aryl hydrocarbon receptor pathway activation enhances gastric cancer cell invasiveness likely through a c-Jun-dependent induction of matrix metalloproteinase-9. BMC Cell Biol. 2009, 16: 10-27.
Bui LC, Tomkiewicz C, Chevallier A, Pierre S, Bats AS, Mota S, Raingeaud J, Pierre J, Diry M, Transy C, Garlatti M, Barouki R, Coumoul X: Nedd9/Hef1/Cas-L mediates the effects of environmental pollutants on cell migration and plasticity. Oncogene. 2009, 28 (41): 3642-3651.
Ambolet-Camoit A, Bui LC, Pierre S, Chevallier A, Marchand A, Coumoul X, Garlatti M, Andreau K, Barouki R, Aggerbeck M: 2,3,7,8-tetrachlorodibenzo-p-dioxin counteracts the p53 response to a genotoxicant by upregulating expression of the metastasis marker agr2 in the hepatocarcinoma cell line HepG2. Toxicol Sci. 2010, 115 (2): 501-512.
Zhao QT, Yue SQ, Cui Z, Wang Q, Cui X, Zhai HH, Zhang LH, Dou KF: Potential involvement of the cyclooxygenase-2 pathway in hepatocellular carcinoma-associated angiogenesis. Life Sci. 2007, 80 (5): 484-492.
Akkız H, Bayram S, Bekar A, Akgöllü E, Ülger Y: Functional polymorphisms of cyclooxygenase-2 gene and risk for hepatocellular carcinoma. Mol Cell Biochem. 2011, 347 (1–2): 201-208.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/556/prepub
Acknowledgements
We thank the Center for Resources, Research, and Development of Kaohsiung Medical University for support with instrumentation and the Laboratory Animal Center of Kaohsiung Medical University for assistance with animal experiments.
Funding
This work was supported by the National Science Council, Taiwan [grant number 102-2628-B-037-011-MY3 and 102-2632-B-037-001-MY3]. Kaohsiung Medical University (Hospital) Research Fund [grant numbers KMUH102-2R22, KMUH101-1R27, KMUH100-0R24]. National Sun Yat-Sen University-KMU Joint Research Project (#NSYSUKMU 102-P029).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CFT conducted the experiments and wrote the manuscript. THH and CYH analyzed the data. JNL, EMT and PLK participated in the study design. YCW conducted the animal experiments. FJL consulted on the double immunogold transmission electron microscopy experiment. KKK and HLW helped to draft the manuscript. All authors approved the final version of the manuscript.
Eing-Mei Tsai and Po-Lin Kuo contributed equally to this work.
Electronic supplementary material
12885_2013_4746_MOESM1_ESM.tiff
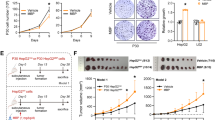
Additional file 1: Figure S1: Effects of BBP on AhR expression of hepatocellular carcinoma cell lines. Huh7, HepG2, and PLC cells were treated with BBP (1 μM) for 24 hours and AhR protein levels were then analyzed by immunoblotting. β-actin was used as an internal control. (TIFF 512 KB)
12885_2013_4746_MOESM2_ESM.tiff
Additional file 2: Figure S2: Effects of BBP on angiogenesis, migration and invasion. (A) Effect of conditioned media from Huh7 and PLC cells on HUVEC tube formation. After Huh7 and PLC cells were treated with BBP (1 μM) for 1 day, the culture medium was changed to the fresh medium and the cells were cultured for 1 day. Conditioned medium was collected from each culture dish. HUVEC were treated with 20% conditioned medium and incubated for 16 hours. HUVEC were imaged after staining with Calcein-AM (top). Total tube lengths were determined by MetaMorph software (bottom). Scale bar: 200 μm. The asterisks indicates a significant difference between control and test groups, as analyzed by Student’s t-test (*p < 0.05). (B) Transwell migration assay. (C) Transwell invasion assay. Huh7 and PLC cells were seeded on inserts and treated with BBP (1 μM). Scale bars: 100 μm. (D) Huh7 and PLC cells were harvested to prepare whole cell lysates for AhR protein levels were measured by immunoblotting, β-actin was used as an internal control. (TIFF 5 MB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tsai, CF., Hsieh, TH., Lee, JN. et al. Benzyl butyl phthalate induces migration, invasion, and angiogenesis of Huh7 hepatocellular carcinoma cells through nongenomic AhR/G-protein signaling. BMC Cancer 14, 556 (2014). https://doi.org/10.1186/1471-2407-14-556
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2407-14-556