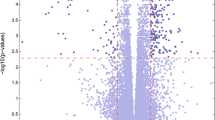
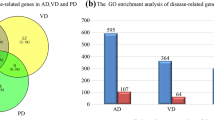
Abstract
Two of the most prevalent central neuron system disorders are Alzheimer (AD) and Parkinson’s disease (PD). Interestingly, despite their differences in both pathological and molecular basis of the diseases, they exhibit some degrees of similarities. Here, we have conducted a comparative systems-level analysis study for these diseases. Cohort cortex samples from healthy control cases and AD/PD patients were obtained, then we have applied weighted gene co-expression network analysis (WGCNA). Network analysis identified key modules of genes related to each of these diseases. Gene ontology enrichment of the modules showed the involvement of both disease-specific and shared biological processes, including chemical synaptic transmission, nervous system development, and immune responses that are involved in both AD and PD. Surprisingly, the expression patterns for the gene members of the shared modules were strikingly identical. Additionally, we have identified a set of novel genes, including INPP4A, CREG2, ABI3, MYO1F, NAPB, NXN, DOCK6, CPSF6, and IKZF1. While these genes are implicated in either AD or PD modules as shown in Table 2, their potential functional relevance to both diseases warrants further investigation. In conclusion, besides unveiling the presence of high molecular level similarities between AD and PD, for the first time, several novel genes have been proposed that can open a new opportunity for diagnostic or treatment applications.






Similar content being viewed by others
Data availability
All data used in this study is freely available and can be obtained from NCBI using above-mentioned GSE codes “GSE36980” and “GSE68719”.
Abbreviations
- AD:
-
Alzheimer's disease
- PD:
-
Parkinson’s disease
- WGCNA:
-
Weighted gene co-expression network analysis
References
Lang AE, Lozano AM. Parkinson's disease. N Engl J Med 1998; 339: 1130-43. doi: https://doi.org/10.1056/NEJM199810153391607
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease Report of the NINCDS‐ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34: 939-. https://doi.org/10.1212/wnl.34.7.939
Clark CM, Ewbank D, Lee VM-Y, Trojanowski JQ. Molecular pathology of Alzheimer's disease: neuronal cytoskeletal abnormalities. Blue Books of Practical Neurology 1998; 19: 285–304.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology 1998; 50: 318-. doi: https://doi.org/10.1212/wnl.50.2.318
Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet 2006; 7: 306. doi: https://doi.org/10.1038/nrg1831
Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol 2011; 70: 532-40. doi: https://doi.org/10.1002/ana.22615.
Lodato S, Arlotta P. Generating neuronal diversity in the mammalian cerebral cortex. Annu Rev Cell Dev Biol 2015; 31: 699-720. doi: https://doi.org/10.1146/annurev-cellbio-100814-125353
TAYLOR AE, Saint-Cyr J, Lang A. Frontal lobe dysfunction in Parkinson's disease: The cortical focus of neostriatal outflow. Brain 1986; 109: 845–83.
DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol 1990; 27: 457-64. doi: https://doi.org/10.1002/ana.410270502
Geroldi C, Akkawi NM, Galluzzi S, Ubezio M, Binetti G, Zanetti O, et al. Temporal lobe asymmetry in patients with Alzheimer's disease with delusions. J Neurol Neurosurg Psychiatry 2000; 69: 187-91. doi: https://doi.org/10.1136/jnnp.69.2.187
Liang WS, Reiman EM, Valla J, Dunckley T, Beach TG, Grover A, et al. Alzheimer's disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci 2008; 105: 4441-6. doi: https://doi.org/10.1073/pnas.070925910
Wen Z, Liu Z-P, Liu Z, Zhang Y, Chen L. An integrated approach to identify causal network modules of complex diseases with application to colorectal cancer. J Am Med Inform Assoc 2013; 20: 659-67. doi: https://doi.org/10.1136/amiajnl-2012-001168
Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol 2005; 4. https://doi.org/10.2202/1544-6115.1128
Hokama M, Oka S, Leon J, Ninomiya T, Honda H, Sasaki K, et al. Altered expression of diabetes-related genes in Alzheimer's disease brains: the Hisayama study. Cereb Cortex 2013; 24: 2476-88. doi: https://doi.org/10.1093/cercor/bht101
Dumitriu A, Golji J, Labadorf AT, Gao B, Beach TG, Myers RH, et al. Integrative analyses of proteomics and RNA transcriptomics implicate mitochondrial processes, protein folding pathways and GWAS loci in Parkinson disease. BMC Med Genomics 2015; 9: 5. doi: https://doi.org/10.1186/s12920-016-0164-y
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008; 9: 559. doi: https://doi.org/10.1186/1471-2105-9-559
Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2008; 4: 44. doi: https://doi.org/10.1038/nprot.2008.211
Johnson SC. Hierarchical clustering schemes. Psychometrika 1967; 32: 241-54. doi: https://doi.org/10.1007/BF02289588
Bastian M, Heymann S, Jacomy M. Gephi: an open source software for exploring and manipulating networks. Icwsm 2009; 8: 361-2.
Yu H, Kim PM, Sprecher E, Trifonov V, Gerstein M. The importance of bottlenecks in protein networks: correlation with gene essentiality and expression dynamics. PLoS Comput Biol 2007; 3: e59. https://doi.org/10.1371/journal.pcbi.0030059
Calderone A, Formenti M, Aprea F, Papa M, Alberghina L, Colangelo AM, et al. Comparing Alzheimer’s and Parkinson’s diseases networks using graph communities structure. BMC Syst Biol 2016; 10: 25. doi: https://doi.org/10.1186/s12918-016-0270-7
Ping L, Duong DM, Yin L, Gearing M, Lah JJ, Levey AI, et al. Global quantitative analysis of the human brain proteome in Alzheimer’s and Parkinson’s Disease. Scientific data 2018; 5: 180036. https://doi.org/10.1038/sdata.2018.36
Stern Y, Richards M, Sano M, Mayeux R. Comparison of cognitive changes in patients with Alzheimer's and Parkinson's disease. Arch Neurol 1993; 50: 1040-5. doi: https://doi.org/10.1001/archneur.1993.00540100035011
Chatterjee P, Roy D, Bhattacharyya M, Bandyopadhyay S. Biological networks in Parkinson’s disease: an insight into the epigenetic mechanisms associated with this disease. BMC Genomics 2017; 18: 721. doi: https://doi.org/10.1186/s12864-017-4098-3
Miller JA, Oldham MC, Geschwind DH. A systems level analysis of transcriptional changes in Alzheimer's disease and normal aging. J Neurosci 2008; 28: 1410-20. doi: https://doi.org/10.1523/JNEUROSCI.4098-07.2008
Braak H, Rüb U, Schultz C, Tredici KD. Vulnerability of cortical neurons to Alzheimer's and Parkinson's diseases. J Alzheimers Dis 2006; 9: 35-44. doi: https://doi.org/10.3233/JAD-2006-9S305
Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE. Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb Perspect Med 2012; 2:9381. doi: https://doi.org/10.1101/cshperspect.a009381
Marttinen M, Kurkinen KM, Soininen H, Haapasalo A, Hiltunen M. Synaptic dysfunction and septin protein family members in neurodegenerative diseases. Mol Neurodegener 2015; 10: 16. doi: https://doi.org/10.1186/s13024-015-0013-z
Palubinsky AM, Martin JA, McLaughlin B. The role of central nervous system development in late-onset neurodegenerative disorders. Dev Neurosci 2012; 34: 129-39. doi: https://doi.org/10.1159/000336828
Doty KR, Guillot-Sestier M-V, Town T. The role of the immune system in neurodegenerative disorders: adaptive or maladaptive? Brain Res 2015; 1617: 155-73. doi: https://doi.org/10.1016/j.brainres.2014.09.008
Lin J-X, Li P, Liu D, Jin HT, He J, Rasheed MAU, et al. Critical Role of STAT5 transcription factor tetramerization for cytokine responses and normal immune function. Immunity 2012; 36: 586-99. doi: https://doi.org/10.1016/j.immuni.2012.02.017
Tiwari PC, Pal R. The potential role of neuroinflammation and transcription factors in Parkinson disease. Dialogues Clin Neurosci 2017; 19: 71. doi: https://doi.org/10.31887/DCNS.2017.19.1/rpal
Theuns J, Van Broeckhoven C. Transcriptional regulation of Alzheimer’s disease genes: implications for susceptibility. Hum Mol Genet 2000; 9: 2383-94. doi: https://doi.org/10.1093/hmg/9.16.2383
Ma Q-L, Yang F, Calon F, Ubeda OJ, Hansen JE, Weisbart RH, et al. p21-activated kinase-aberrant activation and translocation in Alzheimer disease pathogenesis. J Biol Chem 2008; 283: 14132-43. doi: https://doi.org/10.1074/jbc.M708034200
Chandrasekaran S, Bonchev D. A network view on Parkinson's disease. Comput Struct Biotechnol J 2013; 7: e201304004. https://doi.org/10.5936/csbj.201304004
Antonell A, Lladó A, Altirriba J, Botta-Orfila T, Balasa M, Fernández M, et al. A preliminary study of the whole-genome expression profile of sporadic and monogenic early-onset Alzheimer's disease. Neurobiol Aging 2013; 34: 1772-8. doi: https://doi.org/10.1016/j.neurobiolaging.2012.12.026
Parker JG, Wanat MJ, Soden ME, Ahmad K, Zweifel LS, Bamford NS, et al. Attenuating GABAA receptor signaling in dopamine neurons selectively enhances reward learning and alters risk preference in mice. J Neurosci 2011; 31: 17103-12. doi: https://doi.org/10.1523/JNEUROSCI.1715-11.2011
Nikolić M. The Pak1 kinase: an important regulator of neuronal morphology and function in the developing forebrain. Mol Neurobiol 2008; 37: 187. doi: https://doi.org/10.1007/s12035-008-8032-1
Jeanneau C, Mathieu S, Sigaud R, PROROK-HAMON M, Ouafik L. Elucidating the roles of Alzheimer disease-associated proteases and the signal-peptide peptidase-like 3 (SPPL3) in the shedding of glycosyltransferases. bioRxiv 2018; 317214. https://doi.org/10.1101/317214
Fedele CG, Ooms LM, Ho M, Vieusseux J, O'Toole SA, Millar EK, et al. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc Natl Acad Sci 2010; 107: 22231-6. doi: https://doi.org/10.1073/pnas.1015245107
Sasaki J, Kofuji S, Itoh R, Momiyama T, Takayama K, Murakami H, et al. The PtdIns (3, 4) P 2 phosphatase INPP4A is a suppressor of excitotoxic neuronal death. Nature 2010; 465: 497. doi: https://doi.org/10.1038/nature09023
Willemen HL, Kavelaars A, Prado J, Maas M, Versteeg S, Nellissen LJ, et al. Identification of FAM173B as a protein methyltransferase promoting chronic pain. PLoS Biol 2018; 16: e2003452. https://doi.org/10.1371/journal.pbio.2003452
Hroudová J, Singh N, Fišar Z. Mitochondrial dysfunctions in neurodegenerative diseases: relevance to Alzheimer’s disease. Biomed Res Int 2014; 2014. https://doi.org/10.1155/2014/175062
Ma SL, Tang NLS, Tam CWC, Lui VWC, Suen EWC, Chiu HFK, et al. Association between HLA-A alleles and Alzheimer’s disease in a southern Chinese community. Dement Geriatr Cogn Disord 2008; 26: 391-7. doi: https://doi.org/10.1159/000164275
Obulesu M, Lakshmi MJ. Apoptosis in Alzheimer’s disease: an understanding of the physiology, pathology and therapeutic avenues. Neurochem Res 2014; 39: 2301-12. doi: https://doi.org/10.1007/s11064-014-1454-4
Kunita R, Otomo A, Ikeda J-E. Identification and characterization of novel members of the CREG family, putative secreted glycoproteins expressed specifically in brain. Genomics 2002; 80: 456-60. doi: https://doi.org/10.1006/geno.2002.6857
Büttner N, Johnsen SA, Kügler S, Vogel T. Af9/Mllt3 interferes with Tbr1 expression through epigenetic modification of histone H3K79 during development of the cerebral cortex. Proc Natl Acad Sci 2010; 200912041. https://doi.org/10.1073/pnas.0912041107
Rendón WO, Martínez-Alonso E, Tomás M, Martínez-Martínez N, Martínez-Menárguez JA. Golgi fragmentation is Rab and SNARE dependent in cellular models of Parkinson’s disease. Histochem Cell Biol 2013; 139: 671-84. doi: https://doi.org/10.1007/s00418-012-1059-4
Mosley RL, Hutter-Saunders JA, Stone DK, Gendelman HE. Inflammation and adaptive immunity in Parkinson’s disease. Cold Spring Harb Perspect Med 2012; 2: a009381. https://doi.org/10.1101/cshperspect.a009381
Pellegrini L, Wetzel A, Grannó S, Heaton G, Harvey K. Back to the tubule: microtubule dynamics in Parkinson’s disease. Cell Mol Life Sci 2017; 74: 409-34. doi: https://doi.org/10.1007/s00018-016-2351-6
Sims R, Van Der Lee SJ, Naj AC, Bellenguez C, Badarinarayan N, Jakobsdottir J, et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer's disease. Nat Genet 2017; 49: 1373. doi: https://doi.org/10.1038/ng.3916
Kim SV, Mehal WZ, Dong X, Heinrich V, Pypaert M, Mellman I, et al. Modulation of cell adhesion and motility in the immune system by Myo1f. Science 2006; 314: 136-9. doi: https://doi.org/10.1126/science.1131920
Yoo BC, Cairns N, Fountoulakis M, Lubec G. Synaptosomal proteins, beta-soluble N-ethylmaleimide-sensitive factor attachment protein (beta-SNAP), gamma-SNAP and synaptotagmin I in brain of patients with Down syndrome and Alzheimer’s disease. Dement Geriatr Cogn Disord 2001; 12: 219-25. doi: https://doi.org/10.1159/000051261
Triplett JC, Zhang Z, Sultana R, Cai J, Klein JB, Büeler H, et al. Quantitative expression proteomics and phosphoproteomics profile of brain from PINK1 knockout mice: insights into mechanisms of familial Parkinson's disease. J Neurochem 2015; 133: 750-65. doi: https://doi.org/10.1111/jnc.13039
Walden H, Muqit MM. Ubiquitin and Parkinson's disease through the looking glass of genetics. Biochem J 2017; 474: 1439-51. doi: https://doi.org/10.1042/BCJ20160498
Schweig JE, Yao H, Beaulieu-Abdelahad D, Ait-Ghezala G, Mouzon B, Crawford F, et al. Alzheimer’s disease pathological lesions activate the spleen tyrosine kinase. Acta Neuropathol Commun 2017; 5: 69. doi: https://doi.org/10.1186/s40478-017-0472-2
Imamura K, Takeshima T, Kashiwaya Y, Nakaso K, Nakashima K. D‐β‐hydroxybutyrate protects dopaminergic SH‐SY5Y cells in a rotenone model of Parkinson's disease. J Neurosci Res 2006; 84: 1376-84. doi: https://doi.org/10.1002/jnr.21021
Arenas E. Wnt signaling in midbrain dopaminergic neuron development and regenerative medicine for Parkinson's disease. J Mol Cell Biol 2014; 6: 42-53. doi: https://doi.org/10.1093/jmcb/mju001
Kim H, Calatayud C, Guha S, Fernández-Carasa I, Berkowitz L, Carballo-Carbajal I, et al. The Small GTPase RAC1/CED-10 Is Essential in Maintaining Dopaminergic Neuron Function and Survival Against α-Synuclein-Induced Toxicity. Mol Neurobiol 2018; 1–20. https://doi.org/10.1007/s12035-018-0881-7
Dehay B, Bové J, Rodríguez-Muela N, Perier C, Recasens A, Boya P, et al. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci 2010; 30: 12535-44. doi: https://doi.org/10.1523/JNEUROSCI.1920-10.2010
Lu AT, Hannon E, Levine ME, Hao K, Crimmins EM, Lunnon K, et al. Genetic variants near MLST8 and DHX57 affect the epigenetic age of the cerebellum. Nat Commun 2016; 7: 10561. doi: https://doi.org/10.1038/ncomms10561
Rhinn H, Qiang L, Yamashita T, Rhee D, Zolin A, Vanti W, et al. Alternative α-synuclein transcript usage as a convergent mechanism in Parkinson's disease pathology. Nat Commun 2012; 3: 1084. doi: https://doi.org/10.1038/ncomms2032
Li MD, Burns TC, Morgan AA, Khatri P. Integrated multi-cohort transcriptional meta-analysis of neurodegenerative diseases. Acta Neuropathol Commun 2014; 2: 1. doi: https://doi.org/10.1186/s40478-014-0093-y
Zhang Y, James M, Middleton FA, Davis RL. Transcriptional analysis of multiple brain regions in Parkinson's disease supports the involvement of specific protein processing, energy metabolism, and signaling pathways, and suggests novel disease mechanisms. Am J Med Genet B Neuropsychiatr Genet 2005; 137: 5-16. doi: https://doi.org/10.1002/ajmg.b.30195
Hong L, Sklar LA. Targeting GTPases in Parkinson’s disease: comparison to the historic path of kinase drug discovery and perspectives. Front Mol Neurosci 2014; 7: 52. doi: https://doi.org/10.3389/fnmol.2014.00052
Acknowledgements
The author thanks Mr. Rasoul Godini (currently a Ph.D. student at Monash University, Australia) for his help with WGCNA analysis and Ms. Fatemeh Hadi (Ph.D. graduate, Razi University, Iran) for confirming the genes in the modules that has been included in Table 2 of this manuscript.
Author information
Authors and Affiliations
Contributions
Not applicable
Corresponding author
Ethics declarations
Ethics approval and Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing of interests
The authors declare that the research was conducted in the absence of any commercial or financial interest.
Additional information
Highlight study
• Network analysis identified key modules of genes related to both AD and PD.
• Chemical transmission, nervous development, and immune responses are affected.
• INPP4A, CREG2, ABI3, MYO1F, NAPB, NXN, DOCK6, CPSF6, and IKZF1 were identified.
• There are system-level similarities between AD and PD.
Supplementary Information
Supplementary file 1
(PPTX 165 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fallahi, H., Radak, M. & Yadegari, Z.S. Uncovering systems-level molecular similarities between Alzheimer’s and Parkinson’s diseases. Neurosci Behav Physi 53, 1300–1318 (2023). https://doi.org/10.1007/s11055-023-01484-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01484-8