Abstract
The ongoing battle against viral infections highlighted so recently by the COVID-19 pandemic demonstrates the need to develop new approaches using nanotechnology in antiviral strategies. Nanoparticles have emerged as promising tools in the fight against viral outbreaks, offering various options for application such as biosensors, vaccine nanoparticles, disinfectants, and functionalized nanoparticles. In this comprehensive review, we evaluate the role of nanoparticles in pandemic control, exploring their potential applications, benefits, and associated risks. We first discuss the importance of nanotechnology in viral outbreak management, particularly in vaccine development. Although lipid nanoparticles play a crucial role in mRNA vaccines, there are concerns about their potential side effects. Although functionalization of protective face masks using metallic nanoparticles has emerged as a sustainable alternative to disposable masks, reducing waste production and enhancing virus filtration, improper disposal of such masks leads to environmental contamination and potential ecological harm. Second, we address the potential adverse effects associated with nanoparticle-based vaccines containing polyethylene glycol and other vaccine components, which trigger autoimmune diseases and alter menstrual cycles. To manage outbreaks effectively, we must minimize such potential risks and environmental impacts. Thus, when developing effective strategies for future pandemic control, it is crucial to understand the advantages and challenges associated with nanoparticle usage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout the twentieth century, viral infections significantly impacted global health, causing millions of deaths worldwide. To combat these diseases, nanotechnology has emerged as a promising approach in the development of antiviral agents such as biosensors, nanoprobes, virus-like particles (VLPs), and functionalized nanoparticles [1,2,3]. The COVID-19 pandemic highlighted the importance of nanotechnology in the battle against viral infections, particularly in the development of vaccines. In turn, ongoing discoveries of new virus variants highlight the importance of being prepared to combat potential future pandemics. Recently, for example, three new COVID-related virus variants were discovered in bats in Laos [4].
In this review, we evaluate the role of nanoparticles in pandemic control and discuss their potential applications, and also the alarms and risks associated with their use. We also draw on the insights learned from the COVID-19 outbreak regarding the use of nanoparticles in managing viral outbreaks. For instance, although antiviral face masks containing metal nanoparticles were proposed as a more sustainable alternative to disposable masks, since they reduce the amount of non-biodegradable waste material [5], their improper disposal has contributed to the release of metal nanoparticles into the environment. One recent example was their release into Colombian and Southern Brazilian waters [6, 7]. Improper disposal may also cause ecological harm [8]. A comprehensive understanding of the advantages and risks associated with nanoparticle utilization is therefore essential to their responsible and effective use.
Another significant application of nanoparticles in the fight against COVID-19 is the use of lipid nanoparticle platforms for mRNA vaccines. Although the widespread use of lipid nanoparticles containing mRNA vaccines has contributed significantly to pandemic control [9], the long-term safety and activity of the vaccine-containing nanoparticles are unclear. Neither have the logistics associated with transport — such as special equipment for storage during transport to remote areas, and the costs this involves — been addressed so far.
Below, we explore the various potentials of nanoparticles in viral outbreak management. More specifically, we discuss the use of functionalized face masks for preventing viral infections, the significance of nanoparticle-based drug delivery systems for antiviral therapeutics, and the role of nanoparticles in diagnostic platforms for rapid and accurate viral detection. We also address the concerns and risks associated with the use of nanoparticles, including their potential ecological impacts and other long-term safety considerations. We highlight the critical role of nanoparticles in tackling viral outbreaks, showcasing their potential applications and stressing the importance of their responsible and ethical use. Through proper consideration of the benefits and risks intricately linked with nanoparticle usage, we hope to ensure the development of effective strategies for future pandemic control.
Nanotechnology applications in SARS-CoV-2 transmission prevention
Functionalization of protective face masks using nanoparticles
The improper disposal of non-reusable face masks has become an environmental concern, as these masks often contain non-biodegradable materials and pathogenic particles in their filter layer. Over time, the mask weathering caused by factors such as mechanical stress, UV-light, or quartz particles causes the release of microplastics from the masks into the environment [10].
Surgical masks usually consist of three layers, with a central filter layer. To reduce waste production, this filter layer can be functionalized with metallic nanoparticles composed of silver, zinc, or copper, which interfere with viral reproductive cycles, thereby helping to improve virus filtration over that of ordinary masks [11,12,13,14,15]. Metallic nanoparticles can be used to functionalize face masks through the addition of substances such as photo-sensitizing nanoparticles, which produce reactive oxygen species (ROS) upon exposure to specific wavelengths of light, thereby effectively destroying pathogenic membranes, proteins, and nucleic acids after each mask use [16]. Plasma-based nanoparticles with photo-thermal efficiency, such as graphene, silver, and gold nanoparticles, are able to self-disinfect when exposed to light, and to absorb any moisture present in the mask [12, 17]. Graphene-derivatives add other properties to functionalized face masks, such as resistance to smog, mechanical and abrasion stress, and UV light [18]. Positively charged polymer nanoparticles have strong virucidal properties that reform and fluidize the lipid content of viral membranes, particularly in lipid-raft areas [19]. Similarly, biodegradable polysaccharide-based materials successfully combat COVID-19 in facial mask layers, achieving complete decomposition in soil within 4 weeks [20, 21].
Various nanotechnology applications in SARS-CoV-2 transmission prevention
As well as functionalizing protective face masks, metallic nanoparticles can be applied in mouthwash and nose rinses, offering new opportunities for combating viral infections. As silver nanoparticles (AgNPs) inhibited SARS-CoV-2 in pre-clinical studies [22, 23], they may have potential for wider application. Whereas chemical disinfectants need high concentrations of the active substance, metallic nanoparticles can be used in low concentrations, producing less harmful byproducts and being more effective than standard disinfectants [24]. Another application of nanotechnology involves spraying nano-sized electrostatic atomized water particles (NEAWPs) onto an electrode on which water molecules have first condensed. This significantly reduces the environmental virus count [25]. Such use of nanoparticles is particularly important because the excessive use of traditional disinfectants during the pandemic increased levels of quaternary ammonium compounds in water and soil, thereby posing an environmental threat [26].
Nanoparticles as antiviral therapeutic agents
The main antiviral agents against SARS-CoV-2 infection, i.e., remdesivir, zanamivir, oseltamivir, and abacavir, are specific for HIV and/or influenza, but not for SARS-CoV-2 infections [27, 28]. The use of nanoparticles in COVID-19 treatment strategies is promising since the nanoparticles do combat SARS-CoV-2. For example, iron oxide nanoparticles interfere with the S1 subunit of the RBD domain [29], and amyloid-like proteins from LCB1 and LCB3 sequences of the S protein self-assemble into multivalent spherical nanoparticles, competitively blocking viral interaction with the angiotensin-converting enzyme 2 (ACE2) receptor [30]. Similarly, linear polyglycerol sulfate and its fullerene-conjugated derivative can block virus entry into host cells [31]. Biological nanovesicles from human lung spheroid cells that present ACE2, as cell-mimicking nanodecoys, are promising, since they absorb viruses and prevent their attachment to the host cells [32].
Controlled-release systems of antivirals using nanoparticles
Nanoparticles provide a controlled-release system of antivirals to reduce side effects, increase bioavailability, improve circulation time, or ameliorate delivery of the antivirals [33]. For example, polymeric nanoparticles made of poly-ε-caprolactone (PCL) or poly-lactic glycolic acid-conjugated-poly-ethylene glycol (PLGA-PEG) decorated with ACE2 ligands successfully encapsulate remdesivir, playing dual antiviral roles through competitive interference with SARS-CoV-2 in ACE2 binding, and through targeted drug delivery to lung cells [34]. The anti-COVID efficacy of the drug is enhanced by PLGA-lipid hybrid nanoparticles encapsulating fluoxetine hydrochloride [35]. Table 1 provides information on selected types of nanoparticle that are used against different coronaviruses.
Environmental challenges and urgent action
Adverse effects associated with nanoparticles in personal protective equipment
Despite some positive aspects of metal nanoparticle usage in personal protective equipment (PPEs) such as masks, potentially adverse effects of metal nanoparticles on ecosystems and their fate in the mask-washing process have not been thoroughly investigated. Their entry into the ecosystem may have unintended consequences.
Due to their high surface-to-volume ratio and reactive surface, nanoparticles are prone to interfere with biological processes. While their environmental impact, particularly that of nanoparticles originating from PPEs, has raised concerns about possible adverse effects on the ecosystem, these effects are not entirely negative. As well as aiding the removal of heavy metals and organic pollutants in wastewater treatment, metal nanoparticles can degrade microplastics and nanoplastics, and produce H2O and CO2 as end-products of degradation [8, 36, 37]. Nonetheless, the prolonged and continuous use of nanoparticles in PPEs leads to the accumulation of toxic levels of degradation products, posing risks to various organisms, and to the entire ecosystem [38]. In aquatic conditions, metal nanoparticles derived from PPEs can interact with other pollutants. The detrimental effects in organisms range from inflammation to cellular damage that further exacerbate any impact on the environment [8]. A further problem is the improper disposal of PPEs in landfills, dumpsites, marine environments, or public spaces. This can cause animals to mistakenly recognize such PPE waste as food, resulting in their inadvertent and potentially harmful ingestion.
All in all, urgent action is required to address a range of significant environmental challenges. To promote proper disposal practices and prevent the dissemination of nanoparticles into the environment, specific recycling guidelines tailored to nanotechnology products should be established and enforced [19]. Before the SARS-CoV-2 pandemic, nanoparticles entered the environment mainly through household usage, industrial waste, or laboratory penetration. However, during the initial stages of the pandemic, when it was believed that the virus could be transmitted through surfaces, the use of antiseptic and disinfectant agents skyrocketed, increasing the release of nanoparticles into the environment. Now, in the post-SARS-CoV-2 period, the main source of nanoparticle pollution is the widespread uncontrolled abandonment of personal protective equipment.
Adverse effects of nanoparticles on plants and microorganisms
During the SARS-CoV-2 pandemic, the worldwide demand for masks reached over 4 billion daily, all while recycling programs for masks were inadequately planned [39]. Several countries used reusable masks containing carbon nanotubes, and/or silver (Ag), silicon dioxide (SiO2), zinc oxide (ZnO), or nanoparticles titanium dioxide (TiO2). Disposal of nanosilver from these masks was found to pose ecological hazards, inhibiting plant growth and photosynthesis [40]. Engineered nanoparticles commonly penetrate the roots of plants, resulting in phytotoxicity [41]. As nanoparticles generate reactive ions interacting with nutrients and inorganic compounds in plants, they cause chlorosis and wilting [42, 43]. Small-sized nanoparticles such as TiO2 pass through protective layers like the cuticle, cell wall, and cell membrane [44], impairing the growth of seedlings crops, the uptake of minerals, and chlorophyll synthesis [45]. ZnO nanoparticles reduce chlorophyll production in bulb onions, as well as crop growth and development [46,47,48]. Ag nanoparticles increase the activity of antioxidant enzymes, reduce chlorophyll content, and impair photosynthesis in tomatoes [49, 50].
Similarly adverse effects of nanoparticles are observed not only in plants, but also in bacteria and aquatic animals [51]. For example, ZnO-based nanoparticles induce genetic mutations in Caenorhabditis elegans, resulting in offspring toxicity [52, 53]. In sea water, TiO2 nanoparticles released from sunscreens cause severe damage to gill filaments, hampering aquatic animal reproduction [54, 55]. Overall, it is therefore clear that the adverse effects of nanoparticles eventually disrupt the food chain for higher organisms.
Adverse effects of nanoparticles on higher organisms
The generation of reactive oxygen species is a biological process. Their excessive generation causes oxidative stress, leading to inflammation, diabetes, cancer, and other degenerative diseases [56, 57]. Excessive reactive oxygen species causes free-radical production, lipid peroxidation, genotoxicity, and apoptosis [58]. Nanoparticles accumulate in various organs and have overall systemic effects [59]. Some inorganic nanoparticles such as TiO2, SiO2, ZnO, and Fe2O3 dissolve in the acidic environment of the stomach [60]. Through absorption into the skin, lungs, and liver [61], they also impair human health.
Toxicity caused by silver nanoparticles (AgNPs) in vitro depends on the surface coating and the concentration of AGNPs it contains. In vivo, AgNPs enter the bloodstream and accumulate in organs, where they cause cytotoxicity [62]. The generation of Ag+ ions from AgNPs and oxidative stress both lead to apoptosis via translocation of mitochondrial cytochrome C into the cytosol [63], or to necrosis by reducing sulfhydryl groups [64, 65]. Table 2 provides an overview of anti-COVID-19-related cytotoxic effects caused by AgNPs and other types of nanoparticles in vitro and in vivo, and the compendial and noncompendial tests used.
Direct contact with titanium dioxide nanoparticles (TiO2NPs) affects skin cells in various ways, for example, by impairing their viability, proliferation, and differentiation [66]. TiO2NPs penetrate into the deep layers of the skin are known to be released in sweat [67,68,69]. Inhalation of TiO2NPs poses a significant health risk: due to the lower protection provided by the olfactory bulb than by the blood–brain barrier, nano-sized materials can penetrate the brain faster through the olfactory nerve than through systemic injection [70, 71].
Being in direct contact with the skin and the air we breathe, protective masks containing Ag and TiO2 nanoparticles are potentially harmful. The presence of these nanoparticles has been shown to significantly inhibit the growth rate of human osteoblasts, indicating that the adverse effects of the masks are not limited to the skin, inhalation, or brain [72].
The leaching of Ag+ or Cu2+ ions from metal-impregnated masks has also been linked to potential health risks for humans [73]. Exposure to these nanoparticles through ingestion, inhalation, or dermal penetration can cause toxicity [74]. Ingestion is followed by exposure to the complex and harsh condition of the gastrointestinal tract, i.e., pH variations, gastric salts, ions, and enzymes. These interactions modify the composition of nanoparticles, leading to biomolecule adsorption and aggregation [75,76,77].
Although nanoparticles have toxic effects on the immune system and are involved in oxidative stress-related disorders, many people attribute these disorders to factors such as air pollution. This has led to proposals for public education on proper disposal of personal protective equipment in government-provided trash containers. Long-term monitoring of coastal waste and citizen initiatives for litter collection in populated areas have also been suggested [78,79,80], as has the recycling of carbon powders from masks for use in batteries [81] or renewable fuels [82], and the promotion of reusable alternatives and cellulose-fiber textiles. Potential disposal methods also include incineration and optimized pyrolysis [82, 83].
The toxicity of metal nanoparticles varies according to the size, surficial coating, and shape of the nanoparticles [84]. The solubility of AgNPs is inversely proportional to the size of the nanoparticle. Due to increased dissolution and cell penetration efficacy, small nanoparticles exhibit high toxicity, with a strong attachment to DNA also causing DNA damage. Use AgNPs sized more than 20 nm shows less genotoxicity [85, 86]. As metal nanoparticles sized less than 100 nm cause increased toxicity in vivo, the recommended ranges lie between 100 and 150 nm [87, 88].
Toxicity is also influenced by the type of nanoparticle coating. Coating AgNPs with polyvinylpyrrolidone (PVP) has been found to have a greater toxicity and tissue uptake than citrate coatings, while positively charged polymers such as chitosan enhance the toxicity of AgNPs more than citrate-stabilized particles do. A bovine serum albumin (BSA) coating of gold nanoparticles (AuNPs) also leads to greater toxicity and poorer renal clearance than a glutathione (GSH) coating. On the other hand, coating AuNPs with PEG reduces nanoparticle toxicity and appears to be a suitable coating option [85, 89,90,91,92].
Nanoparticles in vaccines and risk assessment
The WHO defines vaccines as pharmaceutical formulations that activate the immune system in order to produce specific antibodies, thereby generating protective immunity against a disease caused by a pathogen [93]. The conventional vaccines developed since the late eighteenth century rely on the discovery of antibodies in patients who have recovered from infections. To elicit an immune response, these use attenuated or inactivated pathogens and purified pathogen fragments [94]. Second-generation vaccines are produced using recombinant DNA technology in bacteria or in cell cultures [95].
Recently, a third generation of vaccines has emerged, which introduces the gene encoding the protective antigen into a host cell. By improving antigen processing and its presentation to antigen-presenting cells (APCs), this enhances the activation of CD4+ and CD8+ cells. This recent advance in vaccine technology holds promise for eliciting protective immune responses against the virus [96].
To overcome the limitations of conventional vaccines, i.e., attenuated or inactivated viruses, alternative options such as RNA- or DNA-based vaccines have also been sought recently. These new RNA- or DNA-based vaccine production technologies aim both to improve reactivity and efficacy and to reduce the cost of vaccines. They can also be used to effectively treat other diseases, such as cancer. But whereas vaccines need to be delivered to the right places in the body in a suitable form so as to prepare the immune system to combat an invading pathogen effectively, most vaccine molecules are prone to degradation. Due to limited accessibility and poor cell permeation [97], they may not be recognized efficiently by the immune system. A crucial role in enhancing the effectiveness of vaccines is played by delivery systems based on nanoformulations. By tailoring nanoencapsulation, vaccines can be delivered with precision and stability [96].
These delivery systems contribute to the in vivo behavior of vaccines in various ways: they protect vaccines from enzymatic degradation, improve their pharmacokinetic properties through surface engineering techniques such as PEGylation, enable active targeting to specific organs or cell types, and engineer controlled release of vaccines [93, 98, 99].
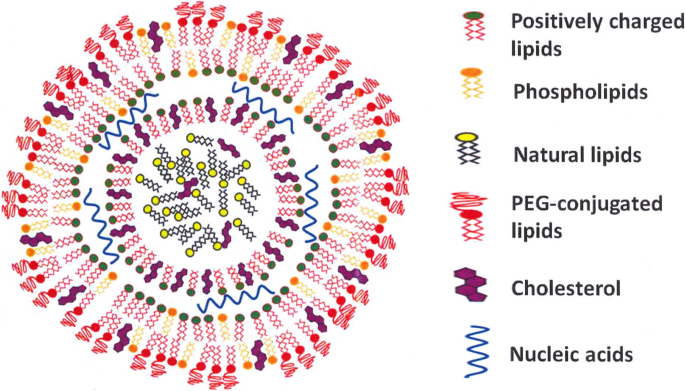
Lipid nanoparticles, self-assembling protein nanoparticles, virus-like particles, liposomes, and cationic nanoemulsion vaccines have been designed against SARS-CoV-2 [100]. The most prominent vaccines against SARS-CoV-2 are lipid-based nanoparticles (LNPs), which have been designed as drug nanocarriers for nucleic acid delivery [101]. By protecting fragile and unstable nucleic acids from degradation by nucleases, LNPs can increase the half-life of nucleic acids in the blood circulation. Charge-reversible LNPs contain ionizable lipids, either positively or negatively charged, that allow the LNPs to remain neutrally charged in the bloodstream, effective encapsulation of nucleic acids in the LNPs, and a high degree of endosomal escape of LNPs (Fig. 1) [102].
Schematic illustration of an mRNA-based SARS-CoV-2 lipid nanoparticle vaccine. Different components of the vaccine are visualized, i.e., positively charged lipids, phospholipids, natural lipids, PEGylated lipids, cholesterol, and nucleic acids. This figure is modified from those published in [105]. PEG, polyethylene glycol
LNPs contain two other main components: cholesterol, a neutral phospholipid, and PEG-lipid, which protects LNPs from phagocytosis and aggregation in the blood circulation and also during manufacturing and storage. In vaccine formulations, the PEG-lipid also ensures that the LNP maintains the desired diameter (200 nm) [103]. A factor that is crucial to efficient nucleic acid delivery is the complete escape of nucleic acids from the endosomal compartment after LNP internalization. By increasing the diffusibility of PEG-lipids, the addition of distearoylphosphatidylcholine (DSPC) and dioleoylphosphatidylethanolamine (DOPE) lipids to nanoparticles enhances endosomal escape [104].
To produce viral proteins, leading anti-COVID vaccine developers such as Moderna, Pfizer/BioNTech, CureVac, Walvax, Sanofi, Pasteur, and Entos Pharmaceuticals all use cationic LNPs to deliver mRNA or DNA encapsulated into host cells. Although mRNA vaccines are more prone to instability and functional defects than DNA vaccines, they are preferred due to their higher immunogenicity, their direct translation in the cytosol, and their higher loading potential into LNPs [106,107,108]. To achieve the same level of efficiency, self-amplifying mRNA-LNP vaccines such as those developed by Imperial College London and Arcturus/Duke-NUS require ten times less mRNA than mRNA vaccines. However, they have less flexibility in nucleotide modification than their mRNA counterparts [102, 109].
Most LNP-derived vaccines currently available induce immune responses against the Spike protein (S protein). Interestingly, the receptor binding (RBD) and N-terminal (NTD) domains of the S protein are targeted by the most potent of the 61 monoclonal antibodies isolated from infected patients [110]. As anti-NTD antibodies inhibit and anti-RBD antibodies neutralize viral infections [111], the presentation of one of the virus protein domains is preferred above presentation of the whole protein for optimal immunity against new COVID-19 variants.
Due to the need for expensive low-temperature storage required by the SARS-CoV-2 vaccines currently available, their distribution poses challenges in developing countries. As the mechanical stresses caused by shaking might lead to aggregation and mRNA degradation in LNPs, vaccines also need to be administered promptly after preparation [112,113,114]. By enhancing the long-term stability of mRNA-LNPs, freeze-drying offers a solution to both these problems. However, if freeze-drying is to be successful, vaccine structure should not be affected by the lyoprotectants and by temperature stress. A new generation of the Pfizer/BioNTech vaccine is currently being prepared in lyophilized (freeze-dried) form [115].
Adverse effects associated with SARS-CoV-2 nanoparticle vaccines
Mild to moderate side effects are experienced after vaccinations. Compared to conventional vaccines, Pfizer and Moderna vaccines have been shown to cause more serious allergic reactions, including anaphylaxis. While side effects such as flushing and transient dyspnea were also observed in some of these mRNA vaccines, they were not considered to be allergic reactions [116]. Similar side effects were reported in earlier clinical safety studies of mRNA vaccines against influenza [116].
Although the rate of allergic reactions for LNP vaccines containing mRNA cargo is generally around 1.31 (95% CI, 0.90–1.84) per million doses, the number of severe immune reactions may be higher with booster doses [117]. LNPs induce inflammation, especially in non-adherent cells, due to the higher availability of cell surface receptors than in adherent cells [118, 119]. The main suspect for anaphylactic reactions in mRNA vaccines is the coating polymer, PEG, which alters the water solubility of the vaccine-containing nanoparticles [120]. Although PEG is widely used in cosmetics, food, medication, and pharmaceutical agents, its use in vaccine technology is rare [121].
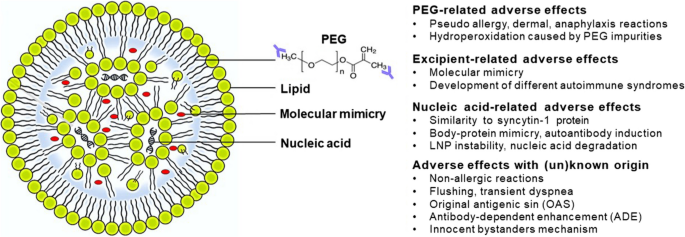
Initially, PEG molecules were thought to be safe and biologically inert, but nowadays PEG and PEG-like polymers are not considered to be as safe as initially thought [122]. An immune response mediated by anti-PEG IgG antibodies may develop in allergic individuals, particularly females [123]. These antibodies can target the PEG backbone or specifically bind to PEG terminal functional groups [124]. In the presence of reactive oxygen species, anti-PEG antibodies detrimentally affect the respiratory chain and signal transduction pathways, and also disrupt cell membranes [125]. In vivo, oxidation of PEG, especially of the PEG low-molecular polymer chains, produces toxic molecules, i.e., glycolic acid and hydroxy acid metabolites [126]. PEGylated nanoparticles cause pseudoallergic reactions such as complement-activation-related pseudo allergy (CARPA) [127] and toxic or immunogenic responses, particularly with booster doses. Anaphylactic responses to PEG occur in 2–8 cases per year worldwide, which has led the clinical use of two PEGylated pharmaceuticals to be abandoned [128,129,130]. The concentration of PEG in mRNA vaccines is much lower than in PEGylated drugs, and intramuscular administration induces less inflammation [131]. While anaphylactic reactions are caused not only by PEG, allergic reactions are also caused by vaccine components such as polysorbate 80 in the vaccines developed by AstraZeneca and Johnson [132]. On the other hand, polysorbate 80 is considered to be safer than PEG [128]. Figure 2 provides a schematic illustration of the various SARS-CoV-2 vaccines, and Table 3 a summary of the side effects associated with them.
Categorized adverse effects associated with different components of nucleic acid-based lipid nanoparticle vaccines. PEG, excipient, or nucleic-acid-related adverse effects, and adverse effects of known and unknown origin are indicated. Formation of anti-PEG backbone auto-antibodies (blue). PEG, polyethylene glycol
The side effects of PEGylated vaccines and polysorbate-containing vaccines include urticaria, dizziness, diarrhea, wheezing, and tachycardia [133]. Dermal side effects, such as erythema or swelling, are slightly more common with mRNA vaccines than with adenoviral vaccines (10–15% versus 5–7% of the patients) [134,135,136]. Rare side effects of viral vector vaccines include thrombosis and thrombocytopenia. When there is cross-reactivity between PEG and polysorbates, immediate hypersensitivity reactions occur [137, 138]. For approximately 6 months, mRNA and adenoviral vaccines can both cause changes in menstrual cycles, such as dysmenorrhea, alterations in frequency, volume, or cessation of bleeding. Women with pre-existing platelet disorders [139], those taking estrogen-based contraceptives [140], and those with thrombocytopenia [141] are all at a higher risk for such changes in their menstrual cycle. Messenger RNA- and viral vaccines affect the menstrual cycle, but the strongest changes are observed with mRNA-LNP vaccines [142, 143].
A major concern with the use of nanoparticle vaccines is that they trigger autoimmune diseases. SARS-CoV-2 mRNA-NPs vaccines trigger autoimmune liver diseases, Guillain-Barré syndrome, IgA nephropathy, myocarditis, optical neuromyelitis, autoimmune polyarthritis, Graves’ disease, type 1 diabetes mellitus, and systemic lupus erythematosus [144,145,146,147,148,149]. A second concern is the possibility of reverse transcription of mRNA vaccines in liver cells, which has been observed in vitro [150], although genotoxicity in vivo remains debatable [151]. A third concern is the phenomenon of original antigenic sin (OAS), which occurs when antibodies from previous infections or vaccinations hinder the neutralization of newly mutated antigens, particularly the omicron antigen variant [152]. A fourth concern is the concept of antibody-dependent enhancement (ADE), where low antibody titers bind to virus particles without neutralizing them, thereby facilitating virus entry into macrophages and enhancing respiratory disease responses. ADE has been linked to SARS-CoV-2 mRNA-NP vaccines [153].
A fifth concern has also arisen regarding the cross-reaction of vaccine-induced antibodies against syncytin-1, a placental protein similar to the SARS-CoV-2 spike protein, which activates the immune system and impacts female pregnancy [154]. Another significant consequence that can arise, mainly in young men, after a second dose of mRNA-based vaccines is myocarditis; this has an incidence of 12.6 cases per million. Sex hormones can contribute to the development of myocarditis [155], which is not only related to molecular mimicry of S protein, self-antigens, or the formation of autoantibodies, but may also be caused by vaccine adjuvants, activation of “innocent bystanders,” or induction of autoantibodies [149].
A question we are currently unable to answer is whether the S protein should be replaced by other viral proteins as the immunogenic target to develop vaccines. New generations of vaccines such as spike-trimers and spike-ferritin in liposomes will continue to be based on spike proteins [156, 157]. To address and mitigate the side effects of nanoparticle-based vaccines, certain modifications might be considered. PEG chain length and topological configuration affect immunogenicity. Pre-treatment with small amounts of high-molecular-weight PEG reduces anti-PEG reactions, and in animal models, short and hyperbranched PEG polymers, such as poly(oligo-ethylene glycol) methacrylate, exhibit decreased interaction with anti-PEG IgG and IgM antibodies [158]. To prevent inflammation and side effects, glyceryl monostearate (GMS) should not be included in LNPs [118]. Promising alternatives for PEG are polyglycerol polyricinoleate, polysarcosine, polyhydroxypropylmethacrylamide, polysulfobetaine, and polycarboxybetaine polymers [159]. Replacing PEG with polysulfobetaine coating results in higher biological activity of insulin; in nude mice, a dextran coating eliminated toxicity and liver stress of iron oxide nanoparticles [160, 161]. Polyesters like polycarbonates and polyphosphoesters might also be viable alternatives to PEG, since they degrade in vivo into non-toxic fragments, and can be easily produced using ring-opening polymerization (ROP) [162].
More preclinical studies are needed to evaluate vaccines. As two-dimensional in vitro studies may not always completely capture the complex immune environment, and as the phenotype and expression of receptors on cells may be influenced by culture conditions [163], the prediction of vaccine performance systems might be improved by three-dimensional cell culture systems [164] and/or standardized in vitro culture systems [164]. And as small rodents are anatomically different from humans, non-human primates might be more reliable for in vivo studies [165].
When evaluating nucleic acid vaccines, it is essential to assess not only the quantitative distribution of DNA or mRNA cargo, but also protein expression. This will help to monitor the distribution, retention, and release pattern of the delivered DNA or mRNA, providing a predictive tool for vaccine safety. In addition, valuable insight into the tissue localization of delivered nanoparticles is provided by information on vaccine distribution in lymph nodes, organs, and APCs [100]. Although a skin-sensitization test is recommended before vaccination with PEG and polysorbate [121, 128, 166, 167], the number of positive cases in skin tests is considerably lower than the number of sensitive cases after vaccine administration [168]. Protocols for graded dosing of vaccines have been developed for hypersensitive individuals, such as those with basophil disorders and uncontrolled asthma. Allergic individuals are advised to receive a second dose of a different vaccine, or, in some cases, heterologous prime-boost vaccines are recommended [169,170,171]. Finally, in Fig. 3, we propose several solutions that will minimize the disadvantages associated with nanoparticle use.
Conclusions
The use of nanoparticles in combatting viral infections has proved to represent a promising and valuable approach in the realm of global health. The COVID-19 pandemic underscored the significance of nanotechnology in vaccine development, infection prevention, and therapeutic strategies. By offering innovative solutions — including functionalized face masks, antiviral therapeutics, and diagnostic platforms — nanoparticles have already showcased their potential in pandemic control. However, the potential risks and challenges associated with their use still require attention, particularly in vaccine development.
Existing LNP formulations have played a crucial role in the rapid development of SARS-CoV-2 vaccines. Unfortunately, vaccine-induced immunity against SARS-CoV-2 is of limited duration, and, to enhance vaccine safety, adverse effects such as anaphylaxis and autoimmune reactions call for modifications in nanoparticle design such as after receiving primary doses, individuals with a history of COVID-19 vaccine anaphylaxis should not receive booster doses of the same vaccine.
The development of long-lasting and immunogenic nanoparticle formulations against SARS-CoV-2 is crucial. To prevent nanoparticle aggregation, surface modification of LNPs is also vital. It is also possible that alternative coating materials, such as shorter-length PEG polymers or other synthetic or natural polymers, may help to minimize side effects and enhance vaccine safety.
Whatever their promise in combating viral infections, the main concerns raised by metal nanoparticles involve their entry into the ecosystem. However, they can also aid in wastewater treatment, microplastic degradation, and environmentally friendly H2O and CO2 production. If responsible nanoparticle use is to be ensured, it is imperative to achieve better control of their toxicity through modifications of nanoparticle size, surface coating, and shape, and also to stimulate public education and proper disposal practices for nanoparticle-based personal protective equipment. Although multiple factors determine whether nanoparticles are toxic, very little information is available on their toxicity, which is sometimes related to the specific drug delivery, and/or to the physical characteristics of the nanoparticles (i.e., their size, surface area, charge, shape, and composition). The adverse effects associated with the use of nanoparticles such as LNPs containing PEG (PEGylated LNPs; Fig. 2) limit the use of LNPs in clinical applications. PEG is an FDA-approved compound that is used in pharmacochemical and personal care products. A solution to problems involving its toxicity in clinical uses may be provided by LNP modification, such as by replacing PEG with natural polymers.
The lessons learned from the COVID-19 pandemic have shed light on the importance of responsible and ethical nanoparticle use. In the pursuit of future pandemic control and global health protection, it is essential to continue harnessing the potential of nanotechnology while simultaneously remaining cautious and well-informed. By prioritizing the safety of nanoparticle-based products and vaccines, we will be able to ensure that nanotechnology remains a valuable tool in our fight against viral outbreaks, with minimized risks and enhanced benefits for both human health and the environment.
References
Kausar S, Said Khan F, Ishaq Mujeeb Ur Rehman M, Akram M, Riaz M, Rasool G, Hamid Khan A, Saleem I, Shamim S, Malik A (2021) A review: mechanism of action of antiviral drugs. Int J Immunopathol Pharmacol 35:731–738. https://doi.org/10.1177/2F20587384211002621
Figueiró Longo JP, Muehlmann LA (2021) How has nanomedical innovation contributed to the COVID-19 vaccine development? Nanomedicine (Lond) 16:731–738. https://doi.org/10.2217/nnm-2021-0035
Chen L, Liang J (2020) An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater Sci Eng C 112:110924. https://doi.org/10.1016/j.msec.2020.110924
Mallapaty S (2021) Laos bats host closest known relatives of virus behind COVID. Nature 597:603. https://doi.org/10.1038/d41586-021-02596-2
Sportelli MC, Izzi M, Kukushkina EA, Hossain SI, Picca RA, Ditaranto N, Cioffi N (2020) Can nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials 10:731–738. https://doi.org/10.3390/nano10040802
Silva LF, Dotto GL, Pinto D, Oliveira ML (2021) Nanoparticles and interfaces with toxic elements in fluvial suspended sediment. Mar Pollut Bull 168:112405. https://doi.org/10.1016/j.marpolbul.2021.112405
Oliveira ML, Dotto GL, Pinto D, Neckel A, Silva LF (2021) Nanoparticles as vectors of other contaminants in estuarine suspended sediments: natural and real conditions. Mar Pollut Bull 168:112429. https://doi.org/10.1016/j.marpolbul.2021.112429
López AF, Fabiani M, Lassalle V, Spetter C, Severini MF (2022) Critical review of the characteristics, interactions, and toxicity of micro/nanomaterials pollutants in aquatic environments. Mar Pollut Bull 174:731–738. https://doi.org/10.1016/j.marpolbul.2021.113276
Krantz MS, Phillips EJ (2022) COVID-19 mRNA vaccine safety during the first 6 months of roll-out in the USA. Lancet Infect Dis 35:731–738. https://doi.org/10.1016/S1473-3099(22)00123-2
De-la-Torre GE, Pizarro-Ortega CI, Dioses-Salinas DC, Ammendolia J, Okoffo ED (2021) Investigating the current status of COVID-19 related plastics and their potential impact on human health. Curr Opin Toxicol 35:731–738. https://doi.org/10.1016/j.cotox.2021.08.002
Campos EV, Pereira AE, De Oliveira JL, Carvalho LB, Guilger-Casagrande M, De Lima R, Fraceto LF (2020) How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J Nanobiotechnology 18:1–23. https://doi.org/10.1186/s12951-020-00685-4
Seidi F, Deng C, Zhong Y, Liu Y, Huang Y, Li C, Xiao H (2021) Functionalized masks: powerful materials against COVID-19 and future pandemics. Small 17:2102453. https://doi.org/10.1002/smll.202102453
Bradley D (2020) Copper against COVID. Mater Today (Kidlington, England) 40:2. https://doi.org/10.1016/j.mattod.2020.09.016
Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, Svistunov AA, Petrakis D, Spandidos DA, Aaseth J (2020) Zinc and respiratory tract infections: perspectives for COVID-19. Int J Mol Med 46:17–26. https://doi.org/10.3892/ijmm.2020.4575
Sousa BC, Cote DL (2020) Antimicrobial copper cold spray coatings and SARS-CoV-2 surface inactivation. MRS Adv 5:2873–2880. https://doi.org/10.1557/adv.2020.366
Joost U, Juganson K, Visnapuu M, Mortimer M, Kahru A, Nõmmiste E, Kisand V, Ivask A (2015) Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: effects on Escherichia coli cells and fatty acids. J Photochem Photobiol B Biol 142:178–185. https://doi.org/10.1016/j.jphotobiol.2014.12.010
Xiao MF, Zeng C, Li SH, Yuan FL (2021) Applications of nanomaterials in COVID-19 pandemic. Rare Met 41:1–13. https://doi.org/10.1007/s12598-021-01789-y
Bhattacharjee S, Joshi R, Chughtai AA, Macintyre CR (2019) Graphene modified multifunctional personal protective clothing. Adv Mater Interfaces 6:1900622. https://doi.org/10.1002/admi.201900622
Palmieri V, De Maio F, De Spirito M, Papi M (2021) Face masks and nanotechnology: keep the blue side up. Nano Today 37:101077. https://doi.org/10.1016/j.nantod.2021.101077
El-Atab N, Qaiser N, Badghaish H, Shaikh SF, Hussain MM (2020) Flexible nanoporous template for the design and development of reusable anti-COVID-19 hydrophobic face masks. ACS Nano 14:7659–7665. https://doi.org/10.1021/acsnano.0c03976
Choi S, Jeon H, Jang M, Kim H, Shin G, Koo JM, Lee M, Sung HK, Eom Y, Yang HS (2021) Biodegradable, efficient, and breathable multi-use face mask filter. Adv Sci 8:2003155. https://doi.org/10.1002/advs.202003155
Almanza-Reyes H, Moreno S, Plascencia-López I, Alvarado-Vera M, Patrón-Romero L, Borrego B, Reyes-Escamilla A, Valencia-Manzo D, Brun A, Pestryakov A (2021) Evaluation of silver nanoparticles for the prevention of SARS-CoV-2 infection in health workers: in vitro and in vivo. Plos One 16:e0256401. https://doi.org/10.1371/journal.pone.0256401
ClinicalTrials.gov (2021) Evaluation of silver nanoparticles for the prevention of COVID-19 (COVID-19). (NCT04894409). https://clinicaltrials.gov/ct2/show/NCT04894409
Talebian S, Wallace GG, Schroeder A, Stellacci F, Conde J (2020) Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat Nanotechnol 15:618–621. https://doi.org/10.1038/s41565-020-0751-0
Yasugi M, Komura Y, Ishigami Y (2022) Mechanisms underlying inactivation of SARS-CoV-2 by nano-sized electrostatic atomized water particles. J Nanopart Res 24:99. https://doi.org/10.1007/s11051-022-05485-5
Hora PI, Pati SG, McNamara PJ, Arnold WA (2020) Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ Sci Technol Lett 7:622–631. https://doi.org/10.1021/acs.estlett.0c00437
Riva L, Yuan S, Yin X, Martin-Sancho L, Matsunaga N, Pache L, Burgstaller-Muehlbacher S, De Jesus PD, Teriete P, Hull MV (2020) Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 586:113–119. https://doi.org/10.1038/s41586-020-2577-1
De Clercq E (2006) Antiviral agents active against influenza A viruses. Nat Rev Drug Discov 5:1015–1025. https://doi.org/10.1038/nrd2175
Abo-Zeid Y, Ismail NS, McLean GR, Hamdy NM (2020) A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur J Pharm Sci 153:105465. https://doi.org/10.1016/j.ejps.2020.105465
Behbahanipour M, Benoit R, Navarro S, Ventura S (2023) Oligobinders: bioengineered soluble amyloid-like nanoparticles to bind and neutralize SARS-CoV-2. ACS Appl Mater Interfaces 15:11444–11457. https://doi.org/10.1021/acsami.2c18305
Page TM, Nie C, Neander L, Povolotsky TL, Sahoo AK, Nickl P, Adler JM, Bawadkji O, Radnik J, Achazi K (2023) Functionalized fullerene for inhibition of SARS-CoV-2 variants. Small 19:2206154. https://doi.org/10.1002/smll.202206154
Li Z, Wang Z, Dinh P-UC, Zhu D, Popowski KD, Lutz H, Hu S, Lewis MG, Cook A, Andersen H (2021) Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19. Nat Nanotechnol 16:942–951. https://doi.org/10.1038/s41565-021-00923-2
Milovanovic M, Arsenijevic A, Milovanovic J, Kanjevac T, Arsenijevic N (2017) Nanoparticles in antiviral therapy. In: Antimicrobial nanoarchitectonics. Elsevier, pp 383–410. https://doi.org/10.1016/B978-0-323-52733-0.00014-8
Sanna V, Satta S, Hsiai T, Sechi M (2022) Development of targeted nanoparticles loaded with antiviral drugs for SARS-CoV-2 inhibition. Eur J Med Chem 231:114121. https://doi.org/10.1016/j.ejmech.2022.114121
Khater SE, El-Khouly A, Abdel-Bar HM, Al-Mahallawi AM, Ghorab DM (2021) Fluoxetine hydrochloride loaded lipid polymer hybrid nanoparticles showed possible efficiency against SARS-CoV-2 infection. Int J Pharm 607:121023. https://doi.org/10.1016/j.ijpharm.2021.121023
Naseem T, Durrani T (2021) The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: a review. Environ Chem Ecotoxicol 3:59–75. https://doi.org/10.1016/j.enceco.2020.12.001
Uheida A, Mejía HG, Abdel-Rehim M, Hamd W, Dutta J (2021) Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. J Hazard Mater 406:124299. https://doi.org/10.1016/j.jhazmat.2020.124299
Hydzik P (2012) Nanoparticles toxicity--selective examples. Przegl Lek 69:486–489. PMID: 23243914 https://europepmc.org/article/med/23243914
Shirvanimoghaddam K, Czech B, Yadav R, Gokce C, Fusco L, Delogu LG, Yilmazer A, Brodie G, Al-Othman AK, Al-Tamimi AK (2022) Facemask global challenges: the case of effective synthesis, utilization, and environmental sustainability. Sustainability 14:737. https://doi.org/10.3390/su14020737
Vishwakarma K, Upadhyay N, Singh J, Liu S, Singh VP, Prasad SM, Chauhan DK, Tripathi DK, Sharma S (2017) Differential phytotoxic impact of plant-mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front Plant Sci 8:1501. https://doi.org/10.1016/j.aquatox.2016.04.019
Dietz K-J, Herth S (2011) Plant nanotoxicology. Trends Plant Sci 16:582–589. https://doi.org/10.1016/j.tplants.2011.08.003
Begum P, Fugetsu B (2012) Phytotoxicity of multi-walled carbon nanotubes on red spinach (Amaranthus tricolor L) and the role of ascorbic acid as an antioxidant. J Hazard Mater 243:212–222. https://doi.org/10.1016/j.jhazmat.2012.10.025
Slomberg DL, Schoenfisch MH (2012) Silica nanoparticle phytotoxicity to Arabidopsis thaliana. Environ Sci Technol 46:10247–10254. https://doi.org/10.1021/es300949f
Khan M, Khan MSA, Borah KK, Goswami Y, Hakeem KR, Chakrabartty I (2021) The potential exposure and hazards of metal-based nanoparticles on plants and environment, with special emphasis on ZnO NPs, TiO2 NPs, and AgNPs: a review. Environ Adv 6:100128. https://doi.org/10.1016/j.envadv.2021.100128
Dağhan H (2018) Effects of TiO2 nanoparticles on maize (Zea mays L.) growth, chlorophyll content, and nutrient uptake. Appl Ecol Environ Res 16:6873–6883. https://doi.org/10.15666/aeer/1605_68736883
Lin D, Xing B (2008) Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol 42:5580–5585. https://doi.org/10.1021/es800422x
Monica RC, Cremonini R (2009) Nanoparticles and higher plants. Caryologia 62:161–165. https://doi.org/10.1080/00087114.2004.10589681
Raskar S, Laware S (2014) Effect of zinc oxide nanoparticles on cytology and seed germination in onion. Int J Curr Microbiol App Sci 3:467–473. https://www.ijcmas.com/vol-3-2/S.V.Raskar%20and%20S.L.Laware.pdf
Li CC, Wang YJ, Dang F, Zhou DM (2016) Mechanistic understanding of reduced AgNP phytotoxicity induced by extracellular polymeric substances. J Hazard Mater 308:21–28. https://doi.org/10.1016/j.jhazmat.2016.01.036
Wang C, Jiang K, Wu B, Zhou J, Lv Y (2018) Silver nanoparticles with different particle sizes enhance the allelopathic effects of Canada goldenrod on the seed germination and seedling development of lettuce. Ecotoxicology 27:1116–1125. https://doi.org/10.1007/s10646-018-1966-9
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84:415–430. https://doi.org/10.1016/j.aquatox.2007.07.009
Huang CW, Li SW, Liao VH-C (2017) Chronic ZnO-NPs exposure at environmentally relevant concentrations results in metabolic and locomotive toxicities in Caenorhabditis elegans. Environ Pollut 220:1456–1464. https://doi.org/10.1016/j.envpol.2016.10.086
Bundschuh M, Filser J, Lüderwald S, McKee MS, Metreveli G, Schaumann GE, Schulz R, Wagner S (2018) Nanoparticles in the environment: where do we come from, where do we go to? Environ Sci Europe 30:1–17. https://doi.org/10.1186/s12302-018-0132-6
Labille J, Slomberg D, Catalano R, Robert S, Apers-Tremelo M-L, Boudenne J-L, Manasfi T, Radakovitch O (2020) Assessing UV filter inputs into beach waters during recreational activity: a field study of three French Mediterranean beaches from consumer survey to water analysis. Sci Total Environ 706:136010. https://doi.org/10.1016/j.scitotenv.2019.136010
Wang J, Zhu X, Zhang X, Zhao Z, Liu H, George R, Wilson-Rawls J, Chang Y, Chen Y (2011) Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemosphere 83:461–467. https://doi.org/10.1016/j.chemosphere.2010.12.069
Bodamyali T, Stevens CR, Blake DR, Winyard PG (2000) Reactive oxygen/nitrogen species and acute inflammation: a physiological process. In: Free Radicals and Inflammation. Springer, pp 11–16. https://doi.org/10.1007/978-3-0348-8482-2_2
Jakubczyk K, Dec K, Kałduńska J, Kawczuga D, Kochman J, Janda K (2020) Reactive oxygen species-sources, functions, oxidative damage. Pol Merkur Lekarski 48:124–127
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84. https://doi.org/10.1016/j.biocel.2006.07.001
Natarajan P, Tomich JM (2020) Understanding the influence of experimental factors on bio-interactions of nanoparticles: towards improving correlation between in vitro and in vivo studies. Arch Biochem Biophys 694:108592. https://doi.org/10.1016/j.abb.2020.108592
Sohal IS, Cho YK, O’Fallon KS, Gaines P, Demokritou P, Bello D (2018) Dissolution behavior and biodurability of ingested engineered nanomaterials in the gastrointestinal environment. ACS Nano 12:8115–8128. https://doi.org/10.1021/acsnano.8b02978
De Matteis V (2017) Exposure to inorganic nanoparticles: routes of entry, immune response, biodistribution and in vitro/in vivo toxicity evaluation. Toxics 5:29. https://doi.org/10.3390/toxics5040029
Yang L, Ji W, Mao M, Huang J-n (2020) An updated review on the properties, fabrication and application of hybrid-nanofluids along with their environmental effects. J Clean Prod 257:120408. https://doi.org/10.1016/j.jclepro.2020.120408
Hsin Y-H, Chen C-F, Huang S, Shih T-S, Lai P-S, Chueh PJ (2008) The apoptotic effect of nanosilver is mediated by a ROS-and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett 179:130–139. https://doi.org/10.1016/j.toxlet.2008.04.015
ATSDR (2014) Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological profile for silver. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=539&tid=97. Accessed 26 March 2014
Rezvani E, Rafferty A, McGuinness C, Kennedy J (2019) Adverse effects of nanosilver on human health and the environment. Acta Biomater 94:145–159. https://doi.org/10.1016/j.actbio.2019.05.042
Kiss B, Bíró T, Czifra G, Tóth BI, Kertész Z, Szikszai Z, Kiss ÁZ, Juhász I, Zouboulis CC, Hunyadi J (2008) Investigation of micronized titanium dioxide penetration in human skin xenografts and its effect on cellular functions of human skin-derived cells. Exp Dermatol 17:659–667. https://doi.org/10.1111/j.1600-0625.2007.00683.x
Lademann J, Weigmann H-J, Rickmeyer C, Barthelmes H, Schaefer H, Mueller G, Sterry W (1999) Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Skin Pharmacol Physiol 12:247–256. https://doi.org/10.1159/000066249
Bennat C, Müller-Goymann C (2000) Skin penetration and stabilization of formulations containing microfine titanium dioxide as physical UV filter. Int J Cosmet Sci 22:271–283. https://doi.org/10.1046/j.1467-2494.2000.00009.x
Mavon A, Miquel C, Lejeune O, Payre B, Moretto P (2007) In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen. Skin Pharmacol Physiol 20:10–20. https://doi.org/10.1159/000096167
Christensen FM, Johnston HJ, Stone V, Aitken RJ, Hankin S, Peters S, Aschberger K (2011) Nano-TiO2–feasibility and challenges for human health risk assessment based on open literature. Nanotoxicology 5:110–124. https://doi.org/10.3109/17435390.2010.504899
Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C (2004) Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 16:437–445. https://doi.org/10.1080/08958370490439597
Gutwein LG, Webster TJ (2004) Increased viable osteoblast density in the presence of nanophase compared to conventional alumina and titania particles. Biomaterials 25:4175–4183. https://doi.org/10.1016/j.biomaterials.2003.10.090
Pollard ZA, Karod M, Goldfarb JL (2021) Metal leaching from antimicrobial cloth face masks intended to slow the spread of COVID-19. Sci Rep 11:19216. https://doi.org/10.1038/s41598-021-98577-6
Mercer RR, Scabilloni JF, Wang L, Battelli LA, Antonini JM, Roberts JR, Qian Y, Sisler JD, Castranova V, Porter DW (2018) The fate of inhaled nanoparticles: detection and measurement by enhanced dark-field microscopy. Toxicol Pathol 46:28–46. https://doi.org/10.1177/0192623317732321
Coreas R, Cao X, DeLoid GM, Demokritou P, Zhong W (2020) Lipid and protein corona of food-grade TiO2 nanoparticles in simulated gastrointestinal digestion. NanoImpact 20:100272. https://doi.org/10.1016/j.impact.2020.100272
Baimanov D, Wang J, Zhang J, Liu K, Cong Y, Shi X, Zhang X, Li Y, Li X, Qiao R (2022) In situ analysis of nanoparticle soft corona and dynamic evolution. Nat Commun 13:5389. https://doi.org/10.1038/s41467-022-33044-y
Zhou H, McClements DJ (2022) Recent advances in the gastrointestinal fate of organic and inorganic nanoparticles in foods. Nanomaterials 12:1099. https://doi.org/10.3390/nano12071099
Li L, Huang J, Almutairi AW, Lan X, Zheng L, Lin Y, Chen L, Fu N, Lin Z, Abomohra AE-F (2021) Integrated approach for enhanced bio-oil recovery from disposed face masks through co-hydrothermal liquefaction with Spirulina platensis grown in wastewater. Biomass Conv Bioref 13:11109–11120. https://doi.org/10.1007/s13399-021-01891-2
Li S, Ding J, Zheng X, Sui Y (2021) Beach tourists behavior and beach management strategy under the ongoing prevention and control of the COVID-19 pandemic: a case study of Qingdao. China Ocean Coast Manage 215:105974. https://doi.org/10.1016/j.ocecoaman.2021.105974
Hatami T, Rakib MRJ, Madadi R, De-la-Torre GE, Idris AM (2022) Personal protective equipment (PPE) pollution in the Caspian Sea, the largest enclosed inland water body in the world. Sci Total Environ 824:153771. https://doi.org/10.1016/j.scitotenv.2022.153771
Lee G, Lee ME, Kim SS, Joh H-I, Lee S (2022) Efficient upcycling of polypropylene-based waste disposable masks into hard carbons for anodes in sodium ion batteries. J Ind Eng Chem 105:268–277. https://doi.org/10.1016/j.jiec.2021.09.026
Zhao X, Klemeš JJ, You F (2022) Energy and environmental sustainability of waste personal protective equipment (PPE) treatment under COVID-19. Renew Sustain Energy Rev 153:111786. https://doi.org/10.1016/j.rser.2021.111786
Aragaw TA, Mekonnen BA (2021) Current plastics pollution threats due to COVID-19 and its possible mitigation techniques: a waste-to-energy conversion via pyrolysis. Environ Syst Res 10. https://doi.org/10.1186/s40068-020-00217-x
Sani A, Cao C, Cui D (2021) Toxicity of gold nanoparticles (AuNPs): a review. Biochem Biophys Rep 26:100991. https://doi.org/10.1016/j.bbrep.2021.100991
Kim K-T, Truong L, Wehmas L, Tanguay RL (2013) Silver nanoparticle toxicity in the embryonic zebrafish is governed by particle dispersion and ionic environment. Nanotechnology 24:115101. https://doi.org/10.1093/toxsci/kft081
Park MV, Neigh AM, Vermeulen JP, de la Fonteyne LJ, Verharen HW, Briedé JJ, van Loveren H, de Jong WH (2011) The effect of particle size on the cytotoxicity, inflammation, developmental toxicity, and genotoxicity of silver nanoparticles. Biomaterials 32:9810–9817. https://doi.org/10.1016/j.biomaterials.2011.08.085
Renwick L, Brown D, Clouter A, Donaldson K (2004) Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med 61:442–447. https://doi.org/10.1136/oem.2003.008227
Sager TM, Kommineni C, Castranova V (2008) Pulmonary response to intratracheal instillation of ultrafine versus fine titanium dioxide: role of particle surface area. Part Fibre Toxicol 5:17. https://doi.org/10.1186/1743-8977-5-17
Hunt PR, Keltner Z, Gao X, Oldenburg SJ, Bushana P, Olejnik N, Sprando RL (2014) Bioactivity of nanosilver in Caenorhabditis elegans: effects of size, coat, and shape. Toxicol Rep 1:923–944. https://doi.org/10.1016/j.toxrep.2014.10.020
Nativo P, Prior IA, Brust M (2008) Uptake and intracellular fate of surface-modified gold nanoparticles. ACS Nano 2:1639–1644. https://doi.org/10.1021/nn800330a
Cruje C, Chithrani B (2015) Integration of peptides for enhanced uptake of pegylated gold nanoparticles. J Nanosci Nanotechnol 15:2125–2131. https://doi.org/10.1166/jnn.2015.10321
Zhang X-D, Wu D, Shen X, Liu P-X, Fan F-Y, Fan S-J (2012) In vivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials 33:4628–4638. https://doi.org/10.1016/j.biomaterials.2012.03.020
Pollard AJ, Bijker EM (2021) A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 21:83–100. https://doi.org/10.1038/s41577-020-00497-5
Riedel S (2005) Edward Jenner and the history of smallpox and vaccination. Baylor University Med Center Proc 2005. Taylor & Francis. https://doi.org/10.1080/08998280.2005.11928028
Nascimento I, Leite L (2012) Recombinant vaccines and the development of new vaccine strategies. Braz J Med Biol Res 45:1102–1111. https://pubmed.ncbi.nlm.nih.gov/22948379/, https://doi.org/10.1590/s0100-879x2012007500142
Lozano D, Larraga V, Vallet-Regí M, Manzano M (2023) An overview of the use of nanoparticles in vaccine development. Nanomaterials 13:1828. https://doi.org/10.3390/nano13121828
Delany I, Rappuoli R, De Gregorio E (2014) Vaccines for the 21st century. EMBO Mol Med 6:708–720. https://doi.org/10.1002/emmm.201403876
Kirtane AR, Verma M, Karandikar P, Furin J, Langer R, Traverso G (2021) Nanotechnology approaches for global infectious diseases. Nat Nanotechnol 16:369–384. https://doi.org/10.1038/s41565-021-00866-8
Gregory AE, Titball R, Williamson D (2013) Vaccine delivery using nanoparticles. Front Cell Infect Microbiol 3:13. https://doi.org/10.3389/fcimb.2013.00013
Lee J, Kim D, Byun J, Wu Y, Park J, Oh Y-K (2022) In vivo fate and intracellular trafficking of vaccine delivery systems. Adv Drug Deliv Rev 186:114325. https://doi.org/10.1016/j.addr.2022.114325
Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Du X, Hope MJ, Madden TD (2019) The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol 14:1084–1087. https://doi.org/10.1038/s41565-019-0591-y
Buschmann MD, Carrasco MJ, Alishetty S, Paige M, Alameh MG, Weissman D (2021) Nanomaterial delivery systems for mRNA vaccines. Vaccines 9:65. https://doi.org/10.3390/vaccines9010065
Evers MJ, Kulkarni JA, van der Meel R, Cullis PR, Vader P, Schiffelers RM (2018) State-of-the-art design and rapid-mixing production techniques of lipid nanoparticles for nucleic acid delivery. Small Methods 2:1700375. https://doi.org/10.1002/smtd.201700375
Hou X, Zaks T, Langer R, Dong Y (2021) Lipid nanoparticles for mRNA delivery. Nat Rev Mater 7:65. https://doi.org/10.1038/s41578-021-00400-1
Kulkarni JA, Witzigmann D, Thomson SB, Chen S, Leavitt BR, Cullis PR, van der Meel R (2020) The current landscape of nucleic acid therapeutics. Nat Nanotechnol 15:963–963. https://doi.org/10.1038/s41565-021-00898-0
Klauer AA, van Hoof A (2012) Degradation of mRNAs that lack a stop codon: a decade of nonstop progress. Wiley Interdiscip Rev RNA 3:649–660. https://doi.org/10.1002/wrna.1124
Pardi N, Hogan MJ, Porter FW, Weissman D (2018) mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov 17:261–279. https://doi.org/10.1038/nrd.2017.243
Kulkarni JA, Witzigmann D, Thomson SB, Chen S, Leavitt BR, Cullis PR, van der Meel R (2021) The current landscape of nucleic acid therapeutics. Nat Nanotechnol 16:630–643. https://doi.org/10.1038/s41565-021-00898-0
Bloom K, van den Berg F, Arbuthnot P (2021) Self-amplifying RNA vaccines for infectious diseases. 28:117–129. https://doi.org/10.1038/s41434-020-00204-y
Liu L, Wang P, Nair MS, Yu J, Rapp M, Wang Q, Luo Y, Chan JF-W, Sahi V, Figueroa A (2020) Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 584:450–456. https://doi.org/10.1038/s41586-020-2571-7
Gamelin FX, Baquet G, Berthoin S, Thevenet D, Nourry C, Nottin S, Bosquet L (2021) Scientific rationale for developing potent RBD-based vaccines targeting COVID-19. npj Vaccines 6:128. https://doi.org/10.1038/s41541-021-00393-6
European Medicines Agency (EMA). (2021). Comirnaty EPAR public assessment report. CHMP Committee for Medicinal Products for Human Use. https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf. Accessed 19 February 2021
Selmin F, Musazzi UM, Franzè S, Scarpa E, Rizzello L, Procacci P, Minghetti P (2021) Pre-drawn syringes of Comirnaty for an efficient COVID-19 mass vaccination: demonstration of stability. Pharmaceutics 13:1029. https://doi.org/10.3390/pharmaceutics13071029
Gruber MF (2020) Emergency use authorization (EUA) for an unapproved product review memorandum, in FDA.gov. https://www.fda.gov/media/144416/download. Accessed 20 November 2020
Dolgin E (2020) COVID-19 vaccines poised for launch, but impact on pandemic unclear. Nat Biotechnol. https://doi.org/10.1038/d41587-020-00022-y.Accessed25November2020
Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ, Laska ME, Smith M, Almarsson Ö, Thompson J (2017) Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther 25:1316–1327. https://doi.org/10.1016/j.ymthe.2017.03.035
Reuben RC, Adogo LY (2021) SARS-CoV-2 vaccines–induced thrombotic thrombocytopenia: should we consider immuno-hypersensitivity? Rev Saude Publica 55:70. https://doi.org/10.11606/s1518-8787.2021055003855
Winter E, Pizzol CD, Locatelli C, Crezkynski-Pasa TB (2016) Development and evaluation of lipid nanoparticles for drug delivery: study of toxicity in vitro and in vivo. J Nanosci Nanotechnol 16:1321–1330. https://doi.org/10.1166/jnn.2016.11667
Ng KK, Lovell JF, Zheng G (2011) Lipoprotein-inspired nanoparticles for cancer theranostics. Acc Chem Res 44:1105–1113. https://doi.org/10.1021/ar200017e
Sellaturay P, Nasser S, Islam S, Gurugama P, Ewan PW (2021) Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy 51:861. https://doi.org/10.1111/cea.13874
Banerji A, Wickner PG, Saff R, Stone CA Jr, Robinson LB, Long AA, Wolfson AR, Williams P, Khan DA, Phillips E (2021) mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract 9:1423–1437. https://doi.org/10.1016/j.jaip.2020.12.047
Ulbricht J, Jordan R, Luxenhofer R (2014) On the biodegradability of polyethylene glycol, polypeptoids, and poly (2-oxazoline)s. Biomaterials 35:4848–4861. https://doi.org/10.1016/j.biomaterials.2014.02.029
Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, Rosser EC, Webb K, Deakin CT (2020) Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ICU admission. Nat Commun 11:6317. https://doi.org/10.1038/s41467-020-19741-6
Saifer MG, Williams LD, Sobczyk MA, Michaels SJ, Sherman MR (2014) Selectivity of binding of PEGs and PEG-like oligomers to anti-PEG antibodies induced by methoxypolyethylene glycol-proteins. Mol Immunol 57:236–246. https://doi.org/10.1016/j.molimm.2013.07.014
Yang Q, Lai SK (2015) Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7:655–677. https://doi.org/10.1002/wnan.1339
Zhang P, Sun F, Liu S, Jiang S (2016) Anti-PEG antibodies in the clinic: current issues and beyond PEGylation. J Control Release 244:184–193. https://doi.org/10.1016/j.jconrel.2016.06.040
Zhou Z-H, Stone CA, Jakubovic B, Phillips EJ, Sussman G, Park J, Hoang U, Kirshner SL, Levin R, Kozlowski S (2021) Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract 9:1731-1733.e3. https://doi.org/10.1016/j.jaip.2020.11.011
Stone CA Jr, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, Hemler JA, Phillips EJ (2019) Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract 7:1533-1540.e8. https://doi.org/10.1016/j.jaip.2018.12.003
Hershfield MS, Ganson NJ, Kelly SJ, Scarlett EL, Jaggers DA, Sundy JS (2014) Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res Ther 16:R63. https://doi.org/10.1186/ar4500
Market (2017) Pegloticase: withdrawal of its European marketing authorisation is welcome. Prescrire Int 26:71. https://www.ncbi.nlm.nih.gov/pubmed/30730621, https://www.ema.europa.eu/en/documents/public-statement/public-statement-krystexxa-withdrawal-marketing-authorisation-european-union_en.pdf
Vrieze J (2020) Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Scienceinsider. https://doi.org/10.1126/science.abg2359, https://www.science.org/content/article/suspicions-grow-nanoparticles-pfizer-s-covid-19-vaccine-trigger-rare-allergic-reactions. Accessed 21 Dec 2020
Coors EA, Seybold H, Merk HF, Mahler V (2005) Polysorbate 80 in medical products and non-immunologic anaphylactoid reactions. Ann Allergy Asthma Immunol 95:593–599. https://doi.org/10.1016/S1081-1206(10)61024-1
Carpenter T, Konig J, Hochfelder J, Siegel S, Gans M (2022) Polyethylene glycol and polysorbate testing in 12 patients before or after coronavirus disease 2019 vaccine administration. Ann Allergy Asthma Immunol 128:99–101. https://doi.org/10.1016/j.anai.2021.10.009
Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B (2021) Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med 384:2187–2201. https://doi.org/10.1056/NEJMoa2101544
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384:403–416. https://doi.org/10.1056/NEJMoa2035389
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Marc GP, Moreira ED, Zerbini C (2020) Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. https://doi.org/10.1056/NEJMoa2034577
Schwede K, Simon JC, Treudler R (2019) A case of severe immediate type reaction to macrogol and polysorbate 60 after intravaginal drug application. Eur J Dermatol 29:329–331. https://doi.org/10.1684/ejd.2019.3541
Wenande E, Garvey L (2016) Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy 46:907–922. https://doi.org/10.1111/cea.12760
Rajpurkar M, O’Brien SH, Haamid FW, Cooper DL, Gunawardena S, Chitlur M (2016) Heavy menstrual bleeding as a common presenting symptom of rare platelet disorders: illustrative case examples. J Pediatr Adolesc Gynecol 29:537–541. https://doi.org/10.1016/j.jpag.2016.02.002
Soltani Hekmat A, Javanmardi K (2021) Possible risk of thrombotic events following Oxford-AstraZeneca COVID-19 vaccination in women receiving estrogen. BioMed Res Int 2021:7702863. https://doi.org/10.1155/2021/7702863
Hunter PR (2021) Thrombosis after COVID-19 vaccination. BMJ 373:n958. https://doi.org/10.1136/bmj.n958
Alghamdi AN, Alotaibi MI, Alqahtani AS, Al Aboud D, Abdel-Moneim AS (2021) BNT162b2 and ChAdOx1 SARS-CoV-2 post-vaccination side-effects among Saudi vaccinees. Front Med 8:1796. https://doi.org/10.3389/fmed.2021.760047
GOV.UK (2022) Coronavirus vaccine - summary of yellow card reporting in vigilance, safety alerts and guidance. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting. Updated 8 March 2023
Rela M, Jothimani D, Vij M, Rajakumar A, Rammohan A (2021) Auto-immune hepatitis following COVID vaccination. J Autoimmun 123:102688. https://doi.org/10.1016/j.jaut.2021.102688
Patrizio A, Ferrari SM, Antonelli A, Fallahi P (2021) A case of Graves’ disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J Autoimmun 125:102738. https://doi.org/10.1016/j.jaut.2021.102738
Zavala-Miranda MF, González-Ibarra SG, Pérez-Arias AA, Uribe-Uribe NO, Mejia-Vilet JM (2021) New-onset systemic lupus erythematosus beginning as class V lupus nephritis after COVID-19 vaccination. Kidney Int 100:1340–1341. https://doi.org/10.1016/j.kint.2021.09.009
Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS (2021) Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus 13:e13426. https://doi.org/10.7759/cureus.13426
Abramson M, Yu SM-W, Campbell KN, Chung M, Salem F (2021) IgA nephropathy after SARS-CoV-2 vaccination. Kidney Med 3:860–863. https://doi.org/10.1016/j.xkme.2021.05.002
Jara LJ, Vera-Lastra O, Mahroum N, Pineda C, Shoenfeld Y (2022) Autoimmune post-COVID vaccine syndromes: does the spectrum of autoimmune/inflammatory syndrome expand? Clin Rheumatol 41:1603–1609. https://doi.org/10.1007/s10067-022-06149-4
Aldén M, Olofsson Falla F, Yang D, Barghouth M, Luan C, Rasmussen M, De Marinis Y (2022) Intracellular reverse transcription of Pfizer-BioNTech COVID-19 mRNA vaccine BNT162b2 in vitro in human liver cell line. Curr Issues Mol Biol 44:1115–1126. https://doi.org/10.3390/cimb44030073
Merchant HA (2022) Comment on Aldén et al. Intracellular reverse transcription of Pfizer-BioNTech COVID-19 mRNA vaccine BNT162b2 in vitro in human liver cell line. Curr Issues Mol Biol 44:1661–1663. https://doi.org/10.3390/cimb44040113
Rijkers GT, van Overveld FJ (2021) The “original antigenic sin” and its relevance for SARS-CoV-2 (COVID-19) vaccination. Clin Immunol Commun 1:13–16. https://doi.org/10.1016/j.clicom.2021.10.001
Hoepel W, Chen H-J, Geyer CE, Allahverdiyeva S, Manz XD, de Taeye SW, Aman J, Mes L, Steenhuis M, Griffith GR (2021) High titers and low fucosylation of early human anti-SARS-CoV-2 IgG promote inflammation by alveolar macrophages. Sci Transl Med 13:eabf8654. https://doi.org/10.1126/scitranslmed.abf8654
Kurdoğlu Z (2021) Do the COVID-19 vaccines cause menstrual irregularities? Int J Womens Health Reprod Sci 9:158–159. https://doi.org/10.15296/ijwhr.2021.29
Bozkurt B, Kamat I, Hotez PJ (2021) Myocarditis with COVID-19 mRNA vaccines. Circulation 144:471–484. https://doi.org/10.1161/CIRCULATIONAHA.121.056135
Seephetdee C, Bhukhai K, Buasri N, Leelukkanaveera P, Lerdwattanasombat P, Manopwisedjaroen S, Phueakphud N, Kuhaudomlarp S, Saphire EO, Thitithanyanont A (2022) Broad neutralization of SARS-CoV-2 variants by circular mRNA producing vFlip-X spike in mice. https://doi.org/10.1101/2022.03.17.484759
ClinicalTrials.gov (2021) SARS-CoV-2-Spike-Ferritin-Nanoparticle (SPFN) vaccine with ALFQ adjuvant for prevention of COVID-19 in healthy adults. (NCT04784767). https://clinicaltrials.gov/ct2/show/NCT04784767
Joh DY, Zimmers Z, Avlani M, Heggestad JT, Aydin HB, Ganson N, Kumar S, Fontes CM, Achar RK, Hershfield MS (2019) Architectural modification of conformal PEG-bottlebrush coatings minimizes anti-PEG antigenicity while preserving stealth properties. Adv Healthc Mater 8:1801177. https://doi.org/10.1002/adhm.201801177
Friedl JD, Nele V, De Rosa G, Bernkop-Schnürch A (2021) Bioinert, stealth, or interactive: how surface chemistry of nanocarriers determines their fate in vivo. Adv Funct Mater 31:2103347. https://doi.org/10.1002/adfm.202103347
Han X, Lu Y, Xie J, Zhang E, Zhu H, Du H, Wang K, Song B, Yang C, Shi Y (2020) Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nat Nanotechnol 15:605–614. https://doi.org/10.1038/s41565-020-0693-6
Xue W, Liu Y, Zhang N, Yao Y, Ma P, Wen H, Huang S, Luo Y, Fan H (2018) Effects of core size and peg coating layer of iron oxide nanoparticles on the distribution and metabolism in mice. Int J Nanomedicine 13:5719–5728. https://doi.org/10.2147/IJN.S165451
Yao X, Qi C, Sun C, Huo F, Jiang X (2023) Poly(ethylene glycol) alternatives in biomedical applications. Nano Today 48:101738. https://doi.org/10.1016/j.nantod.2022.101738
Patente TA, Pinho MP, Oliveira AA, Evangelista GC, Bergami-Santos PC, Barbuto JA (2019) Human dendritic cells: their heterogeneity and clinical application potential in cancer immunotherapy. Front Immunol 9:3176. https://doi.org/10.3389/fimmu.2018.03176
Tezera LB, Bielecka MK, Chancellor A, Reichmann MT, Shammari BA, Brace P, Batty A, Tocheva A, Jogai S, Marshall BG (2017) Dissection of the host-pathogen interaction in human tuberculosis using a bioengineered 3-dimensional model. Elife 6:e21283. https://doi.org/10.7554/eLife.21283
Geisbert TW, Pushko P, Anderson K, Smith J, Davis KJ, Jahrling PB (2002) Evaluation in nonhuman primates of vaccines against Ebola virus. Emerg Infect Dis 8:503–507. https://doi.org/10.3201/eid0805.010284
Vidal Oribe I, Venturini Díaz M, Hernández Alfonso P, del Pozo Gil M, González Mahave I, Lobera Labairu T (2022) Tolerance of SARS-CoV-2 vaccines with polyethylene glycol in allergic patients to polysorbate 80. J Investig Allergol Clin Immunol 32:403–405. https://doi.org/10.18176/jiaci.0772
Broyles AD, Banerji A, Barmettler S, Biggs CM, Blumenthal K, Brennan PJ, Breslow RG, Brockow K, Buchheit KM, Cahill KN (2020) Practical guidance for the evaluation and management of drug hypersensitivity: specific drugs. J Allergy Clin Immunol Pract 8:S16–S116. https://doi.org/10.1016/j.jaip.2020.08.002
Greenhawt M, Shaker M, Golden DB (2021) Peg/polysorbate skin testing has no utility in the assessment of suspected allergic reactions to SARS-CoV-2 vaccines. J Allergy Clin Immunol Pract 9:3321–3322. https://doi.org/10.1016/j.jaip.2021.06.025
Pitlick MM, Gonzalez-Estrada A, Park MA (2022) Graded COVID-19 vaccine administration: a safe alternative to vaccine avoidance. Ann Allergy Asthma Immunol 128:731–733. https://doi.org/10.1016/j.anai.2022.02.024
Liu X, Shaw RH, Stuart AS, Greenland M, Aley PK, Andrews NJ, Cameron JC, Charlton S, Clutterbuck EA, Collins AM (2021) Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral-vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomized, non-inferiority trial. Lancet 398:856–869. https://doi.org/10.1016/S0140-6736(21)01694-9
Yii A, Tay TR, Choo X, Koh M, Tee A, Wang DY (2018) Precision medicine in united airways disease: a “treatable traits” approach. Allergy 73:1964–1978. https://doi.org/10.1111/all.13496
Łoczechin A, Séron K, Barras A, Giovanelli E, Belouzard S, Chen Y-T, Metzler-Nolte N, Boukherroub R, Dubuisson J, Szunerits S (2019) Functional carbon quantum dots as medical countermeasures to human coronavirus. ACS Appl Mater Interfaces 11:42964–42974. https://doi.org/10.1021/acsami.9b15032
Huang X, Li M, Xu Y, Zhang J, Meng X, An X, Sun L, Guo L, Shan X, Ge J (2019) Novel gold nanorod-based HR1 peptide inhibitor for Middle East respiratory syndrome coronavirus. ACS Appl Mater Interfaces 11:19799–19807. https://doi.org/10.1021/acsami.9b04240
Neufurth M, Wang X, Tolba E, Lieberwirth I, Wang S, Schröder HC, Müller WE (2020) The inorganic polymer, polyphosphate, blocks binding of SARS-CoV-2 spike protein to ACE2 receptor at physiological concentrations. Biochem Pharmacol 182:114215. https://doi.org/10.1016/j.bcp.2020.114215
Tang H, Qin H, He S, Li Q, Xu H, Sun M, Li J, Lu S, Luo S, Mao P (2023) Anti-coronaviral nanocluster restrain infections of SARS-CoV-2 and associated mutants through virucidal inhibition and 3CL protease inactivation. Adv Sci 10:2207098. https://doi.org/10.1002/advs.202207098
Gattani V, Dawre S (2023) Development of favipiravir loaded PLGA nanoparticles entrapped in in-situ gel for treatment of COVID-19 via nasal route. J Drug Deliv Sci Technol 79:104082. https://doi.org/10.1016/j.jddst.2022.104082
Idris A, Davis A, Supramaniam A, Acharya D, Kelly G, Tayyar Y, West N, Zhang P, McMillan CL, Soemardy C (2021) A SARS-CoV-2 targeted siRNA-nanoparticle therapy for COVID-19. Mol Ther 29:2219–2226. https://doi.org/10.1016/j.ymthe.2021.05.004
Nadworny PL, Hickerson L, Holley-Harrison HD, Bloom DC, Grams TR, Edwards TG, Schultz GS, Burrell RE (2023) Treatment of infection and inflammation associated with COVID-19, multi-drug resistant pneumonia and fungal sinusitis by nebulizing a nanosilver solution. Nanomed Nanotechnol Biol Med 48:102654. https://doi.org/10.1016/j.nano.2023.102654
Saify Nabiabad H, Amini M, Demirdas S (2022) Specific delivering of RNAi using spike’s aptamer-functionalized lipid nanoparticles for targeting SARS-CoV-2: a strong anti-COVID drug in a clinical case study. Chem Biol Drug Des 99:233–246. https://doi.org/10.1111/cbdd.13978
Pokhrel LR, Williams F, Cook PP, O’Rourke D, Murray G, Akula SM (2022) Preclinical efficacy and safety of novel SNAT against SARS-CoV-2 using a hamster model. Drug Delivery Transl Res 12:3007–3016. https://doi.org/10.1007/s13346-022-01166-x
Zhou Y, Jiang X, Tong T, Fang L, Wu Y, Liang J, Xiao S (2020) High antiviral activity of mercaptoethane sulfonate functionalized Te/BSA nanostars against arterivirus and coronavirus. RSC Adv 10:14161–14169. https://doi.org/10.1039/D0RA01387K
Du T, Zhang J, Li C, Song T, Li P, Liu J, Du X, Wang S (2020) Gold/silver hybrid nanoparticles with enduring inhibition of coronavirus multiplication through multisite mechanisms. Bioconjug Chem 31:2553–2563. https://doi.org/10.1021/acs.bioconjchem.0c00506
Du T, Liang J, Dong N, Lu J, Fu Y, Fang L, Xiao S, Han H (2018) Glutathione-capped Ag2S nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Appl Mater Interfaces 10:4369–4378. https://doi.org/10.1021/acsami.7b13811
Hu C-MJ, Chang W-S, Fang Z-S, Chen Y-T, Wang W-L, Tsai H-H, Chueh L-L, Takano T, Hohdatsu T, Chen H-W (2017) Nanoparticulate vacuolar ATPase blocker exhibits potent host-targeted antiviral activity against feline coronavirus. Sci Rep 7:1343. https://doi.org/10.1038/s41598-017-13316-0
Jensen DMK, Cun D, Maltesen MJ, Frøkjaer S, Nielsen HM, Foged C (2010) Spray drying of siRNA-containing PLGA nanoparticles intended for inhalation. J Control Release 142:138–145. https://doi.org/10.1016/j.jconrel.2009.10.010
Lv X, Wang P, Bai R, Cong Y, Suo S, Ren X, Chen C (2014) Inhibitory effect of silver nanomaterials on transmissible virus-induced host cell infections. Biomaterials 35:4195–4203. https://doi.org/10.1016/j.biomaterials.2014.01.054
Valizadeh H, Abdolmohammadi-Vahid S, Danshina S, Gencer MZ, Ammari A, Sadeghi A, Roshangar L, Aslani S, Esmaeilzadeh A, Ghaebi M (2020) Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int Immunopharmacol 89:107088. https://doi.org/10.1016/j.intimp.2020.107088
Park HH, Park W, Lee YY, Kim H, Seo HS, Choi DW, Kwon HK, Na DH, Kim TH, Choy YB (2020) Bioinspired DNase-I-coated melanin-like nanospheres for modulation of infection-associated NETosis dysregulation. Adv Sci 7:2001940. https://doi.org/10.1002/advs.202001940
Lee YY, Park HH, Park W, Kim H, Jang JG, Hong KS, Lee J-Y, Seo HS, Na DH, Kim TH, Choy YB (2021) Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm. Biomaterials 267:120389. https://doi.org/10.1016/j.biomaterials.2020.120389
Morad R, Akbari M, Rezaee P, Koochaki A, Maaza M, Jamshidi Z (2021) First principle simulation of coated hydroxychloroquine on Ag, Au, and Pt nanoparticles. Sci Rep 11:1–9. https://doi.org/10.1038/s41598-021-81617-6
Niemiec S, Hilton S, Wallbank A, Azeltine-Bannerman M, Louiselle A, Elajaili H, Allawzi A, Xu J, Mattson C, Dewberry L (2020) Cerium oxide nanoparticles conjugated to anti-inflammatory microRNA-146a prevent bleomycin-induced acute lung injury. Nanomed Nanotechnol Biol Med 34:102388. https://doi.org/10.1016/j.nano.2021.102388
Wang G, Gaikwad H, McCarthy M, Gonzalez-Juarrero M, Li Y, Armstrong M, Reisdorph N, Morrison T, Simberg D (2021) Lipid nanoparticle formulation of niclosamide (Nano NCM) effectively inhibits SARS-CoV-2 replication in vitro. Precis Nanomed 4:724–737. https://doi.org/10.33218/001c.18813
Zhang Q, Honko A, Zhou J, Gong H, Downs SN, Vasquez JH, Fang RH, Gao W, Griffiths A, Zhang L (2020) Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett 20:5570–5574. https://doi.org/10.1021/acs.nanolett.0c02278
Starpharma (2020) SPL creates slow-release soluble DEP® remdesivir nanoparticle. https://starpharma.com/assets/asxannouncements/200901%20SPL%20creates%20slow%20release%20soluble%20DEP%20remdesivir%20nanoparticle.pdf
Khaitov M, Nikonova A, Shilovskiy I, Kozhikhova K, Kofiadi I, Vishnyakova L, Nikolskii A, Gattinger P, Kovchina V, Barvinskaia E (2021) Silencing of SARS-CoV-2 with modified siRNA-peptide dendrimer formulation. Allergy 76:2840–2854. https://doi.org/10.1111/all.14850
ClinicalTrials.gov (2021) Treatment of patients with mild coronavirus-19 (COVID-19) disease with methotrexate associated to LDL like nanoparticles (Nano-COVID19). (NCT04610567). https://clinicaltrials.gov/ct2/show/NCT04610567?term=nanoparticle&recrs=adeh&cond=COVID-19&draw=2&rank=5
ClinicalTrials.gov (2020) Efficacy and safety of MTX-loaded nanoparticles to treat severe COVID-19 patients. (NCT04352465). https://clinicaltrials.gov/ct2/show/study/NCT04352465?term=nanoparticle&recrs=abdeh&cond=COVID-19&draw=2&rank=1
ClinicalTrials.gov (2021) Colloidal silver, treatment of COVID-19. (NCT04978025). https://clinicaltrials.gov/ct2/show/NCT04978025?term=nanoparticle&recrs=abdeh&cond=COVID-19&draw=3&rank=19
Gambichler T, Hamdani N, Budde H, Sieme M, Skrygan M, Scholl L, Dickel H, Behle B, Ganjuur N, Scheel C (2021) Bullous pemphigoid after SARS-CoV-2 vaccination: spike protein-directed immunofluorescence confocal microscopy and T cell receptor studies. Br J Dermatol 186:1012–1013. https://doi.org/10.1111/bjd.20890
Cemşitoğlu N, Adışen E, Erdem Ö (2022) Lichen planus after CoronaVac: a rare complication of vaccines. J Eur Acad Dermatol Venereol 36:e202–e204. https://doi.org/10.1111/jdv.17906
Alghamdi FA, Khayyat ST, Alshareef MM, Wa F (2022) New-onset lichen planusiInduced by the Pfizer COVID-19 vaccine. Case reports in dermatological medicine New-onset cutaneous lichen planus triggered by COVID-19 vaccination. Case Rep Dermatol Med 2022:2082445. https://doi.org/10.1155/2022/2082445
Troeltzsch M, Berndt R (2021) Comment on “oral lichen planus following the administration of vector-based COVID-19 vaccine (Ad26. Cov2. S).” Authors’ reply Oral Dis 27:1327–1328. https://doi.org/10.1111/odi.14060
Kha C, Itkin A (2021) New-onset chilblains in close temporal association with mRNA-1273 (Moderna) vaccination. JAAD Case Reports 8:66–67. https://doi.org/10.1016/j.jdcr.2021.03.046
Toom S, Wolf B, Avula A, Peeke S, Becker K (2021) Familial thrombocytopenia flare-up following the first dose of mRNA-1273 COVID-19 vaccine. Am J Hematol 96:E107–E108. https://doi.org/10.1002/Fajh.26128
McMahon DE, Kovarik CL, Damsky W, Rosenbach M, Lipoff JB, Tyagi A, Chamberlin G, Fathy R, Nazarian RM, Desai SR (2022) Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol 86:113–121. https://doi.org/10.1016/j.jaad.2021.09.002
Ackerman M, Henry D, Finon A, Binois R, Esteve E (2021) Persistent maculopapular rash after the first dose of Pfizer-BioNTech COVID-19 vaccine. J Eur Acad Dermatol Venereol 35:e430–e432. https://doi.org/10.1111/jdv.17248
Cyrenne B, Al-Mohammedi F, DeKoven J, Alhusayen R (2021) Pityriasis rosea-like eruptions following vaccination with BNT162b2 mRNA COVID-19 vaccine. J Eur Acad Dermatol Venereol 35:e485–e487. https://doi.org/10.1111/jdv.17342
Balingit A (2023) What is COVID arm? Healthline.com. https://www.healthline.com/health/adult-vaccines/covid-arm
Cirillo N (2021) Reported orofacial adverse effects of COVID-19 vaccines: the knowns and the unknowns. J Oral Pathol Med 50:424–427. https://doi.org/10.1111/jop.13165
Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O (2021) Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford) 60:e8–e9. https://doi.org/10.1093/rheumatology/keab345
Patlolla AK, Hackett D, Tchounwou PB (2015) Genotoxicity study of silver nanoparticles in bone marrow cells of Sprague-Dawley rats. Food Chem Toxicol 85:52–60. https://doi.org/10.1016/j.fct.2015.05.005
Yin N, Liu Q, Liu J, He B, Cui L, Li Z, Yun Z, Qu G, Liu S, Zhou Q (2013) Silver nanoparticle exposure attenuates the viability of rat cerebellum granule cells through apoptosis coupled to oxidative stress. Small 9:1831–1841. https://doi.org/10.1002/smll.201202732
Ziemińska E, Stafiej A, Strużyńska L (2014) The role of the glutamatergic NMDA receptor in nanosilver-evoked neurotoxicity in primary cultures of cerebellar granule cells. Toxicology 315:38–48. https://doi.org/10.1016/j.tox.2013.11.008
Sung JH, Ji JH, Park JD, Yoon JU, Kim DS, Jeon KS, Song MY, Jeong J, Han BS, Han JH (2009) Subchronic inhalation toxicity of silver nanoparticles. Toxicol Sci 108:452–461. https://doi.org/10.1186/1743-8977-7-20
Jia J, Li F, Zhou H, Bai Y, Liu S, Jiang Y, Jiang G, Yan B (2017) Oral exposure to silver nanoparticles or silver ions may aggravate fatty liver disease in overweight mice. Environ Sci Technol 51:9334–9343. https://doi.org/10.1021/acs.est.7b02752
Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, Choi BS, Lim R, Chang HK, Chung YH (2008) Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol 20:575–583. https://doi.org/10.1080/08958370701874663
Van der Zande M, Vandebriel RJ, Van Doren E, Kramer E, Herrera Rivera Z, Serrano-Rojero CS, Gremmer ER, Mast J, Peters RJ, Hollman PC (2012) Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 6:7427–7442. https://doi.org/10.1021/nn302649p
Trickler WJ, Lantz SM, Murdock RC, Schrand AM, Robinson BL, Newport GD, Schlager JJ, Oldenburg SJ, Paule MG, Slikker W Jr (2010) Silver nanoparticle-induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol Sci 118:160–170. https://doi.org/10.1093/toxsci/kfq244
Juling S, Böhmert L, Lichtenstein D, Oberemm A, Creutzenberg O, Thünemann AF, Braeuning A, Lampen A (2018) Comparative proteomic analysis of hepatic effects induced by nanosilver, silver ions, and nanoparticle coating in rats. Food Chem Toxicol 113:255–266. https://doi.org/10.1016/j.fct.2018.01.056
Zhurkov V, Savostikova O, Yurchenko V, Krivtsova E, Kovalenko M, Murav’eva L, Alekseeva A, Belyaeva N, Mikhailova R, Sycheva L (2017) Features of the mutagenic and cytotoxic effects of nanosilver and silver sulfate in mice. Nanotechnol 12:667–672. https://doi.org/10.1134/S1995078017060143
Pratsinis A, Hervella P, Leroux JC, Pratsinis SE, Sotiriou GA (2013) Toxicity of silver nanoparticles in macrophages. Small 9:2576–2584. https://doi.org/10.1002/smll.201202120
Pang S, Gao Y, Wang F, Wang Y, Cao M, Zhang W, Liang Y, Song M, Jiang G (2020) Toxicity of silver nanoparticles on wound healing: a case study of zebrafish fin regeneration model. Sci Total Environ 717:137178. https://doi.org/10.1016/j.scitotenv.2020.137178
Wen H, Dan M, Yang Y, Lyu J, Shao A, Cheng X, Chen L, Xu L (2017) Acute toxicity and genotoxicity of silver nanoparticle in rats. Plos One 12:e0185554. https://doi.org/10.1371/Fjournal.pone.0185554
Lu T, Qu Q, Lavoie M, Pan X, Peijnenburg W, Zhou Z, Pan X, Cai Z, Qian H (2020) Insights into the transcriptional responses of a microbial community to silver nanoparticles in a freshwater microcosm. Environ Pollut 258:113727. https://doi.org/10.1016/j.envpol.2019.113727
Sung JH, Ji JH, Yoon JU, Kim DS, Song Y, Jeong J, Han S, Han JH, Chung YH, Kim J (2008) Lung function changes in Sprague-Dawley rats after prolonged inhalation exposure to silver nanoparticles. Inhalation Toxicol 20:567–574. https://doi.org/10.1080/08958370701874671
Lee S-H, Hwang SH, Park JS, Park H-S, Shin YS (2016) Anaphylaxis to polyethylene glycol (Colyte®) in a patient with diverticulitis. J Korean Med Sci 31:1662–1663. https://doi.org/10.3346/jkms.2016.31.10.1662
Pidaparti M, Bostrom B (2012) Comparison of allergic reactions to Pegasparaginase given intravenously versus intramuscularly. Pediatr Pediatr Blood Cancer 59:436–439. https://doi.org/10.1002/pbc.23380
Moreno A, Pitoc GA, Ganson NJ, Layzer JM, Hershfield MS, Tarantal AF, Sullenger BA (2019) Anti-PEG antibodies inhibit the anticoagulant activity of pegylated aptamers. Cell Chem Biol 26:634-644.e3. https://doi.org/10.1016/j.chembiol.2019.02.001
Sung JH, Ji JH, Park JD, Song MY, Song KS, Ryu HR, Yoon JU, Jeon KS, Jeong J, Han BS (2011) Subchronic inhalation toxicity of gold nanoparticles. Part Fibre Toxicol 8:1–18. https://doi.org/10.1186/1743-8977-8-16
Kim K-T, Zaikova T, Hutchison JE, Tanguay RL (2013) Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol Sci 133:275–288. https://doi.org/10.1093/toxsci/kft081
Hext PM, Tomenson JA, Thompson P (2005) Titanium dioxide: Inhalation toxicology and epidemiology. Ann Occup Hyg 49:461–472. https://doi.org/10.1093/annhyg/mei012
Zhang L, Bai R, Li B, Ge C, Du J, Liu Y, Le Guyader L, Zhao Y, Wu Y, He S (2011) Rutile TiO2 particles exert size and surface coating dependent retention and lesions on the murine brain. Toxicol Lett 207:73–81. https://doi.org/10.1016/j.toxlet.2011.08.001
Wang J, Li Y, Li W, Chen C, Li B, Zhao Y (2008) Biological effect of intranasally instilled titanium dioxide nanoparticles on female mice. NANO 3:279–285. https://doi.org/10.1142/S1793292008001325
Mohammadipour A, Fazel A, Haghir H, Motejaded F, Rafatpanah H, Zabihi H, Hosseini M, Bideskan AE (2014) Maternal exposure to titanium dioxide nanoparticles during pregnancy; impaired memory and decreased hippocampal cell proliferation in rat offspring. Environ Toxicol Pharmacol 37:617–625. https://doi.org/10.1016/j.etap.2014.01.014
Hong F, Ji L, Zhou Y, Wang L (2017) Retracted: pulmonary fibrosis of mice and its molecular mechanism following chronic inhaled exposure to TiO2 nanoparticles. Environ Toxicol 33:711. https://doi.org/10.1002/TOX.22493
Chen J, Dong X, Zhao J, Tang G (2009) In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitoneal injection. J Appl Toxicol 29:330–337. https://doi.org/10.1002/jat.1414
Acknowledgements
The authors thank Dr. Jianfeng Jin and Prof. Jenneke Klein-Nulend for their outstanding help in editing the manuscript. Also, we thank David Alexander for his careful reading of the manuscript.
Author information
Authors and Affiliations
Contributions
B. Zandieh-Doulabi had the idea for the article. F. Araste performed the literature search and data analysis. F. Araste and B. Zandieh-Doulabi drafted the work, and all authors critically revised it.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Araste, F., Bakker, A.D. & Zandieh-Doulabi, B. Potential and risks of nanotechnology applications in COVID-19-related strategies for pandemic control. J Nanopart Res 25, 229 (2023). https://doi.org/10.1007/s11051-023-05867-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05867-3