Abstract
Nanoparticles serve various industrial and domestic purposes which is reflected in their steadily increasing production volume. This economic success comes along with their presence in the environment and the risk of potentially adverse effects in natural systems. Over the last decade, substantial progress regarding the understanding of sources, fate, and effects of nanoparticles has been made. Predictions of environmental concentrations based on modelling approaches could recently be confirmed by measured concentrations in the field. Nonetheless, analytical techniques are, as covered elsewhere, still under development to more efficiently and reliably characterize and quantify nanoparticles, as well as to detect them in complex environmental matrixes. Simultaneously, the effects of nanoparticles on aquatic and terrestrial systems have received increasing attention. While the debate on the relevance of nanoparticle-released metal ions for their toxicity is still ongoing, it is a re-occurring phenomenon that inert nanoparticles are able to interact with biota through physical pathways such as biological surface coating. This among others interferes with the growth and behaviour of exposed organisms. Moreover, co-occurring contaminants interact with nanoparticles. There is multiple evidence suggesting nanoparticles as a sink for organic and inorganic co-contaminants. On the other hand, in the presence of nanoparticles, repeatedly an elevated effect on the test species induced by the co-contaminants has been reported. In this paper, we highlight recent achievements in the field of nano-ecotoxicology in both aquatic and terrestrial systems but also refer to substantial gaps that require further attention in the future.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Nano-based technology has made enormous progress over the last decades, which is underpinned by a 25-fold increase between 2005 and 2010 in the numbers of products that either contain or require nanoparticles (NP) for their production [1]. This development is likely facilitated by their unique general properties (in particular particle size, surface area, surface reactivity, charge, and shape) relative to their bulk or dissolved counterparts. This enables a broad range of possible applications, including cosmetic, pharmaceutical, and medical utilization [2, 3]. Engineered NP consist of carbon-based and inorganic forms, partly with functionalized surfaces [4].
Along with their unique general properties, the high diversity of NPs’ elemental and structural composition has, however, challenged environmental scientists in multiple ways, ranging from NP characterization and fate in complex matrixes [e.g. 5] to individual and combined NP effects in aquatic and terrestrial (eco)systems [e.g. 6]. The crystalline composition of titanium dioxide NP (TiO2 NP), for instance, influences their toxicity for the water flea Daphnia magna when based on the mass concentration—a pattern independent of the initial particle size [7]. Such observations suggest that other or additional dose measures, such as particle number, surface area, or body burden, are needed to adequately reflect the exposure situation [8]. In fact, NPs’ surface area explained a large share of variability in the resulting toxicity among crystalline structures [7].
In this paper, we give a brief overview of recent scientific advances enhancing the understanding of the (i) sources and (ii) fate of NP, (iii) the effects of NP in simplified studies, and (iv) how NP interact with biota in a more complex environment. We consider both aquatic and terrestrial systems but mainly focus on metal-based NP as carbon-based NP are covered elsewhere [9, 10]. We will, however, not specifically cover the methodological developments in the context of NP quantification and properties as this has also been covered elsewhere (see for instance, [11,12,13]). On this basis, we develop recommendations for future research directions in nano-ecotoxicology.
Pathways of nanoparticles into natural ecosystems
It has been anticipated that the increasing application of NP both quantitatively but also in terms of product diversity will lead to a diversification in emission sources into the environment [4]. Key products containing NP are coatings, paints and pigments, catalytic additives, and cosmetics [14]. This chapter will discuss NP emissions from such products, whereas the release process is beyond our scope.
NP can enter the environment a long their life cycle and three emission scenarios are generally considered: (i) release during production of raw material and nano-enabled products; (ii) release during use; and (iii) release after disposal of NP-containing products (waste handling) [15,16,17]. NP emissions can be either directly to the environment or indirectly via a technical system such as wastewater treatment plants (WWTPs) or landfills. Indirect emissions are likely occurring either via the effluent of WWTPs, application of biosolids to soil, or leachates from landfills. It has also been pointed out that NP fate in technical systems such as WWTPs determines whether bare, coated, chemically or physically transformed particles are released, and via which pathway (as effluent or biosolid) [18,19,20,21].
So far, emission and environmental concentration levels have been estimated using material flow models following the NP life cycle [22]. Calculation models assume that produced NP will be released either to waste streams or directly to environmental compartments, and more realistic approaches account for the delayed release during use due to in-use NP stocks. NP emissions are also controlled by (i) ageing or weathering [19, 23,24,25,26], (ii) the fate of the NP during use [20, 27, 28], and (iii) the waste management system [29, 30]. Production volumes, however, may give a good indication of the emission of specific NP. Available data on production volumes differ greatly depending on the way of data collection. TiO2 NP and SiO2 NP are certainly the most relevant materials in terms of worldwide productions volumes (> 10,000 t/a in 2010), followed by CeO2 NP, FeO x NP, AlO x NP, and ZnO NP, and carbon nanotubes (CNT) (100–1000 t/a in 2010). The production volume of Ag NP was estimated with approximately 55 t/a worldwide in 2010 [31].
First attempts to estimate NP emissions during the life cycle indicated that most NP are emitted during use phase and after disposal, e.g. on landfills [30], while during production not more than 2% of the production volume is released [32]. Depending on the type and application of NP, they are either directly released into the environment, or indirectly via technical compartments and waste streams or enter in-use stock causing a delayed release [22, 30, 33,34,35]. The release pattern and masses depend on the NP type and its application. For instance, Sun et al. [22] studied emission patterns in the EU in 2014 of TiO2 NP, ZnO NP, Ag NP, and CNTs considering landfills, sediments, and soil as sink for NP. They found that TiO2 NP accumulate in sludge-treated soils followed by sediments and landfills (approx. 8400 t/a and 7600 t/a and 7000 t/a). The dominating emission pathway of TiO2 NP occurs via wastewater (85% of total TiO2 NP emissions) [30, 47]. For example, TiO2 NP are accumulated in sewage sludge during wastewater treatment, which is in many countries ultimately deployed onto soils. It was estimated that approximately 36% of TiO2 NP emissions occur via this pathway. A lower portion of the sewage sludge is deposited onto landfills directly or after incineration which equals approximately 30% of total emitted TiO2 NP. TiO2 NP emissions via wastewater effluent account for approximately 33% [22]. ZnO NP, which are mostly used in cosmetics, electronics, and medicine accumulate in sediments (1300 t/a), in natural and urban soil (300 t/a), as well as at landfills (200 t/a). The dominating emission pathway occurs, just as for TiO2 NP, via wastewater since both are used in cosmetics [47]. CNTs and Ag NP show different emission patterns. CNTs are predominantly emitted via production and use, and are directly deposited at landfills. Hence, approximately 90% of the CNT production is accumulated in landfills, approximately 10% in soils and < 1% in sediments and air [22]. Ag NP are emitted from production and use to both landfills and wastewater.
Global estimation of NP emissions indicates that landfills (approximately 63–91%) and soils (approximately 8–28%) receive the largest share followed by emissions into the aquatic environment and air (7 and 1.5%, respectively, of the production volumes) [30]. Such estimations allow to identify applications with potentially high environmental implication. A potential increase in outdoor applications of NP may elevate their mass flows directly into the aquatic and terrestrial environment [36]. For example, NP emission from façade paints, such as photocatalytic active compounds, e.g. TiO2 NP, has been demonstrated previously [19, 37, 38].
Besides emission of engineered NP, there are particulate emissions of anthropogenic NP which are not intentionally produced as NP. For instance, particulate emissions from traffic, such as palladium, were identified to be in nanoscale [39, 40]. NP release might also be intended due to their direct application in environmental compartments, for instance, for groundwater remediation such as iron-based NP [41,42,43] or when applying nano-pesticides directly to agricultural fields [44, 45]. Although some information on NP emission is available, it is of high importance to quantify their amounts and concentrations in the environment. Quantification of NP emissions into the aquatic environment has by now, however, been hampered by the lack of appropriate analytical techniques [5].
Predicting and measuring nanoparticles in natural ecosystems
Computational modelling was suggested as a way forward estimating environmental concentrations because straight forward analytical methods were not available for detection of NP in the environment [46, 47]. Material flow models rely on life cycle information and production volumes, which were not necessarily available in sufficient detail, limiting their accuracy [48]. Very recently, more advanced models use probabilistic approaches [49] that consider dynamic input rates, in-use stocks as well as the continuous rise in production volumes [22]. These models allow predicting the time-dependent material flow of specific NP (e.g. TiO2 NP) in technical systems and environmental compartments. These models estimate NP concentrations in surface waters to be in the lower ng/L or µg/L range depending on the type of NP. For instance, mean NP concentrations in surface water were estimated for TiO2 with approximately 2.2 µg/L (Q0.15 0.19 µg/L to Q0.85 4.4 µg/L) and for Ag NP with 1.5 ng/L (Q0.15 0.4 µg/L to Q0.85 2.8 ng/L) for the EU in 2014 [22]. Although these models hardly consider NP-specific fate mechanisms (e.g. sedimentation [50]), first studies assessing the actual presence of NP in the aquatic environment [35, 36, 51, 52] are in consensus with modelling results [16]. For instance, analytical studies revealed TiO2 NP surface water concentrations between 3 ng/L and 1.6 µg/L, confirming the high variation of modelling results in a comparable concentration range. However, analytical limitations, namely the lack of specific and sensitive analytical methods in complex matrixes, did not yet allow to formally assess for the assumption that the increasing production and market volume of NP will ultimately lead to an increase in environmental concentrations.
Complementary analytical techniques have been used to determine and characterize metal-based NP in different environmental compartments [51, 53]. Concentration and size of metal-based NP such Au, Ag, Cu, TiO2, in surface water and soils have been, for example, determined by single particle inductively coupled plasma mass spectrometry (sp-ICP-MS) [53, 54] or fractionation techniques in combination with light scattering and elemental detection [55]. Structural information and information on particle size have been derived from electron microscopy as a complementary technique [51]. Among others, the NP surface chemistry including surface charge or functionalization controls NP fate. Therefore, surface characterization methods are important to understand NP fate processes [56].
For complex types of NP such as core shell structures a multi-element technique, e.g. sp-ICP-Time of Flight (ToF)-MS was developed [57] and has recently been successfully applied to determine engineered CeO2 NP in soil [58]. This technique is of high importance to differentiate between engineered NP and natural NP by detecting impurities in natural NP which are not present in engineered NP. Such analyses will help to validate model outputs on environmental NP concentrations.
In comparison to the analysis of inorganic NP in environmental compartments, organic NP analysis is still in its infancy. In fact, analytical techniques for organic NP have been developed, for example, for fullerenes and CNTs [52, 59] but they are hampered by insufficient selectivity with regard to the high environmental background concentrations of carbon. To improve the analysis of organic NP in complex media such surface water or soil, more efficient extraction methods are needed [60].
Recently, considerable progress has been made overcoming some of the analytical problems (natural particle counterparts, low concentrations, matrix interferences) [58, 61, 62], opening new possibilities that foster our understanding on both sources and fate of NP in the environment. However, future work should focus on the differentiation between engineered and natural NP, the detection of organic NP, and the characterization of the NP surfaces.
Fate of nanoparticles in the environment
NP in the environment undergo ageing processes such as chemical transformation, aggregation, and disaggregation. The interplay between these processes and the NP transport determines the fate and ultimately the ecotoxicological potential of NP [63,64,65]. Since particle properties and environmental conditions control these ageing and transport processes [45, 65, 66], a direct transfer of data to even slightly deviating conditions is difficult and can even be misleading. Here, we analyse the current state of the art regarding NP fate and conclude if this knowledge is sufficient to allow for extrapolations from well-defined and controlled laboratory conditions to complex, real-world scenarios.
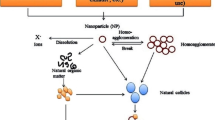
Alterations in chemical speciation, dissolution, degradation, as well as alteration of the surface properties by precipitation and ad- or desorption are important chemical transformation processes of NP, which have frequently been investigated both in aquatic and soil ecosystems (Fig. 1). A general remark in this context: functionalization of NP surfaces, which makes the particles’ properties more beneficial for industrial purposes [67, 68], will strongly control transformation processes in the environment [66, 69, 70]. A thorough characterization of NP surface chemistry (i.e. the formation or loss of coating [71]) over time seems therefore mandatory to understand NP fate [72, 73], but is still highly challenging. The present paper, however, specifically focuses on dissolution, passivation, aggregation, adsorption, sedimentation, and deposition as a selection of the most relevant processes under field conditions (see Fig. 2).
Relative number of publications compared to total number of publications found between 2007 and 2017 in soil and aquatic environments (search criteria: nano* & environ* & system, NOT effect* & NOT *tox*; process search criteria: transp*, agg*, homoagg*, heteroagg*, dissol*, redox*, surface* transfo*, reacti*, deposition*; system search criteria: soil*, aqua*; only web-of-science category environmental science considered)
Chemical transformation
Dissolution of NP (Fig. 2a) is driven directly by the particle chemistry. Dissolution of Ag NP, for instance, requires aerobic conditions. In such environments, an oxide layer (Ag2O) can be formed around the particle, which releases Ag+ [74]. In a range of studies, it was uncovered that Ag NP dissolution rates are triggered by particle-inherent factors including surface coating, size, shape, and state of aggregation, as well as environmental parameters such as pH, dissolved organic carbon, and temperature (see for a more detailed assessment of the mechanisms [11, 75,76,77,78,79,80]). Koser et al. [81] used equilibrium speciation calculations, based on a broad basis of literature data, to successfully predict Ag dissolution in several artificial media. This suggests that the scientific community was indeed capable of advancing the knowledge to a state that allows for reasonably accurate predictions and modelling.
A passivation process frequently occurring under various environmental conditions is the sulfidation of NP (Fig. 2b) that includes Ag NP, ZnO NP, and CuO NP [e.g. 79, 82, 83]. Sulfidation of Ag NP, for instance, can lead to the formation of core–shell Ag0–Ag2S structures or hollow Ag2S NP [84]. The mechanisms of sulfidation are given elsewhere [66, 84,85,86]. Sulfidation leads to nearly inert NP surfaces with consequence for their reactivity (Fig. 2) [84, 87] and thus toxicity [66], while sulfidized NP can still be toxic to microorganisms [88].
Colloidal stability
Colloidal stability of NP is one of the key factors controlling their fate and effects [12, 89]. When released into the environment, NP interact with the variety of dissolved or particulate, inorganic or organic compounds influencing NP aggregation dynamics and thus colloidal stability [90]. Ultimately, exposure conditions are controlled by NP aggregation. By focusing on factors controlling homo-aggregation (interaction between the same NP) and hetero-aggregation (interaction between different NP or between NP and natural colloids such as montmorillonite, maghemite, kaolinite but also microorganisms, algae, and proteins [91, 92]), as well as disaggregation, we also discuss the processes determining NP fate.
Homo-aggregation of NP (Fig. 2c) is positively correlated with their concentration in the media. Since predicted environmental concentrations are rather low (in the pg/L to the low µg/L range for surface waters [16]), homo-aggregation is less likely due to the low probability for collisions. Nonetheless, this factor is relevant—but largely ignored—for laboratory-based ecotoxicological investigations that often use high NP concentrations compared to predicted environmental concentrations [76]. Under field conditions, the ionic strength of the surrounding medium seems more relevant as aggregation rates increase with ionic strength [e.g. 93, 94]. The aggregation dynamics are in most cases characterized by classical Derjaguin-Landau-Verwey-Overbeek (DLVO) theory [95, 96]. Furthermore, multivalent cations are more efficient than monovalent cations [97], whereas the efficiency within both groups of cations depends on their respective identity as judged by critical coagulation concentrations (CCCs): the aggregation of citrate-coated Ag NP was, for instance, more efficient for Ca2+ than Mg2+ [76], which may be explained by the higher ability of Ca2+ to form citrate complexes [98]. At the same time, the impact of cations and anions can be concentration dependent. Whereas high concentrations of Cl− ions enhance the aggregation of Ag NP due to the bridging by AgCl [99], low concentrations may stabilize NP via the formation of negatively charged surface precipitates [76]. Similarly, soils and soil extracts modulate NP aggregation [100].
In addition to ionic strength, homo-aggregation is also influenced by a range of environmental variables. The surface charge of NP changes with pH, which is reflected by the isoelectric point (IEP; i.e. the pH at which NP do not carry a net charge). The IEP varies substantially among commercially available NP [101, 102], suggesting that even at the same pH the fate and thus the interaction of NP with organisms might vary substantially [103]. Furthermore, natural organic matter (NOM) can increase or reduce colloidal stability of NP as a function of its quality and quantity as well as the ionic strength of the medium [e.g. 104]: at low ionic strength, NOM stabilizes negatively charged NP through electrostatic and/or steric forces [76, 100]. Due to the formation of cation-NOM bridges among NP, NOM can enhance aggregation at high ionic strength [e.g. 97]. Positively charged NP may also increase aggregation in the presence of NOM as shown for the combination of negatively charged NOM and TiO2 NP due to charge screening [105]. Until now, the effects of several individual factors like surface coating of NP, ionic strength, and valence and type of cations on the fate of NP are characterized to reasonable degree. However, less is known on how the interplay between these individual factors affects the fate of NP under realistic environmental conditions.
In contrast to homo-aggregation, hetero-aggregation (Fig. 2d) is considered to be of higher environmental relevance given the several orders of magnitude higher concentration of natural colloids [106] relative to NP [16]. Quik et al. [107], for example, indicated that hetero-aggregation is the main mechanism removing CeO2 NP from the water phase through sedimentation. The aggregation kinetics of NP and natural colloids or other NP are particularly fast if they are differently charged [92, 108]. The presence of NOM, in contrast, reduces hetero-aggregation due to electrostatic and steric stabilization [109]. Besides electrostatic forces, bridging by polymers, hydrogen as well as chemical bonding were reported as mechanisms inducing hetero-aggregation. This process is, thus, highly complex and among others triggered by NP surface properties, their ageing status, interacting particulate phases, chemical composition of the surrounding environment, and the properties of natural inorganic, organic and biological colloids. However, only a few publications assessed aggregation in complex field-relevant media [e.g. 93, 100, 110], complicating any conclusion about the general relevance of the processes detailed above. Even less is known about the reversibility of NP aggregation in natural systems [93]. As disaggregation is mainly triggered by changing environmental conditions, experiments in simplified artificial systems are falling too short to properly address the dynamics of aggregation and disaggregation in real aquatic systems. This calls for experimental designs capable of simulating such fluctuating conditions. Furthermore, low and environmentally relevant NP concentrations should be used in future studies to avoid potentially confounding implications of NP homo-aggregation, which is, as outlined above, less likely under currently predicted environmental concentrations of NP compared to hetero-aggregation.
Transport in porous media
Most of the published data on NP mobility in porous media was generated using water saturated artificial columns frequently made of quartz sand [e.g. 111] while only a few involved natural soil [e.g. 92]. Similar to aquatic ecosystems, ionic strength triggers NP’ fate: NP retention and deposition in porous media (Fig. 2) is positively related to the ionic strength of the pore water [112], likely driven by quick NP aggregation [e.g. 111]. At high levels of ionic strength, retention rates can further increase due to the “ripening effect” (i.e., increasing attraction forces between NP in the liquid phase and NP already deposited onto soil [113]). Hetero-aggregation of Ag NP with soil colloids, in contrast, can even enhance NP mobility by the size-exclusion effect [92, 112]. Furthermore, electrostatic and steric repulsion forces induced by NOM often lead to higher mobility [e.g. 112, 114], while a decreasing pH has the opposite effect [e.g. 111].
In contrast to water saturated systems, unsaturated porous media are less frequently but increasingly assessed (see for instance, [115]). Relative to water saturated porous media, unsaturated systems have often a higher retention potential [e.g. 116], while Fang et al. [117] found only marginal differences regarding the retention of TiO2 NP between saturated and unsaturated porous media. This pattern may be explained by the non-equilibrium sorption to solid–water interface and equilibrium sorption to air-water interface [118]. Moreover, the water film straining during drying could increase NP retention [116] and is thus of potentially high relevance in unsaturated porous media. The impact of ionic strength, type of cations, NOM but also pH on NP fate is comparable among aquatic systems and porous media irrespective of whether the latter is saturated or unsaturated. Therefore, we refer to the subchapters above for a more detailed description.
Driven by the few number of studies [e.g. 100] and the partly contradictory outcomes particularly for unsaturated porous media, a reliable prediction of NP fate in soil ecosystems seems difficult. Thus, a more systematic approach is urgently needed uncovering the role of soil properties on NP fate. One important step would be to assess NP fate in natural soils instead of porous media made of artificial substrate such as quartz sand. Although there are certainly challenges from the analytical side, i.e. inorganic NP might occur at high levels in natural soils and form a substantial background contamination, which can be overcome with the help of modelling, those data are likely of higher field relevance. In natural soils, for instance, microorganisms, inorganic and organic particles might form complex bio-geochemical interfaces that interact with NP and as a consequence will influence their fate [119] and toxicity. Those insights can, however, barely be inferred from experiments using highly artificial substrates. On the way to indeed using natural soils, it may however, be feasible to employ more complex artificial soils containing among others also natural organic matter.
In summary, predominantly qualitative information on particle fate in aquatic and porous media is available and key factors which control fate processes have been identified. Particle surface properties and NP concentration are most relevant and need to be characterized carefully—also throughout the experiments. Environmental key factors controlling NP fate processes are ionic strength, type and charge of ions, pH, type and concentration of NOM in all environmental compartments, while the degree of water saturation is an additional factor for porous media. Quantitative systematic data are rare [12, 45, 50, 64] but fundamental for process understanding and ultimately for the development of reliable and predictive fate models. Future studies should, thus, qualitatively and quantitatively assess NP fate under environmentally relevant conditions, reflecting amongst others not only realistic NP concentrations, environmental conditions but also reaction and residence times. These data will certainly support the recent developments in NP fate modelling, namely simulations of individual fate processes and fate predictions in rivers and porous media [64, 120,121,122,123]. As their validation is largely lacking, this aspect should be addressed in future work.
Effects of nanoparticles and their mechanisms of toxicity
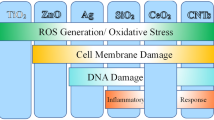
Roughly a decade ago and thus with the initiation of the research field “nano-ecotoxicology”, Moore [124] as well as Hund-Rinke and Simon [125] suggested that NP have the potential to cause harmful effects in biota by the formation of reactive oxygen species (ROS) that could affect biological structures (Fig. 3a). Moore [124] also pointed to the potential of NP to function as carriers for other pollutants (Fig. 2d)—an assumption that will be addressed in more detail in the next chapter. While it is evident from the literature that oxidative stress can indeed be a driver for many NP-induced effects [126], the last decade of research showed that NP have the ability to act via multiple pathways of which the induction of oxidative stress is one. In the following, we briefly highlight the current state of the art with regard to central aspects supposedly driving the ecotoxicity of NP.
No mechanism of toxicity can be considered as generic for all NP [126]. Oxidative stress is, however, a frequently reported phenomenon [127, 128]. Just to name a few other relevant mechanisms, physiological implications that can go as far as reproductive failure by modifying hormones or hatching enzymes were reported [129, 130]. Those effects indicate implications in population development and suggest the potential for transgenerational effects [131, 132]. In addition, algae [133] and aquatic plants [134] were altered in their photosynthetic pigment composition and showed effects in photosystem II, while we refer to Thwala et al. [135] for a more detailed review. Similarly, several recent reviews have covered NP accumulation in terrestrial plants which can cause biochemical and physiological changes [136,137,138]: Cao et al. for instance, documented impacts on carbon fixation as well as water use efficiency during photosynthesis in response to CeO2 NP exposure [139]. The latter may have indirect effects on soil organisms via implications in soil moisture. Besides this massive diversity regarding the mechanisms of toxicity among NP, species and ecosystems, some more general questions attracted attention among researchers, namely the relevance of ions released (dissolved) from NP for NP-induced effects.
Effects of nanoparticles on individuals and populations
Effects of ion-releasing NP
Certain NP are prone to dissolution, i.e. the release of ions from the NP surface, during their entire (aquatic) life cycle (Figs. 2, 3) [140]. In such cases, researchers were interested in uncovering the relevance but also the mechanism of toxicity induced by those ions released from NP. Against this background, Ag NP have frequently been assessed, suggesting that Ag ions released from these NP explained a large proportion of the observed toxicity for various test organisms [e.g. 141–143] and soil microbial communities [144]. Additionally, the mechanisms of toxicity, for example, for snails [143] and periphyton [141], were largely comparable between Ag ions and Ag NP. These observations contradict other findings, highlighting more severe implications by Ag NP than what could be explained exclusively by the Ag ions measured [145]. Differences in gene expression and transcriptomic profile point towards distinct mechanisms of toxicity in aquatic [146, 147] and terrestrial organisms [148]. Nonetheless and in line with the extensive literature review by Völker et al. [149] it may be suggested that Ag NP and Ag ions share common mechanisms of action. This conclusion also implies that the Ag NP-induced toxicity, which largely depends on the particle surface properties, diameter, and exposure time [150], can mainly be explained by the quantity of released ions [sensu 151, 152]. Ag NP are, moreover, sulfidized (see above, Fig. 2b) in wastewater treatment plants and trapped in sewage sludge. As a consequence of the usage of sewage sludge as fertilizer for crop production in various countries, soil organisms are directly exposed to Ag2S NP, Ag NP, and Ag+ ions. The form of Ag directly influences the location and speciation (metallic, ionic, thiol, NP) in which Ag is stored in wheat roots [153]. In cucumber and wheat, Ag2S NP remained in their NP form and were translocated from the roots to leaf tissue, reducing plant growth and activating plant defence mechanisms [154]. Internalization (Fig. 3c) or physical adherence, such as biological surface coating (inhibiting, e.g. photosynthesis, nutrient uptake or movement) are also considered as possible mechanisms of toxicity for “inert” NP, i.e. those not releasing toxic ions (Fig. 3d) [155, 156].
CuO NP exposure resulted in a different pattern relative to Cu ions. This was shown for protein regulation and gene expression in marine mussels [157] and zebrafish gills [146]. Similarly, Mortimer et al. [158] indicated CuO particle-related effects in the membranes’ fatty acid composition of protozoa pointing towards distinct mechanisms of toxicity induced by Cu ions relative to CuO NP. Also in wheat grown on sand, CuO NP reduced root length and simultaneously increased root hair length with no effect on shoot growth, while Cu ions reduced both root and shoot growth [159]. A recent study by Pradhan et al. [160], however, suggests that aquatic fungi from metal contaminated ecosystems have a higher tolerance towards CuO NP driven by elevated enzymatic activity. This observation may indicate a specific adaptation of fungi towards Cu ions, supporting fungi to withstand CuO NP stress, which might indicate a common mechanism of toxicity. On the other hand, a more generic defence mechanism might have been strengthened by the long-term adaptation towards metal stress in general, which resulted in an evolution of co-tolerance towards CuO NP.
Similar to CuO NP, the mechanism of toxicity induced by ZnO NP seems to deviate from its ionic counterpart. Fernandez-Cruz et al. [161], for instance, suggested that cytotoxic effects in fish cells are mainly particle-related, which is supported by studies uncovering difference in gene expression [162, 163] and thus different mechanisms of toxicity [164]. Recent evidence with soil nematodes (Caenorhabditis elegans) indicates a higher ecotoxicological potential of ZnO NP relative to Zn2+ ions [165].
It is hence not possible to draw a generic conclusion regarding the relevance of toxic metal ions released from NP relative to the effects induced by the NP themselves. This relationship is rather driven by the NP identity and coating, the biological system, and the environmental conditions (e.g. complexation with NOM) under which the studies are performed. For instance, gene expression profiling in enchytraeids (Enchytraeus crypticus) suggested deviating mechanisms of toxicity for different Ag NP products [166]. Also the uptake and toxicity of Ag NP in earthworms are triggered by the particle coating. Makama et al. [167] reported size-dependent effects only for PVP-coated Ag NP. The uptake of Ti through the roots into basil was highest when exposed to TiO2 NP with hydrophobic relative to hydrophilic coatings, causing alterations in the content of several essential elements and sugar [168]. A long-term study with two agricultural soils and different plant species looked at the effects of ZnO NP [169]. In this study, acidic soils stimulated Zn accumulation, ROS production, and photosynthetic pigments of beans relative to calcareous systems, while the opposite pattern was observed in tomatoes [169].
Effects of NP not releasing toxic ions
For NP that are only marginally or not releasing toxic metal ions during the aquatic life cycle, “biological surface coating” (i.e. the attachment or adsorption of NP to the organisms’ outer surface, Fig. 3d) is suggested as potential toxicity trigger [170]. The acute toxicity of TiO2 NP and Fe3O4 NP in daphnids, for instance, was attributed to a physical inhibition of moulting, ultimately inducing death [155, 156]. At the same time, biological surface coating could alter daphnids’ swimming behaviour with potential chronic effects [171]. These effects are usually more pronounced at smaller initial particle sizes, while evidence suggest that the surface area is the driving force for toxicity [7, 172, 173].
Nanoparticle-induced effects over multiple generations
As highlighted earlier, aquatic invertebrates have shown an increase in sensitivity in filial generations as a consequence of the exposure of the parental [131] or subsequent generations [132] towards TiO2 NP. Similarly, at Au NP concentrations inducing only negligible mortality in the parental generation of the soil organism C. elegans, the reproduction of subsequent generations (F1–F4) was substantially impaired [174]. In a full lifespan test, CuO NP caused more severe effects compared to a standard test duration [175]. Also, plants exposed to CeO2 NP in their parental generation showed response in the filial generation with implications in the grains’ nutrient composition, growth, and physiology [176]. Despite the current lack of mechanistic understanding regarding the underlying processes, these insights support the idea of long-term effects in natural populations of aquatic and terrestrial organisms. Importantly, such effects are not covered by most standardized test systems. More systematic research is needed addressing the mechanistic basis of these phenomena as well the evolutionary potential of populations and communities to adapt to these emerging stressors.
Interactions of nanoparticles in a complex environment
Impact of natural organic material on NP‑induced effects
In the environment, NP will interact with their abiotic surrounding, which influences their fate and ecotoxicological potential [81, 177]. The relevance of natural organic molecules attaching to NP (Fig. 2e) has recently been reviewed elsewhere [178], which is why we give only a few examples here. Dissolved organic matter (DOM) coats NP and thereby stabilizes particle size [179] due to steric or electrostatic repulsion [180]. These processes are more effective with increasing hydrophobicity or aromaticity of the DOM, ultimately reducing their ecotoxicological potential—likely by reducing the availability of reactive surfaces [181]. In situations where artificial (e.g. polyvinylpyrrolidone, gum arabic or citrate) or natural OM coats Ag NP, the release of potentially toxic ions into the surrounding environment [66, 182] or the NP bioavailability [183] is reduced. On the contrary, humic acid elevated the release of ions from Pb NP [184]. Bicho et al. [185] reported that soil and soil–water extracts could elevate effects of NP (here Europium polyoxometalates encapsulated in SiO2 NP) relative to their absence.
Thus, it becomes apparent that soil properties influence the toxicity of NP to soil organisms. Especially the organic matter content, soil texture, ionic composition, and pH affect the fate and bioavailability of NP [112, 186, 187], which leads to differences in toxicity. With a decrease in organic carbon in standard Lufa soils, the toxic effects of PVP-coated Ag NP to E. crypticus increased [188]. Less toxicity of AgNP was also observed for an entire test battery of dicotyledonous and monocotyledonous plants, Collembola and earthworms in soil with higher silt than sand content [189]. With a rise in soil pH, ZnO NP effects on Folsomia candida reproduction decreased [190].
When considering time as a factor, ageing of NP in presence of DOM for 48 h reduced or did not influence the toxicity of Ag and ZnO NP, respectively [191]. In contrast, the ageing of TiO2 NP in the presence of DOM for 1 and 3 days slightly increased toxicity for daphnids, while ageing for longer time periods (e.g. 6 days) reduced NP-induced effects substantially as NP agglomerates exceeded the size range retained by daphnids’ filter apparatus [192]. Similarly, Collembola (F. candida) showed more severe effects in their reproductive output with increasing ageing duration of Ag NP in spiked sewage sludge [193]. It is suspected that this is due to the continuous release of Ag ions from Ag NP.
Algal cells fed to invertebrates may enhance the uptake and toxicity of NP [194]. The adsorption of NP to algal cell surfaces (Fig. 2f) could accelerate their sedimentation (Fig. 2g), which forces pelagic consumers to invest more energy in collecting their food near the sediment [195]. Bundschuh et al. [196], in contrast, uncovered an increase in Daphnia growth and partly reproduction after allowing an interaction between algae and TiO2 NP for 1–3 days. Moreover, NP ingested together with the food could negatively influence digestion by clogging the gut with negative consequences on life history strategies of primary consumers in autotrophic food webs [195]. Similar patterns have been observed for detritus-based food webs [197]. Altogether, these observations suggest highly complex interactions between organic material (irrespective whether of particulate or dissolved nature), NP and biota, but are also pointing to a lack of mechanistic understanding.
NP-triggered alterations in the ecotoxicity of co-occurring contaminants
Aquatic and terrestrial ecosystems are also commonly exposed to mixtures of chemical stressors, which raised concerns about the potential of NP to act as carriers for organic and inorganic chemical stressors of anthropogenic origin [198]. Schwab et al. [199] reported, for instance, an elevated herbicide (diuron)-induced toxicity applied at environmentally relevant concentrations in the presence of carbon-based NP. Similarly, the acute toxicity of the insecticide bifenthrin was increased in the presence of fullerene NP, while its chronic effects were not affected [200]. In line with these observations, a recent review suggested that carbon-based NP could also act as Trojan horse for metal ions. The potential of carbon-based NP to act as “Trojan horse” for other contaminants strongly depends on the characteristics of the surrounding environment (see for a more detailed discussion [201]). This conclusion is challenged by Sanchis et al. [202], reporting for most combinations of carbon-based NP and organic co-contaminants antagonistic effects for daphnids and bacteria.
Also, the combined exposure of metallic NP with other chemical stressors delivered contradictory results. The presence of TiO2 NP reduced the uptake of phenols [203] and polycyclic aromatic hydrocarbons [204], which, however, did not necessarily reduce the combined toxic effects of those organic chemicals and TiO2 NP, suggesting a significant contribution of the NP to the biological responses. On the contrary, the accumulation of perfluorooctanesulfonate in fish was facilitated in the presence of TiO2 NP. This pattern was particularly pronounced at the bottom of the experimental systems as a consequence of the adsorption of perfluorooctanesulfonate onto TiO2 NP surfaces and the subsequent aggregation and deposition of perfluorooctanesulfonate-loaded NP [205]. Similarly, TiO2 NP increase the uptake of metal ions [206] from the water phase and at high concentrations of OM also from sediments [207], in biota ultimately elevating biological responses.
Other publications indicate the opposite pattern, namely a reduction in metal ion-induced effects in the presence of nTiO2 or Al2O3 NP in algae [208, 209] or mussels [210], with an even more pronounced reduction in the presence of DOM [211]. Follow-up studies suggested that the direction and magnitude of effects caused by a combined exposure of TiO2 NP and metal ions are triggered by the charge of the most toxic metal ion [212]. Although the underlying mechanisms are not well understood yet [213] and the NP concentrations used in those experiments often exceed environmentally relevant concentrations by at least one order of magnitude, interactions of NP with co-contaminants will occur most likely under field conditions. The relevance of such interactions in both direction and magnitude for effects caused by co-contaminants in wildlife remains to be resolved.
NP effects in communities and consequences for trophic interaction
McKee and Filser [214] reviewed interactions of metal-based NP in soils and pointed out the relevance of species interactions for fate and bioavailability of these NP, next to abiotic parameters. They stated that particularly biotic interactions might explain the negative consequences of NP on ecosystem processes such as carbon dioxide emission, nitrogen or phosphorous fluxes [215] that could not be detected from short-term, single species tests [214]. Button et al. [216], for instance, did not detect any negative effects on microbial community structure and function in wetland systems when exposed for 28 days to ionic Ag, citrate- and PVP-coated Ag NP (at 100 µg/L). These microbial communities, however, developed an Ag resistance, indicating an existing potential to adapt to such emerging stressors by elevating the production of extracellular polymers [217], while the costs of this adaptation remain unclear. Evidence is increasing that nitrifiers are among the most sensitive microorganisms towards Ag NP [218,219,220], even when the NP are sulfidized [88]. Nitrite production rate was also reduced by about 30% in the presence of 200 mg magnetite (Fe3O4) NP/L [219], a concentration considered as rather low in soils remediated from organic contamination [41]. This relatively low-effect threshold (given that iron oxides are mostly being considered harmless to beneficial) is supported by a range of further studies reporting sometimes surprisingly strong negative effects on microorganisms exposed to iron oxide NP [214, 221, 222].
When actively spraying Ag NP as growth promoter on several plant species, root and shoot mass of cowpea and Brassica increased, while only for the latter in a dose-dependent manner [218]. For wheat, in turn, shoot mass remained stable and root mass decreased with increasing Ag NP exposure [218]. These authors also reported increased bacterial counts and higher abundances of P-solubilizing bacteria in pure soil (without plants) following Ag NP exposure while at higher Ag NP concentration the abundance of N-fixing bacteria decreased. In the rhizosphere of plants treated with Ag NP, however, the microbial responses varied with plant species and thus deferred from the pure soil [218]. An experiment with Fe3O4 NP and Zea mays documented a reduction in the diversity of arbuscular mycorrhizal fungi accompanied by a decreased catalase activity, plant biomass, phosphorous content, carbon transfer to the fungi, and impaired root colonization and phosphatase activity [221]. These examples emphasize that the interactions between soil microorganisms, plants, and NP are highly complex, warranting further research. Addressing the underlying processes will ultimately support a well-informed decision making about potential environmental risks of NP when applied to agricultural fields.
Recent studies highlight the potential for indirect effects of NP on invertebrate species via microorganisms. Antisari et al. [223] reported for Co NP a reduction in soil microbial biomass and changes in its community composition, which may partly explain alterations in earthworms’ fatty acid composition. The uptake of TiO2 NP via contaminated food seems to be of high relevance for terrestrial [224, 225] and aquatic invertebrates [226]. This direct uptake pathway increases NP exposure and might influence the food quality through impacts on food-associated microorganisms.
In aquatic systems, structural [227, 228] and functional [141, 229] changes, such as photosynthetic efficiency and leaf decomposition were reported, although often at rather high concentrations. These examples suggest that NP can indeed affect species but also species interactions [230] at various trophic levels. At the same time, neither the mechanisms driving these changes nor the consequences for the wider food web or whole ecosystems have yet even been addressed.
Conclusion
Over the past decade, our understanding of sources, fate, and effects of NP in the environment has made significant progress. Besides the call to consider environmental relevant concentrations of NP as well as to monitor the fate of NP during biological testing, there are multiple open questions that need further consideration. A more systematic approach is urgently needed uncovering the role of soil properties (including saturated and unsaturated systems) for NP fate and thus the risk of groundwater contamination by NP. We have, for instance, learned that ions released from NP can in some situations fully explain the effects observed in organisms. It is currently, however, not possible to properly describe under which conditions this simplified assumption should be rejected and other mechanisms need to be considered. The impact of coating—either as intended functionalization or based on natural processes—on the fate and effects of NP is currently underrepresented in literature (but see, [231]). As those coatings are likely of high field relevance, we strongly recommend their inclusion in future projects. Although most studies highlight effects at relatively high NP concentrations, more recent approaches document sublethal implications at field-relevant levels particularly over multiple generations (as reviewed in [177]). Thus, the impact of NP under current and future exposure scenarios (including co-exposure to other stressors) on communities, ecosystems, ecosystem functions as well as interactions across ecosystem boundaries deserves special attention. Particularly for sparingly soluble or insoluble NP that may accumulate in certain environmental compartments (e.g. sediments) over time, investigations covering multiple years of (repeated) exposure and assessment are suggested to properly assess their potential long-term implications in aquatic and terrestrial ecosystems. This aspect directly links to the acknowledgement of NP-induced alterations in horizontal and vertical trophic interactions with food webs.
References
PEN (2013) The Project on emerging nanotechnologies. http://www.nanotechproject.org/. Accessed 14 June 2013
Ito A, Shinkai M, Honda H, Kobayashi T (2005) Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng 100:1–11
Semenzin E, Lanzellotto E, Hristozov D, Critto A, Zabeo A, Giubilato E, Marcomini A (2015) Species sensitivity weighted distribution for ecological risk assessment of engineered nanomaterials: the n-TiO2 case study. Environ Toxicol Chem 34:2644–2659
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150(1):5–22
von der Kammer F, Ferguson PL, Holden PA, Masion A, Rogers KR, Klaine SJ, Koelmans AA, Horne N, Unrine JM (2012) Analysis of engineered nanomaterials in complex matrices (environment and biota): general considerations and conceptual case studies. Environ Toxicol Chem 31(1):32–49
Klaine SJ, Alvarez PJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27(9):1825–1851
Seitz F, Rosenfeldt RR, Schneider S, Schulz R, Bundschuh M (2014) Size-, surface- and crystalline structure composition-related effects of titanium dioxide nanoparticles during their aquatic life cycle. Sci Total Environ 493:891–897
Petersen EJ, Diamond SA, Kennedy AJ, Goss GG, Ho K, Lead J, Hanna SK, Hartmann NB, Hund-Rinke K, Mader B, Manier N, Pandard P, Salinas ER, Sayre P (2015) Adapting OECD aquatic toxicity tests for use with manufactured nanomaterials: key issues and consensus recommendations. Environ Sci Technol 49(16):9532–9547
Freixa A, Acuña V, Sanchís J, Farré M, Barceló D, Sabater S (2018) Ecotoxicological effects of carbon based nanomaterials in aquatic organisms. Sci Total Environ 619–620:328–337
Petersen EJ, Zhang LW, Mattison NT, O’Carroll DM, Whelton AJ, Uddin N, Nguyen T, Huang QG, Henry TB, Holbrook RD, Chen KL (2011) Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ Sci Technol 45(23):9837–9856
Furtado LM, Bundschuh M, Metcalfe CD (2016) Monitoring the environmental fate and transformation of silver nanoparticles in natural waters. UB/TIB Hann 97:449–455
Schaumann GE, Phillippe A, Bundschuh M, Metrevelli G, Klitzke S, Rakcheev D, Grün A, Kumahor S, Kühn M, Baumann T, Lang F, Manz W, Schulz R, Vogel H-J (2015) Understanding the fate and biological effects of Ag- and TiO2-nanoparticles in the environment: the quest for advanced analytics and interdisciplinary concepts. Sci Total Environ 535:3–19
Leopold K, Philippe A, Wörle K, Schaumann GE (2016) Analytical strategies to the determination of metal-containing nanoparticles in environmental samples. Trends Anal Chem 84:107–120
Hansen SF, Heggelund LR, Besora PR, Mackevica A, Boldrin A, Baun A (2016) Nanoproducts—what is actually available to European consumers? Environ Sci Nano 3(1):169–180
Gottschalk F, Sonderer T, Scholz RW, Nowack B (2009) Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions. Environ Sci Technol 43(24):9216–9222
Gottschalk F, Sun TY, Nowack B (2013) Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ Pollut 181:287–300
Tolaymat T, El Badawy A, Genaidy A, Abdelraheem W, Sequeira R (2017) Analysis of metallic and metal oxide nanomaterial environmental emissions. J Clean Prod 143:401–412
Al-Kattan A, Wichser A, Vonbank R, Brunner S, Ulrich A, Zuin S, Arroyo Y, Golanski L, Nowack B (2015) Characterization of materials released into water from paint containing nano-SiO2. Chemosphere 119:1314–1321
Kaegi R, Sinnet B, Zuleeg S, Hagendorfer H, Mueller E, Vonbank R, Boller M, Burkhardt M (2010) Release of silver nanoparticles from outdoor facades. Environ Pollut 158(9):2900–2905
Kaegi R, Voegelin A, Sinnet B, Zuleeg S, Hagendorfer H, Burkhardt M, Siegrist H (2011) Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ Sci Technol 45(9):3902–3908
Zuin S, Gaiani M, Ferrari A, Golanski L (2013) Leaching of nanoparticles from experimental water-borne paints under laboratory test conditions. J Nanopart Res 16(1):2185
Sun TY, Bornhoft NA, Hungerbuhler K, Nowack B (2016) Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ Sci Technol 50(9):4701–4711
Bressot C, Manier N, Pagnoux C, Aguerre-Chariol O, Morgeneyer M (2017) Environmental release of engineered nanomaterials from commercial tiles under standardized abrasion conditions. J Hazard Mater 322:276–283
Duncan TV, Pillai K (2015) Release of engineered nanomaterials from polymer nanocomposites: diffusion, dissolution and desorption. ACS Appl Mater Interfaces 7(1):2–19
Olabarrieta J, Zorita S, Peña I, Rioja N, Monzón O, Benguria P, Scifo L (2012) Aging of photocatalytic coatings under a water flow: long run performance and TiO2 nanoparticles release. Appl Catal B 123:182–192
Wohlleben W, Meyer J, Muller J, Muller P, Vilsmeier K, Stahlmecke B, Kuhlbusch TAJ (2016) Release from nanomaterials during their use phase: combined mechanical and chemical stresses applied to simple and multi-filler nanocomposites mimicking wear of nano-reinforced tires. Environ Sci Nano 3(5):1036–1051
Kinsinger N, Honda R, Keene V, Walker SL (2015) Titanium dioxide nanoparticle removal in primary prefiltration stages of water treatment: role of coating, natural organic matter, source water, and solution chemistry. Environ Eng Sci 32(4):292–300
Mitrano DM, Rimmele E, Wichser A, Erni R, Height M, Nowack B (2014) Presence of nanoparticles in wash water from conventional silver and nano-silver textiles. ACS Nano 8(7):7208–7219
Gottschalk F, Ort C, Scholz RW, Nowack B (2011) Engineered nanomaterials in rivers—exposure scenarios for Switzerland at high spatial and temporal resolution. Environ Pollut 159(12):3439–3445
Keller AA, McFerran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15(6):1692
Piccinno F, Gottschalk F, Seeger S, Nowack B (2012) Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res 14(9):1109
Gottschalk F, Nowack B (2011) The release of engineered nanomaterials to the environment. J Environ Monit 13:1145–1155
Kim B, Murayama M, Colman BP, Hochella MF (2012) Characterization and environmental implications of nano- and larger TiO2 particles in sewage sludge, and soils amended with sewage sludge. J Environ Monit 14(4):1129–1137
Ma R, Levard C, Judy JD, Unrine JM, Durenkamp M, Martin B, Jefferson B, Lowry GV (2014) Fate of zinc oxide and silver nanoparticles in a pilot wastewater treatment plant and in processed biosolids. Environ Sci Technol 48(1):104–112
Mitrano DM, Mehrabi K, Dasilva YAR, Nowack B (2017) Mobility of metallic (nano)particles in leachates from landfills containing waste incineration residues. Environ Sci Nano 4(2):480–492
Baalousha M, Yang Y, Vance ME, Colman BP, McNeal S, Xu J, Blaszczak J, Steele M, Bernhardt E, Hochella MF (2016) Outdoor urban nanomaterials: the emergence of a new, integrated, and critical field of study. Sci Total Environ 557:740–753
Bossa N, Chaurand P, Levard C, Borschneck D, Miche H, Vicente J, Geantet C, Aguerre-Chariol O, Michel FM, Rose J (2017) Environmental exposure to TiO2 nanomaterials incorporated in building material. Environ Pollut 220:1160–1170
Kaegi R, Ulrich A, Sinnet B, Vonbank R, Wichser A, Zuleeg S, Simmler H, Brunner S, Vonmont H, Burkhardt M, Boller M (2008) Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment. Environ Pollut 156(2):233–239
Ermolin MS, Fedotov PS, Ivaneev AI, Karandashev VK, Fedyunina NN, Eskina VV (2017) Isolation and quantitative analysis of road dust nanoparticles. J Anal Chem 72(5):520–532
Prichard HM, Fisher PC (2012) Identification of platinum and palladium particles emitted from vehicles and dispersed into the surface environment. Environ Sci Technol 46(6):3149–3154
Filser J, Arndt D, Baumann J, Geppert M, Hackmann S, Luther EM, Pade C, Prenzel K, Wigger H, Arning J, Hohnholt MC, Koser J, Kuck A, Lesnikov E, Neumann J, Schutrumpf S, Warrelmann J, Baumer M, Dringen R, von Gleich A, Swiderek P, Thoming J (2013) Intrinsically green iron oxide nanoparticles? From synthesis via (eco-)toxicology to scenario modelling. Nanoscale 5(3):1034–1046
Weil M, Meissner T, Busch W, Springer A, Kuhnel D, Schulz R, Duis K (2015) The oxidized state of the nanocomposite Carbo-Iron (R) causes no adverse effects on growth, survival and differential gene expression in zebrafish. Sci Total Environ 530:198–208
Weil M, Meissner T, Springer A, Bundschuh M, Hübler L, Schulz R, Duis K (2016) Oxidized Carbo-Iron causes reduced reproduction and lower tolerance of juveniles in the amphipod Hyalella azteca. Aquat Toxicol 181:94–103
Kah M (2015) Nanopesticides and nanofertilizers: emerging contaminants or opportunities for risk mitigation? Front Chem 3:64
Wagner S, Gondikas A, Neubauer E, Hofmann T, von der Kammer F (2014) Spot the difference: engineered and natural nanoparticles in the environment-release, behavior, and fate. Angew Chem Int Ed 53(46):12398–12419
Boxall AB, Tiede K, Chaudhry Q (2007) Engineered nanomaterials in soils and water: how do they behave and could they pose a risk to human health? Nanomedicine 2(6):919–927
Mueller NC, Nowack B (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42(12):4447–4453
Caballero-Guzman A, Nowack B (2016) A critical review of engineered nanomaterial release data: are current data useful for material flow modeling? Environ Pollut 213:502–517
Sun TY, Gottschalk F, Hungerbuhler K, Nowack B (2014) Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ Pollut 185:69–76
Baalousha M, Cornelis G, Kuhlbusch TAJ, Lynch I, Nickel C, Peijnenburg W, van den Brink NW (2016) Modeling nanomaterial fate and uptake in the environment: current knowledge and future trends. Environ Sci Nano 3(2):323–345
Gondikas AP, von der Kammer F, Reed RB, Wagner S, Ranville JF, Hofmann T (2014) Release of TiO2 nanoparticles from sunscreens into surface waters: a one-year survey at the old Danube recreational lake. Environ Sci Technol 48:5415–5422
Bauerlein PS, Emke E, Tromp P, Hofman JAMH, Carboni A, Schooneman F, de Voogt P, van Wezel AP (2017) Is there evidence for man-made nanoparticles in the Dutch environment? Sci Total Environ 576:273–283
Gondikas A, von der Kammer F, Kaegi R, Borovinskaya O, Neubauer E, Navratilova J, Praetorius A, Cornelis G, Hofmann T (2018) Where is the nano? Analytical approaches for the detection and quantification of TiO2 engineered nanoparticles in surface waters. Environ Sci Nano Press. https://doi.org/10.1039/c1037en00952f
Laborda F, Bolea E, Cepria G, Gomez MT, Jimenez MS, Perez-Arantegui J, Castillo JR (2016) Detection, characterization and quantification of inorganic engineered nanomaterials: a review of techniques and methodological approaches for the analysis of complex samples. Anal Chim Acta 904:10–32
Philippe A, Schaumann GE (2014) Evaluation of hydrodynamic chromatography coupled with UV–visible, fluorescence and inductively coupled plasma mass spectrometry detectors for sizing and quantifying colloids in environmental media. PLoS ONE 9(2):0090559
Grainger DW, Castner DG (2008) Nanobiomaterials and nanoanalysis: opportunities for improving the science to benefit biomedical technologies. Adv Mater 20:867–877
Borovinskaya O, Gschwind S, Hattendorf B, Tanner M, Gunther D (2014) Simultaneous mass quantification of nanoparticles of different composition in a mixture by microdroplet generator-ICPTOFMS. Anal Chem 86(16):8142–8148
Praetorius A, Gundlach-Graham A, Goldberg E, Fabienke W, Navratilova J, Gondikas A, Kaegi R, Gunther D, Hofmann T, von der Kammer F (2017) Single-particle multi-element fingerprinting (spMEF) using inductively-coupled plasma time-of-flight mass spectrometry (ICP-TOFMS) to identify engineered nanoparticles against the elevated natural background in soils. Environ Sci Nano 4(2):307–314
Gogos A, Kaegi R, Zenobi R, Bucheli TD (2014) Capabilities of asymmetric flow field-flow fractionation coupled to multi-angle light scattering to detect carbon nanotubes in soot and soil. Environ Sci Nano 1(6):584–594
Petersen EJ, Flores-Cervantes DX, Bucheli TD, Elliott LCC, Fagan JA, Gogos A, Hanna S, Kagi R, Mansfield E, Bustos ARM, Plata DL, Reipa V, Westerhoff P, Winchester MR (2016) Quantification of carbon nanotubes in environmental matrices: current capabilities, case studies, and future prospects. Environ Sci Technol 50(9):4587–4605
Navratilova J, Praetorius A, Gondikas A, Fabienke W, von der Kammer F, Hofmann T (2015) Detection of engineered copper nanoparticles in soil using single particle ICP-MS. Int J Environ Res Public Health 12(12):15756–15768
Nowack B, Baalousha M, Bornhoft N, Chaudhry Q, Cornelis G, Cotterill J, Gondikas A, Hassellov M, Lead J, Mitrano DM, von der Kammer F, Wontner-Smith T (2015) Progress towards the validation of modeled environmental concentrations of engineered nanomaterials by analytical measurements. Environ Sci Nano 2(5):421–428
Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R (2008) Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol 42(23):8959–8964
Dale AL, Casman EA, Lowry GV, Lead JR, Viparelli E, Baalousha M (2015) Modeling nanomaterial environmental fate in aquatic systems. Environ Sci Technol 49(5):2587–2593
Peijnenburg WJGM, Baalousha M, Chen JW, Chaudry Q, Von der Kammer F, Kuhlbusch TAJ, Lead J, Nickel C, Quik JTK, Renker M, Wang Z, Koelmans AA (2015) A Review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment. Crit Rev Environ Sci Technol 45(19):2084–2134
Levard C, Hotze EM, Lowry GV, Brown GE (2012) Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ Sci Technol 46(13):6900–6914
Sekine R, Khaksar M, Brunetti G, Donner E, Scheckel KG, Lombi E, Vasilev K (2013) Surface immobilization of engineered nanomaterials for in situ study of their environmental transformations and fate. Environ Sci Technol 47(16):9308–9316
Sivry Y, Gelabert A, Cordier L, Ferrari R, Lazar H, Juillot F, Menguy N, Benedetti MF (2014) Behavior and fate of industrial zinc oxide nanoparticles in a carbonate-rich river water. Chemosphere 95:519–526
Stankus DP, Lohse SE, Hutchison JE, Nason JA (2011) Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environ Sci Technol 45(8):3238–3244
Li Y, Zhang W, Niu JF, Chen YS (2013) Surface-coating-dependent dissolution, aggregation, and reactive oxygen species (ROS) generation of silver nanoparticles under different irradiation conditions. Environ Sci Technol 47(18):10293–10301
Auffan M, Pedeutour M, Rose J, Masion A, Ziarelli F, Borschneck D, Chaneac C, Botta C, Chaurand P, Labille J, Bottero JY (2010) Structural degradation at the surface of a TiO2-based nanomaterial used in cosmetics. Environ Sci Technol 44(7):2689–2694
Baer DR, Engelhard MH, Johnson GE, Laskin J, Lai JF, Mueller K, Munusamy P, Thevuthasan S, Wang HF, Washton N, Elder A, Baisch BL, Karakoti A, Kuchibhatla SVNT, Moon D (2013) Surface characterization of nanomaterials and nanoparticles: important needs and challenging opportunities. J Vac Sci Technol A 31(5):50820
Barton LE, Auffan M, Bertrand M, Barakat M, Santaella C, Masion A, Borschneck D, Olivi L, Roche N, Wiesner MR, Bottero JY (2014) Transformation of pristine and citrate-functionalized CeO2 nanoparticles in a laboratory-scale activated sludge reactor. Environ Sci Technol 48(13):7289–7296
Elzey S, Grassian VH (2010) Agglomeration, isolation and dissolution of commercially manufactured silver nanoparticles in aqueous environments. J Nanopart Res 12(5):1945–1958
Mitrano DM, Ranville JF, Bednar A, Kazor K, Hering AS, Higgins CP (2014) Tracking dissolution of silver nanoparticles at environmentally relevant concentrations in laboratory, natural, and processed waters using single particle ICP-MS (spICP-MS). Environ Sci Nano 1(3):248–259
Metreveli G, Frombold B, Seitz F, Grün A, Phillippe A, Rosenfeldt RR, Bundschuh M, Schulz R, Manz W, Schaumann GE (2016) Impact of chemical composition of ecotoxicological test media on the stability and aggregation status of silver nanoparticles. Environ Sci Nano 3:418–433
Khaksar M, Jolley DF, Sekine R, Vasilev K, Johannessen B, Donner E, Lombi E (2015) In situ chemical transformations of silver nanoparticles along the water-sediment continuum. Environ Sci Technol 49(1):318–325
Dale AL, Lowry GV, Casman EA (2013) Modeling nanosilver transformations in freshwater sediments. Environ Sci Technol 47(22):12920–12928
Brunetti G, Donner E, Laera G, Sekine R, Scheckel KG, Khaksar M, Vasilev K, De Mastro G, Lombi E (2015) Fate of zinc and silver engineered nanoparticles in sewerage networks. Water Res 77:72–84
Liu JY, Hurt RH (2010) Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci Technol 44(6):2169–2175
Koser J, Engelke M, Hoppe M, Nogowski A, Filser J, Thoming J (2017) Predictability of silver nanoparticle speciation and toxicity in ecotoxicological media. Environ Sci Nano 4(7):1470–1483
Lowry GV, Espinasse BP, Badireddy AR, Richardson CJ, Reinsch BC, Bryant LD, Bone AJ, Deonarine A, Chae S, Therezien M, Colman BP, Hsu-Kim H, Bernhardt ES, Matson CW, Wiesner MR (2012) Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland. Environ Sci Technol 46(13):7027–7036
Gogos A, Thalmann B, Voegelin A, Kaegi R (2017) Sulfidation kinetics of copper oxide nanoparticles. Environ Sci Nano 4:1733–1741
Thalmann B, Voegelin A, Morgenroth E, Kaegi R (2016) Effect of humic acid on the kinetics of silver nanoparticle sulfidation. Environ Sci Nano 3(1):203–212
Kaegi R, Voegelin A, Ort C, Sinnet B, Thalmann B, Krismer J, Hagendorfer H, Elumelu M, Mueller E (2013) Fate and transformation of silver nanoparticles in urban wastewater systems. Water Res 47(12):3866–3877
Baalousha M, Arkill KP, Romer I, Palmer RE, Lead JR (2015) Transformations of citrate and Tween coated silver nanoparticles reacted with Na2S. Sci Total Environ 502:344–353
Liu JY, Pennell KG, Hurt RH (2011) Kinetics and mechanisms of nanosilver oxysulfidation. Environ Sci Technol 45(17):7345–7353
Kraas M, Schlich K, Knopf B, Wege F, Kagi R, Terytze K, Hund-Rinke K (2017) Long-term effects of sulfidized silver nanoparticles in sewage sludge on soil microflora. Environ Toxicol Chem 36:3305–3313
Lowry GV, Gregory KB, Apte SC, Lead JR (2012) Transformations of nanomaterials in the environment. Environ Sci Technol 46(13):6893–6899
Sani-Kast N, Labille J, Ollivier P, Slomberg D, Hungerbuhler K, Scheringer M (2017) A network perspective reveals decreasing material diversity in studies on nanoparticle interactions with dissolved organic matter. Proc Natl Acad Sci USA 114(10):E1756–E1765
Wang H, Adeleye AS, Huang Y, Li F, Keller AA (2015) Heteroaggregation of nanoparticles with biocolloids and geocolloids. Adv Coll Interface Sci 226:24–36
Cornelis G, Pang L, Doolette C, Kirby JK, McLaughlin MJ (2013) Transport of silver nanoparticles in saturated columns of natural soils. Sci Total Environ 463–464:120–130
Metreveli G, Philippe A, Schaumann GE (2015) Disaggregation of silver nanoparticle homoaggregates in a river water matrix. Sci Total Environ 535:35–44
Adam V, Loyaux-Lawniczak S, Labille J, Galindo C, del Nero M, Gangloff S, Weber T, Quaranta G (2016) Aggregation behaviour of TiO2 nanoparticles in natural river water. J Nanopart Res 18:13
Derjaguin BV, Landau L (1941) Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog Surf Sci 14:633–662
Verwey EJW, Overbeek JTG (1948) Theory of the stability of lyophobic colloids. Elsevier, Amsterdam
Baalousha M, Nur Y, Romer I, Tejamaya M, Lead JR (2013) Effect of monovalent and divalent cations, anions and fulvic acid on aggregation of citrate-coated silver nanoparticles. Sci Total Environ 354:119–131
Field TB, Coburn J, McCourt JL, McBryde WAE (1975) Composition and stability of some metal citrate and diglycolate complexes in aqueous solution. Anal Chim Acta 74:101–106
Li X, Lenhart JJ, Walker HW (2012) Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir 28:1095–1104
Klitzke S, Metreveli G, Peters A, Schaumann GE, Lang F (2015) The fate of silver nanoparticles in soil solution—sorption of solutes and aggregation. Sci Total Environ 535:54–60
Petosa AR, Jaisi DP, Quevedo IR, Elimelech M, Tufenkji N (2010) Aggregation and deposition of engineered nanomaterials in aquatic environments: role of physicochemical interactions. Environ Sci Technol 44(17):6532–6549
Omar FM, Aziz HA, Stoll S (2014) Aggregation and disaggregation of ZnO nanoparticles: influence of pH and adsorption of Suwannee River humic acid. Sci Total Environ 468:195–201
El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM (2011) Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol 45(11):283–287
Philippe A, Schaumann GE (2014) Interactions of dissolved organic matter with natural and engineered inorganic colloids: a review. Environ Sci Technol 48(16):8946–8962
Zhou DX, Ji ZX, Jiang XM, Dunphy DR, Brinker J, Keller AA (2013) Influence of material properties on TiO2 nanoparticle agglomeration. PLoS ONE 8(11):e81239
Atteia O, Perret D, Adatte T, Kozel R, Rossi P (1998) Characterization of natural colloids from a river and spring in a karstic basin. Environ Geol 34(4):257–269
Quik JTK, Stuart MC, Wouterse M, Peijnenburg W, Hendriks AJ, van de Meent D (2012) Natural colloids are the dominant factors in the sedimentation of nanoparticles. Environ Toxicol Chem 31(5):1019–1022
Huynh KA, McCaffery JM, Chen KL (2012) Heteroaggregation of multiwalled carbon nanotubes and hematite nanoparticles: rates and mechanisms. Environ Sci Technol 46(11):5912–5920
Quik JT, Velzeboer I, Wouterse M, Koelmans AA, van de Meent D (2014) Heteroaggregation and sedimentation rates for nanomaterials in natural waters. Water Res 48:269–279
Tso CP, Zhung CM, Shih YH, Tseng YM, Wu SC, Doong RA (2010) Stability of metal oxide nanoparticles in aqueous solutions. Water Sci Technol 61(1):127–133
Solovitch N, Labille J, Rose J, Chaurand P, Borschneck D, Wiesner MR, Bottero JY (2010) Concurrent aggregation and deposition of TiO2 nanoparticles in a sandy porous media. Environ Sci Technol 44(13):4897–4902
Cornelis G, Hund-Rinke K, Kuhlbusch T, van den Brink N, Nickel C (2014) Fate and bioavailability of engineered nanoparticles in soils: a review. Crit Rev Environ Sci Technol 44:2720–2764
Jiang X, Tong M, Kim H (2012) Influence of natural organic matter on the transport and deposition of zinc oxide nanoparticles in saturated porous media. J Colloid Interface Sci 386(1):34–43
Sagee O, Dror I, Berkowitz B (2012) Transport of silver nanoparticles (AgNPs) in soil. Chemosphere 88(5):670–675
Kumahor SK, Hron P, Metreveli G, Schaumann GE, Klitzke S, Lang F, Vogel HJ (2016) Transport of soil-aged silver nanoparticles in unsaturated sand. J Contam Hydrol 195:31–39
Liu L, Gao B, Wu L, Morales VL, Yang LY, Zhou ZH, Wang H (2013) Deposition and transport of graphene oxide in saturated and unsaturated porous media. Chem Eng J 229:444–449
Fang J, Xu MJ, Wang DJ, Wen B, Han JY (2013) Modeling the transport of TiO2 nanoparticle aggregates in saturated and unsaturated granular media: effects of ionic strength and pH. Water Res 47(3):1399–1408
Kumahor SK, Hron P, Metreveli G, Schaumann GE, Vogel HJ (2015) Transport of citrate-coated silver nanoparticles in unsaturated sand. Sci Total Environ 535:113–121
Hoppe M, Mikutta R, Utermann J, Duijnisveld W, Guggenberger G (2014) Retention of sterically and electrosterically stabilized silver nanoparticles in soils. Environ Sci Technol 48(21):12628–12635
Markus AA, Parsons JR, Roex EWM, de Voogt P, Laane RWPM (2015) Modeling aggregation and sedimentation of nanoparticles in the aquatic environment. Sci Total Environ 506:323–329
de Klein JJM, Quik JTK, Bauerlein PS, Koelmans AA (2016) Towards validation of the NanoDUFLOW nanoparticle fate model for the river Dommel, The Netherlands. Environ Sci Nano 3(2):434–441
Meesters JAJ, Quik JTK, Koelmans AA, Hendriks AJ, van de Meent D (2016) Multimedia environmental fate and speciation of engineered nanoparticles: a probabilistic modeling approach. Environ Sci Nano 3(4):715–727
Praetorius A, Scheringer M, Hungerbuhler K (2012) Development of environmental fate models for engineered nanoparticles—a case study of TiO2 nanoparticles in the Rhine River. Environ Sci Technol 46:6705–6713
Moore MN (2006) Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int 32:967–976
Hund-Rinke K, Simon M (2006) Ecotoxic effect of photocatalytic active nanoparticles TiO2 on algae and daphnids. Environ Sci Pollut Res 13(4):225–232
von Moos N, Slaveykova VI (2014) Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae—state of the art and knowledge gaps. Nanotoxicology 8(6):605–630
Mwaanga P, Carraway ER, van den Hurk P (2014) The induction of biochemical changes in Daphnia magna by CuO and ZnO nanoparticles. Aquat Toxicol 150:201–209
Wu Y, Zhou QF (2012) Dose- and time-related changes in aerobic metabolism, chorionic disruption, and oxidative stress in embryonic medaka (Oryzias latipes): underlying mechanisms for silver nanoparticle developmental toxicity. Aquat Toxicol 124:238–246
Muller EB, Lin SJ, Nisbet RM (2015) Quantitative adverse outcome pathway analysis of hatching in zebrafish with CuO nanoparticles. Environ Sci Technol 49(19):11817–11824
Nair PMG, Park SY, Lee SW, Choi J (2011) Differential expression of ribosomal protein gene, gonadotrophin releasing hormone gene and Balbiani ring protein gene in silver nanoparticles exposed Chironomus riparius. Aquat Toxicol 101(1):31–37
Bundschuh M, Seitz F, Rosenfeldt RR, Schulz R (2012) Titanium dioxide nanoparticles increase sensitivity in the next generation of the water flea Daphnia magna. PLoS ONE 7(11):e48956
Jacobasch C, Völker C, Giebner S, Völker J, Alsenz H, Potouridis T, Heidenreich H, Kayser G, Oehlmann J, Oetken M (2014) Long-term effects of nanoscaled titanium dioxide on the cladoceran Daphnia magna over six generations. Environ Pollut 186:180–186
Zou XY, Li PH, Huang Q, Zhang HW (2016) The different response mechanisms of Wolffia globosa: light-induced silver nanoparticle toxicity. Aquat Toxicol 176:97–105
Jiang HS, Yin LY, Ren NN, Zhao ST, Li Z, Zhi YW, Shao H, Li W, Gontero B (2017) Silver nanoparticles induced reactive oxygen species via photosynthetic energy transport imbalance in an aquatic plant. Nanotoxicology 11(2):157–167
Thwala M, Klaine SJ, Musee N (2016) Interactions of metal-based engineered nanoparticles with aquatic higher plants: a review of the state of current knowledge. Environ Toxicol Chem 35(7):1677–1694
Du WC, Tan WJ, Peralta-Videa JR, Gardea-Torresdey JL, Ji R, Yin Y, Guo HY (2017) Interaction of metal oxide nanoparticles with higher terrestrial plants: physiological and biochemical aspects. Plant Physiol Biochem 110:210–225
Marslin G, Sheeba CJ, Franklin G (2017) Nanoparticles alter secondary metabolism in plants via ROS burst. Front Plant Sci 8:832
Tripathi DK, Shweta, Singh S, Singh S, Pandey R, Singh VP, Sharma NC, Prasad SM, Dubey NK, Chauhan DK (2017) An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity. Plant Physiol Biochem 110:2–12
Cao ZM, Stowers C, Rossi L, Zhang WL, Lombardini L, Ma XM (2017) Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (Glycine max (L.) Merr.). Environ Sci Nano 4(5):1086–1094
Batley GE, Kirby JK, McLaughlin MJ (2013) Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc Chem Res 46(3):854–862
Gil-Allué C, Schirmer K, Tlili A, Gessner MO, Behra R (2015) Silver nanoparticle effects on stream periphyton during short-term exposures. Environ Sci Technol 49(2):1165–1172
Jo HJ, Choi JW, Lee SH, Hong SW (2012) Acute toxicity of Ag and CuO nanoparticle suspensions against Daphnia magna: the importance of their dissolved fraction varying with preparation methods. J Hazard Mater 227–228:301–308
Volker C, Kampken I, Boedicker C, Oehlmann J, Oetken M (2015) Toxicity of silver nanoparticles and ionic silver: comparison of adverse effects and potential toxicity mechanisms in the freshwater clam Sphaerium corneum. Nanotoxicology 9(6):677–685
Doolette CL, Gupta VVSR, Lu Y, Payne JL, Batstone DJ, Kirby JK, Navarro DA, McLaughlin MJ (2016) Quantifying the sensitivity of soil microbial communities to silver sulfide nanoparticles using metagenome sequencing. PLoS ONE 11(8):e0161979
Nair PM, Park SY, Choi J (2013) Evaluation of the effect of silver nanoparticles and silver ions using stress responsive gene expression in Chironomus riparius. Chemosphere 92(5):592–599
Griffitt RJ, Hyndman K, Denslow ND, Barber DS (2009) Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicol Sci 107(2):404–415
Poynton HC, Lazorchak JM, Impellitteri CA, Blalock BJ, Rogers K, Allen HJ, Loguinov A, Heckrnan JL, Govindasmawy S (2012) Toxicogenomic responses of nanotoxicity in Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Environ Sci Technol 46(11):6288–6296
Patricia CS, Nerea GV, Erik U, Elena SM, Eider B, Dario DW, Manu S (2017) Responses to silver nanoparticles and silver nitrate in a battery of biomarkers measured in coelomocytes and in target tissues of Eisenia fetida earthworms. Ecotoxicol Environ Saf 141:57–63
Völker C, Oetken M, Oehlmann J (2013) The biological effects and possible modes of action of nanosilver. Rev Environ Contam Toxicol 233:81–106
Yang YF, Cheng YH, Liao CM (2017) Nematode-based biomarkers as critical risk indicators on assessing the impact of silver nanoparticles on soil ecosystems. Ecol Ind 75:340–351
Hoheisel SM, Diamond S, Mount D (2012) Comparison of nanosilver and ionic silver toxicity in Daphnia magna and Pimephales promelas. Environ Toxicol Chem 31(11):2557–2563
Seitz F, Rosenfeldt RR, Strom K, Metrevelli G, Schaumann GE, Schulz R, Bundschuh M (2015) Effects of silver nanoparticle properties, media pH and dissolved organic matter on toxicity to Daphnia magna. Ecotoxicol Environ Saf 111:263–270
Pradas del Real AE, Vidal V, Carriere M, Castillo-Michel HA, Levard C, Chaurand P, Sarret G (2017) Ag nanoparticles and wheat roots: a complex interplay. Environ Sci Technol. https://doi.org/10.1021/acs.est.1027b00422
Wang P, Lombi E, Sun SK, Scheckel KG, Malysheva A, McKenna BA, Menzies NW, Zhao FJ, Kopittke PM (2017) Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ Sci Nano 4(2):448–460
Dabrunz A, Duester L, Prasse C, Seitz F, Rosenfeldt RR, Schilde R, Schaumann GE, Schulz R (2011) Biological surface coating and molting inhibition as mechanisms of TiO2 nanoparticle toxicity in Daphnia magna. PLoS ONE 6(5):e20112
Baumann J, Koser J, Arndt D, Filser J (2014) The coating makes the difference: acute effects of iron oxide nanoparticles on Daphnia magna. Sci Total Environ 484:176–184
Gomes T, Chora S, Pereira CG, Cardoso C, Bebianno MJ (2014) Proteomic response of mussels Mytilus galloprovincialis exposed to CuO NPs and Cu2+: an exploratory biomarker discovery. Aquat Toxicol 155:327–336
Mortimer M, Kasemets K, Vodovnik M, Marinsek-Logar R, Kahru A (2011) Exposure to CuO nanoparticles changes the fatty acid composition of protozoa Tetrahymena thermophila. Environ Sci Technol 45(15):6617–6624
Adams J, Wright M, Wagner H, Valiente J, Britt D, Anderson A (2017) Cu from dissolution of CuO nanoparticles signals changes in root morphology. Plant Physiol Biochem 110:108–117
Pradhan A, Seena S, Schlosser D, Gerth K, Helm S, Dobritzsch M, Krauss GJ, Dobritzsch D, Pascoal C, Cassio F (2015) Fungi from metal-polluted streams may have high ability to cope with the oxidative stress induced by copper oxide nanoparticles. Environ Toxicol Chem 34(4):923–930
Fernandez-Cruz ML, Lammel T, Connolly M, Conde E, Barrado AI, Derick S, Perez Y, Fernandez M, Furger C, Navas JM (2013) Comparative cytotoxicity induced by bulk and nanoparticulated ZnO in the fish and human hepatoma cell lines PLHC-1 and Hep G2. Nanotoxicology 7(5):935–952
Nair PMG, Chung IM (2015) Alteration in the expression of antioxidant and detoxification genes in Chironomus riparius exposed to zinc oxide nanoparticles. Comp Biochem Phys B 190:1–7
Su GY, Zhang XW, Giesy JP, Musarrat J, Saquib Q, Alkhedhairy AA, Yu HX (2015) Comparison on the molecular response profiles between nano zinc oxide (ZnO) particles and free zinc ion using a genome-wide toxicogenomics approach. Environ Sci Pollut Res 22(22):17434–17442
Marisa I, Matozzo V, Munari M, Binelli A, Parolini M, Martucci A, Franceschinis E, Brianese N, Marin MG (2016) In vivo exposure of the marine clam Ruditapes philippinarum to zinc oxide nanoparticles: responses in gills, digestive gland and haemolymph. Environ Sci Pollut Res 23(15):15275–15293
Huang CW, Li SW, Liao VHC (2017) Chronic ZnO-NPs exposure at environmentally relevant concentrations results in metabolic and locomotive toxicities in Caenorhabditis elegans. Environ Pollut 220:1456–1464
Gomes SIL, Roca CP, Scott-Fordsmand JJ, Amorim MJB (2017) High-throughput transcriptomics reveals uniquely affected pathways: AgNPs, PVP-coated AgNPs and Ag NM300K case studies. Environ Sci Nano 4(4):929–937
Makama S, Piella J, Undas A, Dimmers WJ, Peters R, Puntes VF, van den Brink NW (2016) Properties of silver nanoparticles influencing their uptake in and toxicity to the earthworm Lumbricus rubellus following exposure in soil. Environ Pollut 218:870–878
Tan WJ, Du WC, Barrios AC, Armendariz R, Zuverza-Mena N, Ji ZX, Chang CH, Zink JI, Hernandez-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL (2017) Surface coating changes the physiological and biochemical impacts of nano-TiO2 in basil (Ocimum basilicum) plants. Environ Pollut 222:64–72
Garcia-Gomez C, Obrador A, Gonzalez D, Babin M, Fernandez MD (2017) Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci Total Environ 589:11–24
Oberdörster E, Zhu S, Blickley TM, McClellan-Green P, Haasch ML (2006) Ecotoxicology of carbon-based engineered nanoparticles: effects of fullerene (C60) on aquatic organisms. Carbon 44:1112–1120
Noss C, Dabrunz A, Rosenfeldt RR, Lorke A, Schulz R (2013) Three-dimensional analysis of the swimming behavior of Daphnia magna exposed to nanosized titanium dioxide. PLoS ONE 8(11):e80960
Van Hoecke K, De Schamphelaere KAC, Van der Meeren P, Lucas S, Janssen CR (2008) Ecotoxicity of silica nanoparticles to the green alga Pseudokirchneriella subcapitata: importance of surface area. Environ Toxicol Chem 27(9):1948–1957
Van Hoecke K, Quik JTK, Mankiewicz-Boczek J, De Schamphelaere KAC, Elsaesser A, Van der Meeren P, Barnes C, McKerr G, Howard CV, Van De Meent D, Rydzynski K, Dawson KA, Salvati A, Lesniak A, Lynch I, Silversmit G, De Samber B, Vincze L, Janssen CR (2009) Fate and effects of CeO2 nanoparticles in aquatic ecotoxicity tests. Environ Sci Technol 43(12):4537–4546
Moon J, Kwak JI, Kim SW, An YJ (2017) Multigenerational effects of gold nanoparticles in Caenorhabditis elegans: continuous versus intermittent exposures. Environ Pollut 220:46–52
Goncalves MFM, Gomes SIL, Scott-Fordsmand JJ, Amorim MJB (2017) Shorter lifetime of a soil invertebrate species when exposed to copper oxide nanoparticles in a full lifespan exposure test. Sci Rep UK 7:1355
Rico CM, Johnson MG, Marcus MA, Andersen CP (2017) Intergenerational responses of wheat (Triticum aestivum L.) to cerium oxide nanoparticles exposure. Environ-Sci Nano 4(3):700–711
Bundschuh M, Seitz F, Rosenfeldt RR, Schulz R (2016) Effects of nanoparticles in fresh waters—risks, mechanisms and interactions. Freshw Biol 61:2185–2196
Docter D, Westmeier D, Markiewicz M, Stolte S, Knauer SK, Stauber RH (2015) The nanoparticle biomolecule corona: lessons learned—challenge accepted? Chem Soc Rev 44(17):6094–6121
Hall S, Bradley T, Moore JT, Kuykindall T, Minella L (2009) Acute and chronic toxicity of nano-scale TiO2, particles to freshwater fish, cladocerans, and green algae, and effects of organic and inorganic substrate on TiO2 toxicity. Nanotoxicology 3(2):91–97
Hyung H, Fortner JD, Hughes JB, Kim JH (2007) Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environ Sci Technol 41(1):179–184
Seitz F, Rosenfeldt RR, Müller M, Lüderwald S, Schulz R, Bundschuh M (2016) Quantity and quality of natural organic matter influence the ecotoxicity of titanium dioxide nanoparticles. Nanotoxicology 10(10):1415–1421
Park S, Woodhall J, Ma G, Veinot JGC, Boxall AB (2015) Does particle size and surface functionality affect uptake and depuration of gold nanoparticles by aquatic invertebrates? Environ Toxicol Chem 34(4):850–859
Li CC, Wang YJ, Dang F, Zhou DM (2016) Mechanistic understanding of reduced AgNP phytotoxicity induced by extracellular polymeric substances. J Hazard Mater 308:21–28
Chiang CW, Ng DQ, Lin YP, Chen PJ (2016) Dissolved organic matter or salts change the bioavailability processes and toxicity of the nanoscale tetravalent lead corrosion product PbO2 to medaka fish. Environ Sci Technol 50(20):11292–11301
Bicho RC, Soares AMVM, Nogueira HIS, Amorim MJB (2016) Effects of europium polyoxometalate encapsulated in silica nanoparticles (nanocarriers) in soil invertebrates. J Nanopart Res 18(12):360
Tourinho PS, van Gestel CAM, Lofts S, Svendsen C, Soares AMVM, Loureiro S (2012) Metal-based nanoparticles in soil: fate, behavior, and effects on soil invertebrates. Environ Toxicol Chem 31(8):1679–1692
Handy RD, Cornelis G, Fernandes T, Tsyusko O, Decho A, Sabo-Attwood T, Metcalfe C, Steevens JA, Klaine SJ, Koelmans AA, Horne N (2012) Ecotoxicity test methods for engineered nanomaterials: practical experiences and recommendations from the bench. Environ Toxicol Chem 31(1):15–31
Topuz E, van Gestel CAM (2017) The effect of soil properties on the toxicity and bioaccumulation of Ag nanoparticles and Ag ions in Enchytraeus crypticus. Ecotoxicol Environ Saf 144:330–337
Velicogna JR, Ritchie EE, Scroggins RP, Princz JI (2016) A comparison of the effects of silver nanoparticles and silver nitrate on a suite of soil dwelling organisms in two field soils. Nanotoxicology 10(8):1144–1151
Waalewijn-Kool PL, Ortiz MD, Lofts S, van Gestel CAM (2013) The effect of pH on the toxicity of zinc oxide nanoparticles to Folsomia candida in amended field soil. Environ Toxicol Chem 32(10):2349–2355
Cupi D, Hartmann NB, Baun A (2015) The influence of natural organic matter and aging on suspension stability in guideline toxicity testing of silver, zinc oxide, and titanium dioxide nanoparticles with Daphnia magna. Environ Toxicol Chem 34(3):497–506
Seitz F, Rosenfeldt RR, Lüderwald S, Schulz R, Bundschuh M (2015) Aging of TiO2 nanoparticles transiently increases their toxicity to the pelagic microcrustacean Daphnia magna. PLoS ONE 10(5):e0126021
McKee MS, Engelke M, Zhang X, Lesnikov E, Köser J, Eickhorst T, Filser J (2017) Collembola reproduction decreases with aging of silver nanoparticles in a sewage sludge-treated soil. Front Environ Sci 5:19
Jackson BP, Bugge D, Ranville JF, Chen CY (2012) Bioavailability, toxicity, and bioaccumulation of quantum dot nanoparticles to the amphipod Leptocheirus plumulosus. Environ Sci Technol 46(10):5550–5556
Campos B, Rivetti C, Rosenkranz P, Navas JM, Barata C (2013) Effects of nanoparticles of TiO(2) on food depletion and life-history responses of Daphnia magna. Aquat Toxicol 130–131:174–183
Bundschuh M, Vogt R, Seitz F, Rosenfeldt RR, Schulz R (2016) Do titanium dioxide nanoparticles induce food depletion for filter feeding organisms? A case study with Daphnia magna. Environ Pollut 214:840–846
Rosenfeldt RR, Seitz F, Zubrod JP, Feckler A, Merkel T, Lüderwald S, Bundschuh R, Schulz R, Bundschuh M (2015) Does the presence of titanium dioxide nanoparticles reduce copper toxicity? A factorial approach with the benthic amphipod Gammarus fossarum. Aquat Toxicol 165:154–159
Zhang XZ, Sun HW, Zhang ZY, Niu Q, Chen YS, Crittenden JC (2007) Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere 67(1):160–166
Schwab F, Bucheli TD, Camenzuli L, Magrez A, Knauer K, Sigg L, Nowack B (2013) Diuron sorbed to carbon nanotubes exhibits enhanced toxicity to Chlorella vulgaris. Environ Sci Technol 47(13):7012–7019
Brausch KA, Anderson TA, Smith PN, Maul JD (2010) Effects of functionalized fullerenes on bifenthrin and tribufos toxicity to Daphnia magna: survival, reproduction, and growth rate. Environ Toxicol Chem 29(11):2600–2606
Boncel S, Kyziol-Komosinska J, Krzyewska I, Czupiol J (2015) Interactions of carbon nanotubes with aqueous/aquatic media containing organic/inorganic contaminants and selected organisms of aquatic ecosystems—a review. Chemosphere 136:211–221
Sanchis J, Olmos M, Vincent P, Farre M, Barcelo D (2016) New insights on the influence of organic co-contaminants on the aquatic toxicology of carbon nanomaterials. Environ Sci Technol 50(2):961–969
Fang Q, Shi XJ, Zhang LP, Wang QW, Wang XF, Guo YY, Zhou BS (2015) Effect of titanium dioxide nanoparticles on the bioavailability, metabolism, and toxicity of pentachlorophenol in zebrafish larvae. J Hazard Mater 283:897–904
Farkas J, Bergum S, Nilsen EW, Olsen AJ, Salaberria I, Ciesielski TM, Baczek T, Konieczna L, Salvenmoser W, Jenssen BM (2015) The impact of TiO2 nanoparticles on uptake and toxicity of benzo(a)pyrene in the blue mussel (Mytilus edulis). Sci Total Environ 511:469–476
Qiang LW, Pan XY, Zhu LY, Fang SH, Tian SY (2016) Effects of nano-TiO2 on perfluorooctanesulfonate bioaccumulation in fishes living in different water layers: implications for enhanced risk of perfluorooctanesulfonate. Nanotoxicology 10(4):471–479
Fan WH, Cui MM, Liu H, Wang CA, Shi ZW, Tan C, Yang XP (2011) Nano-TiO2 enhances the toxicity of copper in natural water to Daphnia magna. Environ Pollut 159(3):729–734
Ma TW, Wang M, Gong SJ, Tian B (2017) Impacts of sediment organic matter content and pH on ecotoxicity of coexposure of TiO2 nanoparticles and cadmium to freshwater snails Bellamya aeruginosa. Arch Environ Contam Toxicol 72(1):153–165
Chen JY, Qian Y, Li HR, Cheng YH, Zhao MR (2015) The reduced bioavailability of copper by nano-TiO2 attenuates the toxicity to Microcystis aeruginosa. Environ Sci Pollut Res 22(16):12422–12429
Li XM, Zhou SY, Fan WH (2016) Effect of nano-Al2O3 on the toxicity and oxidative stress of copper towards Scenedesmus obliquus. Int J Environ Res Public Health 13(6):575
Della Torre C, Balbi T, Grassi G, Frenzilli G, Bernardeschi M, Smerilli A, Guidi P, Canesi L, Nigro M, Monaci F, Scarcelli V, Rocco L, Focardi S, Monopoli M, Corsi I (2015) Titanium dioxide nanoparticles modulate the toxicological response to cadmium in the gills of Mytilus galloprovincialis. J Hazard Mater 297:92–100
Fan WH, Peng RS, Li XM, Ren JQ, Liu T, Wang XR (2016) Effect of titanium dioxide nanoparticles on copper toxicity to Daphnia magna in water: role of organic matter. Water Res 105:129–137
Rosenfeldt RR, Seitz F, Schulz R, Bundschuh M (2014) Heavy metal uptake and toxicity in the presence of titanium dioxide nanoparticles: a factorial approach using Daphnia magna. Environ Sci Technol 48:6965–6972
Canesi L, Ciacci C, Balbi T (2015) Interactive effects of nanoparticles with other contaminants in aquatic organisms: friend or foe? Mar Environ Res 111:128–134
McKee MS, Filser J (2016) Impacts of metal-based engineered nanomaterials on soil communities. Environ Sci Nano 3(3):506–533
Wang XH, Li J, Liu R, Hai RT, Zou DX, Zhu XB, Luo N (2017) Responses of bacterial communities to CuO nanoparticles in activated sludge system. Environ Sci Technol 51(10):5368–5376
Button M, Auvinen H, Van Koetsem F, Hosseinkhani B, Rousseau D, Weber KP, Du Laing G (2016) Susceptibility of constructed wetland microbial communities to silver nanoparticles: a microcosm study. Ecol Eng 97:476–485
Ergon-Can T, Koseoglu-Imer DY, Algur OF, Koyuncu I (2016) Effect of different nanomaterials on the metabolic activity and bacterial flora of activated sludge medium. Clean Soil Air Water 44(11):1508–1515
Pallavi Mehta CM, Srivastava R, Arora S, Sharma AK (2016) Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 6:254
Michels C, Perazzoli S, Soares HM (2017) Inhibition of an enriched culture of ammonia oxidizing bacteria by two different nanoparticles: silver and magnetite. Sci Total Environ 586:995–1002
Wang JA, Shu KH, Zhang L, Si YB (2017) Effects of silver nanoparticles on soil microbial communities and bacterial nitrification in suburban vegetable soils. Pedosphere 27(3):482–490
Cao JL, Feng YZ, Lin XG, Wang JH, Xie XQ (2017) Iron oxide magnetic nanoparticles deteriorate the mutual interaction between arbuscular mycorrhizal fungi and plant. J Soils Sediments 17(3):841–851
Rashid MI, Shahzad T, Shahid M, Imran M, Dhavamani J, Ismail IMI, Basahi JM, Almeelbi T (2017) Toxicity of iron oxide nanoparticles to grass litter decomposition in a sandy soil. Sci Rep UK 7:41965. https://doi.org/10.1038/srep41965
Antisari LV, Carbone S, Gatti A, Ferrando S, Nacucchi M, De Pascalis F, Gambardella C, Badalucco L, Laudicina VA (2016) Effect of cobalt and silver nanoparticles and ions on Lumbricus rubellus health and on microbial community of earthworm faeces and soil. Appl Soil Ecol 108:62–71
Jemec A, Kos M, Drobne D, Koponen IK, Vuki J, Ferreira NGC, Loureiro S, McShane HVA (2016) In field conditions, commercial pigment grade TiO2 was not harmful to terrestrial isopods but reduced leaf litter fragmentation. Sci Total Environ 571:1128–1135
Garcia-Velasco N, Gandariasbeitia M, Irizar A, Soto M (2016) Uptake route and resulting toxicity of silver nanoparticles in Eisenia fetida earthworm exposed through Standard OECD Tests. Ecotoxicology 25(8):1543–1555
Lüderwald S, Schell T, Seitz F, Rosenfeldt RR, Newton K, Dackermann V, Schulz R, Bundschuh M Exposure pathway depended impacts of silver and titanium dioxide nanoparticles on Gammarus fossarum (in preparation)
Das P, Williams CJ, Fulthorpe RR, Hoque ME, Metcalfe CD, Xenopoulos MA (2012) Changes in bacterial community structure after exposure to silver nanoparticles in natural waters. Environ Sci Technol 46(16):9120–9128
Bour A, Mouchet F, Cadarsi S, Silvestre J, Verneuil L, Baque D, Chauvet E, Bonzom J-M, Pagnout C, Clivot H, Fourquaux I, Tella M, Auffan M, Gauthier L, Pinelli E (2016) Toxicity of CeO2 nanoparticles on a freshwater experimental trophic chain: a study in environmentally relevant conditions through the use of mesocosms. Nanotoxicology 10:245–255
Pradhan A, Seena S, Pascoal C, Cassio F (2011) Can metal nanoparticles be a threat to microbial decomposers of plant litter in streams? Microb Ecol 62:58–68
Kalčíková G, Englert D, Rosenfeldt RR, Seitz F, Schulz R, Bundschuh M (2014) Combined effect of UV-irradiation and TiO2-nanoparticles on the predator-prey interaction of gammarids and mayfly nymphs. Environ Pollut 186:136–140
Pang C, Neubauer N, Boyles M, Brown D, Kanase N, Hristozov D, Fernandes T, Stone V, Wohlleben W, Marcomini A (2017) Releases from transparent blue automobile coatings containing nanoscale copper phthalocyanine and their effects on J774 A1 macrophages. Nanoimpact 7:75–83
Authors’ contributions
MB initiated the paper; all authors contributed to the content in various ways. Therefore, only the main responsibilities for each of the chapters are highlighted. “Introduction”: MB, RS; “Pathways of nanoparticles into natural ecosystems”: SW, MB; “Predicting and measuring nanoparticles in natural ecosystems”: SW; “Fate of nanoparticles in the environment”: GM, SW, GS; “Effects of nanoparticles and their mechanisms of toxicity”: MB, RS; “Effects of nanoparticles on individuals and populations”: JF, MM, MB, RS; “Nanoparticle induced effects over multiple generations”: JF, MM, SL; “Interactions of nanoparticles in a complex environment”: JF, MM, MB, SL; Effects in communities and trophic interaction: JF, MM. Figures: SW, SL. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge JP Zubrod for inviting us to submit this review to this journal. We also thank the reviewers and the editor for their insightful comments improving the quality of this document.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This work has been partly funded within the research group INTERNANO supported by the German Research Foundation (DFG; SCHU2271/5-2; SCHA849/16-2).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bundschuh, M., Filser, J., Lüderwald, S. et al. Nanoparticles in the environment: where do we come from, where do we go to?. Environ Sci Eur 30, 6 (2018). https://doi.org/10.1186/s12302-018-0132-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-018-0132-6