Abstract
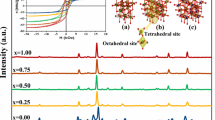
To create the catalytically active ceria-based nanocrystallites with the superior anti-sintering and anti-coking behavior, the peculiarity of genesis and properties of Ni/Ce1-xMxOy nanocrystallites (M = Gd, La, Mg; x = 0–0.5; 1.5 ≤ y ≤ 2.0) were studied by thermal analysis, N2 adsorption, X-ray diffraction, transmission, and scanning electron microscopy. The control of nanocrystallite characteristics was achieved by tuning of metal-support interaction through the application of different synthesis method (polymerizable complex method, sol–gel template method), doping, and conditions of thermal treatment (300-800 °C, oxidizing and reducing atmospheres). From undoped CeO2, the mesoporous nanosized Ce1-xMxOy solid solutions are distinguished by higher surface area (95 vs. 155 m2/g), smaller crystallite size (15 vs. 5 nm), and advanced thermal stability. The supported Ce1-xMxOy material Ni species of different dispersion and reducibility were prepared through regulation of composition and crystallite sizes of CexM1-xOy.The Ni dispersion increases upon a decrease of support crystallite sizes, with an increase of mole fraction of M and in the following row of doping cations: Gd ˂ Mg ˂ La. The presence of La or Mg in the nanocrystallite composition promotes the stability against sintering while small particle size provides the anti-coking resistance. The developed Ni/Ce1-xMxOy nanocrystallites were remarkable for their performance in autothermal conversion of ethanol and stability against carbonaceous deposits.














Similar content being viewed by others
Abbreviations
- BET:
-
Brunauer–Emmett–Teller
- CSR:
-
Coherent scattering region
- DTA:
-
Differential thermal analysis
- EDS:
-
Energy-dispersive spectroscopy
- HRTEM:
-
High-resolution transmission electron microscopy
- HAADF-STEM:
-
High-angle annular dark-field imaging scanning transmission electron microscopy
- OSC:
-
Oxygen storage capacity
- SEM:
-
Scanning electron microscopy
- TGA:
-
Thermogravimetric analyses
- XRD:
-
X-ray diffraction
- XRF:
-
X-ray fluorescence spectroscopy
References
Andana T, Piumetti M, Bensaid S, Veyre L, Thieuleux C, Russo N, Fino D, Quadrelli EA, Pironea R (2017) Ceria-supported small Pt and Pt3Sn nanoparticles for NOx-assisted soot oxidation. Appl Catal B 209:295–310
Aneggi E, Boaro M, Colussi S, de Leitenburg C, Trovarellin A (2016) Ceria-based materials in catalysis: historical perspective and future trends. Handb Phys Chem Rare Earths 50:209–242
Avram D, Sanchez-Dominguez M, Cojocaru B, Florea M, Parvulescu V, Tiseanu C (2015) Toward a unified description of luminescence-local structure correlation in Ln doped CeO2 nanoparticles: roles of Ln ionic radius, Ln concentration, and oxygen vacancies. J Phys Chem C 119:16303–16313
Bengaard HS, Nørskov JK, Sehested J, Clausen BS, Nielsen LP, Molenbroek AM, Rostrup-Nielsen JR (2002) Steam reforming and graphite formation on Ni catalysts. J Catal 209:365–384
Brezesinski T, Antonietti M, Groenewolt M, Pinna N, Smarsly B (2005) The generation of mesostructured crystalline CeO2, ZrO2 and CeO2–ZrO2 films using evaporation-induced self-assembly. New J Chem 29:237–242
Cai W, Wang F, Zhan E, Van Veen AC, Mirodatos C, Shen W (2008) Hydrogen production from ethanol over Ir/CeO2 catalysts: a comparative study of steam reforming, partial oxidation and oxidative steam reforming. J Catal 257:96–107
Cai W, Wang F, Daniel C, van Veen AC, Schuurman Y, Descorme C, Provendier H, Shen W, Mirodatos C (2012) Oxidative steam reforming of ethanol over Ir/CeO2 catalysts: a structure sensitivity analysis. J Catal 286:137–152
Chan SH, Wang HM (2000) Effect of natural gas composition on autothermal fuel reforming products. Fuel Process Technol 64:221–239
Christensen KO, Chen D, Lødeng R, Holmen A (2006) Effect of supports and Ni crystal size on carbon formation and sintering during steam methane reforming. Appl Catal A 314:9–22
Dantas SC, Resend KA, Avila-Neto CN, Noronha FB, Bueno JMC, Hori CE (2016) Nickel supported catalysts for hydrogen production by reforming of ethanol as addressed by in situ temperature and spatial resolved XANES analysis. Int J Hydrog Energy 41:3399–3413
Deng J, Chu W, Wang B, Yanga W, Zhao XS (2016) Mesoporous Ni/Ce1−xNixO2−y heterostructure as an efficient catalyst for converting greenhouse gas to H2 and syngas. Catal Sci Technol 6:851–862
Divins NJ, Casanovas A, Xu W, Senanayake SD, Wiater D, Trovarelli A, Llorca J (2015) The influence of nano-architectured CeOx supports in RhPd/CeO2 for the catalytic ethanol steam reforming reaction. Catal Today 253:99–105
Dong Q, Yin S, Guo C, Sato T (2012) Aluminum-doped ceria-zirconia solid solutions with enhanced thermal stability and high oxygen storage capacity. Nanoscale Res Lett 7:542
Duarte RB, Safonova OV, Krumeich F, Makosch M, van Bokhoven JA (2013) Oxidation state of Ce in CeO2-Promoted Rh/Al2O3catalysts during methane steam reforming: H2O activation and alumina stabilization. ACS Catal 3:1956–1964
Dulgheru P, Sullivan JA (2013) Rare earth (La, Nd, Pr) doped ceria zirconia solid solutions for soot combustion. Top Catal 56:504–510
Farbun IA, Romanova IV, Terikovskaya TE, Dzanashvili DI, Kirillov SA (2007) Complex formation in the course of synthesis of zinc oxide from citrate solutions. Russ J Appl Chem 80(11):1798–1803
Gaki A, Anagnostaki O, Kioupis D, Perraki T, Gakis D, Kakali G (2008) Optimization of LaMO3 (M: Mn, co, Fe) synthesis through the polymeric precursor route. J Alloys Compd 451:305–308
Gosavi PV, Biniwale RB (2012) Effective cleanup of CO in hydrogen by PROX over perovskite and mixed oxides. Int J Hydrog Energy 37:3958–3963
Han X, Yu Y, He H, Shan W (2013) Hydrogen production from oxidative steam reforming of ethanol over rhodium catalysts supported on Ce-La solid solution. Int J Hydrog Energy 38:10293–10304
Hong W, Lijuan Z, Miao L, Yuan L, Xue B (2013) Co/CeO2 for ethanol steam reforming: effect of ceria morphology. J Rare Earths 31(6):565–571
Ismagilov IZ, Matus EV, Kuznetsov VV, Mota N, Navarro RM, Kerzhentsev MA, Ismagilov ZR, Fierro JLG (2013) Nanoscale control during synthesis of Me/La2O3, Me/CexGd1−xOy and Me/CexZr1−xOy (Me = Ni, Pt, Pd, Rh) catalysts for autothermal reforming of methane. Catal Today 210:10–18
Ismagilov IZ, Matus EV, Kuznetsov VV, Kerzhentsev MA, Yashnik SA, Prosvirin IP, Mota N, Navarro RM, Fierro JLG, Ismagilov ZR (2014) Hydrogen production by autothermal reforming of methane over NiPd catalysts: effect of support composition and preparation mode. Int J Hydrog Energy 39:20992–21006
Ismagilov IZ, Matus EV, Nefedova DV, Kuznetsov VV, Yashnik SA, Kerzhentsev MA, Ismagilov MA (2015) Effect of support modification on the physicochemical properties of a NiPd/Al2O3 catalyst for the autothermal reforming of methane. Kinet Catal 56:394–402
Ismagilov ZR, Matus EV, Ismagilov IZ, Sukhova OB, Yashnik SA, Ushakov VA, Kerzhentsev MA (2019) Hydrogen production through hydrocarbon fuel reforming processes over Ni based catalysts. Catal Today 323:166–182
Ivanov VK, Polezhaeva OS, Tret’yakov YD (2010) Nanocrystalline ceria: synthesis, structure-sensitive properties, and promising applications. Russ J Gen Chem 80(3):604–617
Jasinski P, Suzuki T, Anderson HU (2003) Nanocrystalline undoped ceria oxygen sensor. Sens Actuators B: Chem 95:73–77
Karolewicz B, Gajda M, Pluta J, Gorniak A (2016) The effect of Pluronic F127 on the physicochemical properties and dissolution profile of lovastatin solid dispersions. J Therm Anal Calorim 123:2283–2290
Kašpar J, Graziani M, Fornasiero P (2000) Ceria-containing three-way catalysts. Handb Phys Chem Rare Earths 29:159–267
Katta L, Sudarsanam P, Thrimurthulu G, Reddy BM (2010) Doped nanosized ceria solid solutions for low temperature soot oxidation: zirconium versus lanthanum promoters. Appl Catal B: Env 101:101–108
Ke Y, Lai S-Y (2014) Comparison of the catalytic benzene oxidation activity of mesoporous ceria prepared via hard-template and soft-template. Microporous Mesoporous Mater 198:256–262
Kerzhentsev МА, Matus ЕV, Ismagilov IZ, Ushakov VА, Stonkus ОА, Larina ТV, Kozlova GS, Bharali P, Ismagilov ZR (2017) Structural and morphological properties of Ce1-xMxOy (M = Gd, La, Mg) supports for catalysts of autothermal conversion of ethanol. J Struct Chem 58:126–134
Kugai J, Subramani V, Song C, Engelhard MH, Chin Y-H (2006) Effects of nanocrystalline CeO2 supports on the properties and performance of Ni–Rh bimetallic catalyst for oxidative steam reforming of ethanol. J Catal 238:430–440
Li XC, Hu QH, Yang YF, Chen JR, Lai ZH (2008) Effects of sol-gel method and lanthanum addition on catalytic performances of nickel-based catalysts for methane reforming with carbon dioxide. J Rare Earth 26:864–868
Li L, Chen F, Lu J-Q, Luo M-F (2011) Study of defect sites in Ce1-xMxO2-δ (x = 0.2) solid solutions using Raman spectroscopy. J Phys Chem A 115:7972–7977
Li D, Li X, Gong J (2016) Catalytic reforming of oxygenates: state of the art and future prospects. Chem Rev 116:11529–11653
Linganiso LZ, Ramana V, Pendyala R, Jacobs G, Davis BH, Cronauer DC, Kropf AJ, Marshal CL (2011) Low-temperature water–gas shift: doping ceria improves reducibility and mobility of O-bound species and catalyst activity. Catal Lett 141:1723–1731
Liu F, Zhao L, Wang H, Bai X, Liu Y (2014) Study on the preparation of Ni-La-Ce oxide catalyst for steam reforming of ethanol. Int J Hydrog Energy 39:10454–10466
Liu Z, Duchoň T, Wang H, Peterson EW, Zhou Y, Luo S, Zhou J, Matolín V, Stacchiola DJ, Rodriguez JA, Senanayake SD (2015) Mechanistic insights of ethanol steam reforming over Ni-CeOx (111): the importance of hydroxyl groups for suppressing coke formation. J Phys Chem C 119:18248–18256
Matus EV, Nefedova DV, Kuznetsov VV, Ushakov VA, Stonkus OA, Ismagilov IZ, Kerzhentsev MA, Ismagilov ZR (2017) Effect of the support composition on the physicochemical properties of Ni/Ce1-xLaxOy catalysts and their activity in an autothermal methane reforming reaction. Kinet Catal 58:610–621
McFarland EW, Metiu H (2013) Catalysis by doped oxides. Chem Rev 113:4391–4427
Melchionna M, Fornasiero P (2014) The role of ceria-based nanostructured materials in energy applications. Mater Today 17:349–357
Mondal T, Pant KK, Dalai AK (2015) Catalytic oxidative steam reforming of bio-ethanol for hydrogen production over Rh promoted Ni/CeO2-ZrO2 catalyst. Int J Hydrog Energy 40:2529–2544
Montini T, De Rogatis L, Gombac V, Fornasiero P, Graziani M (2007) Rh(1%)@CexZr1-xO2–Al2O3 nanocomposites: Active and stable catalysts for ethanol steam reforming. Appl Catal B: Env 71:125–134
Montini T, Melchionna M, Monai M, Fornasiero P (2016) Fundamentals and catalytic applications of CeO2-based materials. Chem Rev 116:5987–6041
Nahar G, Dupont V (2014) Hydrogen production from simple alkanes and oxygenated hydrocarbons over ceria–zirconia supported catalysts, review. Renew Sust Energ Rev 32:777–796
Nguyen-Phan TD, Song MB, Kim EJ, Shin EW (2009) The role of rare earth metals in lanthanide-incorporated mesoporous titania. Micropor Mesopor Mater 119:290–298
Paier J, Penschke C, Sauer J (2013) Oxygen defects and surface chemistry of ceria: quantum chemical studies compared to experiment. Chem Rev 113:3949–3985
Pinaeva LG, Sadovskaya EM, Ivanova YA, Kuznetsova TG, Prosvirin IP, Sadykov VA, Schuurman Y, van Veen AC, Mirodatos C (2014) Water gas shift and partial oxidation of CH4 over CeO2-ZrO2(-La2O3) and Pt/CeO2-ZrO2(-La2O3): performance under transient conditions. Chem Eng J 257:281–291
Prasad DH, Park SY, Ji H-I, Kim H-R, Son J-W, Kim B-K, Lee H-W, Lee J-H (2012) Structural characterization and catalytic activity of Ce0.65Zr0.25RE0.1O2−δ nanocrystalline powders synthesized by the glycine-nitrate process. J Phys Chem C 116:3467–3476
Quinelato AL, Longo E, Leite ER, Bernardi MIB, Varela JA (2001) Synthesis and sintering of ZrO2-CeO2 powder by use of polymeric precursor based on Pechini process. J Mater Sci 36:3825–3830
Reddy BM, Thrimurthulu G, Katta L (2011) Design of efficient CexM1-xO2-δ (M = Zr, Hf, Tb and Pr) nanosized model solid solutions for CO oxidation. Catal Lett 141:572–581
Rezaei M, Alavi SM, Sahebdelfar S, Yan Z-F (2009) Synthesis of ceria doped nanozirconia powder by a polymerized complex method. J Porous Mater 16:497–505
Rodriguez JA, Grinter DC, Liu Z, Palomino RM, Senanayake SD (2017) Ceria-based model catalysts: fundamental studies on the importance of the metal–ceria interface in CO oxidation, the water–gas shift, CO2 hydrogenation, and methane and alcohol reforming. Chem Soc Rev 46:1824–1841
Shehata N, Meehan K, Hudait M, Jain N (2012) Control of oxygen vacancies and Ce3+ concentrations in doped ceria nanoparticles via the selection of lanthanide element. J Nanopart Res 14:1173
Shido T, Iwasawa Y (1992) Regulation of reaction intermediate by reactant in the water-gas shift reaction on CeO2, in relation to reactant-promoted mechanism. J Catal 136(2):493–503
Shih S-J, Wu Y-Y, Borisenko KB (2011) Control of morphology and dopant distribution in yttrium-doped ceria nanoparticles. J Nanopart Res 13:7021–7028
Sulcova P, Vecera J, Strnadlova L (2012) Study of doped CeO2 prepared by different synthesis. J Therm Anal Calorim 108:519–523
Tai L-W, Lessing PA (1992) Modified resin-intermediate processing of perovskite powders: part 1. Optimization of polymeric precursors. J Mater Res 7:502–510
Theocharis CR, Kyriacou G, Christophidou M (2005) Preparation and characterization of nanoporous ceria containing heteroatoms, with and without a matrix. Adsorption 11:763–767
Vidmar P, Fornasiero P, Kaspar J, Gubitosa G, Graziani M (1997) Effects of trivalent dopants on the redox properties of Ce0.6Zr0.4O2 mixed oxide. J Catal 171:160–168
Vinodkumar T, Durgasri DN, Reddy BM (2013) Design of transition and rare earth metal doped ceria nanocomposite oxides for CO oxidation. Int J Adv Eng Sci Appl Math 5(4):224–231
Vinodkumar T, Rao BG, Reddy BM (2015) Influence of isovalent and aliovalent dopants on the reactivity of cerium oxide for catalytic applications. Catal Today 253:57–64
Wang R, Xu H, Liu X, Ge Q, Li W (2006) Role of redox couples of Rh0/Rhδ+ and Ce4+/Ce3+ in CH4/CO2 reforming over Rh–CeO2/Al2O3 catalyst. Appl Catal A 305:204–210
Wang F, Xu L, Zhang J, Zhao Y, Li H, Li HX, Wu K, Xu GQ, Chen W (2016) Tuning the metal-support interaction in catalysts for highly efficient methane dry reforming reaction. Appl Catal B: Env 180:511–520
Wason MS, Zhao J (2013) Cerium oxide nanoparticles: potential applications for cancer and other diseases. Am J Transl Res 5(2):126–131
Wenjuan S, Hongjuan G, Chang L, Xiaonan W (2012) Controllable preparation of CeO2 nanostructure materials and their catalytic activity. J Rare Earths 30(7):665–669
Wu L, Wiesmann HJ, Moodenbaugh AR, Klie RF, Zhu Y, Welch DO, Suenaga M (2004) Oxidation state and lattice expansion of CeO2-x nanoparticles as a function of particle size, Phys. Rev B: Condens Matter 69:125415
Yan C-H, Yan Z-G, Du Y-P, Shen J, Zhang C, Feng W (2011) Controlled synthesis and properties of rare earth nanomaterials. Handb Phys Chem Rare Earths 41:275–472
Yang J, Lukashuk L, Li H, Fottinger K, Rupprechter G, Schubert U (2014) High surface area ceria for CO oxidation prepared from cerium t-butoxide by combined sol–gel and solvothermal processing. Catal Lett 144:403–412
Yao X, Tang C, Ji Z, Dai Y, Cao Y, Gao F, Dong L, Chen Y (2013) Investigation of the physicochemical properties and catalytic activities of Ce0.67M0.33O2 (M = Zr4+, Ti4+, Sn4+) solid solutions for NO removal by CO. Catal Sci Technol 3:688–698
Zaghib K, Julien CM, Prakash J (2003) New trends in intercalation compounds for energy storage and conversion: Proceedings of the International Symposium
Zanchet D, Santos JB, Damyanova S, Gallo JMR, Bueno JMC (2015) Toward understanding metal-catalyzed ethanol reforming. ACS Catal 5:3841–3863
Zdravković J, Simović B, Golubović A, Poleti D, Veljković I, Šćepanović M, Branković G (2015) Comparative study of CeO2 nanopowders obtained by the hydrothermal method from various precursors. Ceram Int 41:1970–1979
Zhang F, Jin Q, Chan S-W (2004) Ceria nanoparticles: size, size distribution, and shape. J Appl Phys 95(8):4319–4326
Zhang B, Li D, Wang X (2010) Catalytic performance of La–Ce–O mixed oxide for combustion of methane. Catal Today 158:348–353
Zhang T, Tang D, Shao Y, Yu Z (2010a) Kinetics of nanoscale cerium dioxide prepared by Pechini. Process. J Mater Eng Perform (JMEPEG) 19:1220–1224
Zhu J, Zhang D, King KD (2012) Reforming of CH4 by partial oxidation: thermodynamic and kinetic analyses. Fuel 80:899–905
Acknowledgments
The authors are grateful to G.S. Litvak, T.Ya. Efimenko, Dr. E.Y. Gerasimov, Serkova A.N., and Dr. V.A. Ushakov for their assistance with catalyst characterization. This work was conducted within the framework of the budget project No. АААА-А17-117041710090-3 for Boreskov Institute of Catalysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matus, E.V., Okhlopkova, L.B., Sukhova, O.B. et al. Effects of preparation mode and doping on the genesis and properties of Ni/Ce1-xMxOy nanocrystallites (M = Gd, La, Mg) for catalytic applications. J Nanopart Res 21, 11 (2019). https://doi.org/10.1007/s11051-018-4454-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-018-4454-5