Abstract
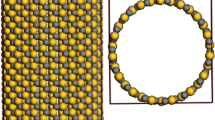
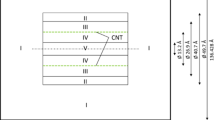
Grand canonical Monte Carlo (GCMC) simulation combined with ab initio quantum mechanics calculations were employed to study methane storage in homogeneous armchair open-ended single-walled silicon nanotubes (SWSiNTs), single-walled carbon nanotubes (SWCNTs), and single-walled silicon carbide nanotubes (SWSiCNTs) in triangular arrays. Two different groups of nanotubes were studied: the first were (12,12) SiNTs, (19,19) CNTs, and (15,15) SiCNTs and the second were (7,7) SiNTs, (11,11) CNTs, and (9,9) SiCNTs with the diameters of 26 and 15 Å for the first and second groups, respectively. The simulations were carried out at different thermodynamic states. The potential energy functions were calculated using ab initio quantum mechanics and then fitted with (12,6) Lennard–Jones potential model as a bridge between first-principles calculations and GCMC simulations. The absolute, excess, and delivery adsorption isotherms of methane were calculated for two groups of nanotubes. The specific surface area and the isosteric heat of adsorption were computed. The radial distribution functions for the adsorbed molecules on different nanotubes were also calculated. Different isotherm models were fitted with the simulation adsorption data. According to the results, the excess uptake value of methane adsorption in (11,11) CNT array exceeded the US Department of Energy target (180 V/V at 298 K and 35 bar). The results also indicate that SiNTs and SiCNTs are not desirable materials compared with corresponding CNTs for natural gas storage.



















Similar content being viewed by others
References
Adisa OO, Cox BJ, Hill JM (2010) Encapsulation of methane in nanotube bundles. Micro Nano Lett IET 5(5):291–295. doi:10.1049/mnl.2010.0075
Allen MP, Tildesley DJ (1987) Computer simulation of liquids. Clarendon, Oxford
Arab M, Picaud F, Devel M, Ramseyer C, Girardet C (2004) Molecular selectivity due to adsorption properties in nanotubes. Phys Rev B 69(16):165401
Arora G, Wagner NJ, Sandler SI (2004) Adsorption and diffusion of molecular nitrogen in single wall carbon nanotubes. Langmuir 20(15):6268–6277. doi:10.1021/la036432f
Bai J, Zeng XC, Tanaka H, Zeng JY (2004) Metallic single-walled silicon nanotubes. Proc Natl Acad Sci USA 101(9):2664–2668
Balilehvand S, Hashemianzadeh S, Razavi S, Karimi H (2012) Investigation of hydrogen and methane adsorption/separation on silicon nanotubes: a hierarchical multiscale method from quantum mechanics to molecular simulation. Adsorption 18(1):13–22. doi:10.1007/s10450-011-9375-x
Barnard AS, Russo SP (2003) Structure and energetics of single-walled armchair and zigzag silicon nanotubes. J Phys Chem B 107(31):7577–7581. doi:10.1021/jp0347421
Bekyarova E, Murata K, Yudasaka M, Kasuya D, Iijima S, Tanaka H, Kahoh H, Kaneko K (2003) Single-wall nanostructured carbon for methane storage. J Phys Chem B 107(20):4681–4684. doi:10.1021/jp0278263
Bienfait M, Zeppenfeld P, Dupont-Pavlovsky N, Muris M, Johnson MR, Wilson T, DePies M, Vilches OE (2004) Thermodynamics and structure of hydrogen, methane, argon, oxygen, and carbon dioxide adsorbed on single-wall carbon nanotube bundles. Phys Rev B 70(3):035410
Blase X, Broyer M, Kéghélian P, Lermé J, Mélinon P, Pellarin M, Perez A, Ray C, Vialle JL (1999) Production and stability of silicon-doped heterofullerenes. Eur Phys J D 9(1):49–54. doi:10.1007/s100530050398
Bradbury WL, Olevsky EA (2011) Synthesis of carbide nanostructures on monolithic agricultural-waste biomass-activated carbon templates. Int J Appl Ceram Technol 8(4):947–952
Buckingham AD, Fowler PW, Hutson JM (1988) Theoretical studies of van der Waals molecules and intermolecular forces. Chem Rev 88(6):963–988. doi:10.1021/cr00088a008
Calbi MM, Cole MW, Gatica SM, Bojan MJ, Stan G (2001) Condensed phases of gases inside nanotube bundles. Rev Mod Phys 73(4):857–865
Cao D, Zhang X, Chen J, Wang W, Yun J (2003) Optimization of single-walled carbon nanotube arrays for methane storage at room temperature. J Phys Chem B 107(48):13286–13292. doi:10.1021/jp036094r
Castrucci P, Scarselli M, De Crescenzi M, Diociaiuti M, Chaudhari PS, Balasubramanian C, Bhave TM, Bhoraskar SV (2006) Silicon nanotubes: synthesis and characterization. Thin Solid Films 508(1–2):226–230. doi:10.1016/j.tsf.2005.07.348
Chen YW, Tang YH, Pei LZ, Guo C (2005) Self-assembled silicon nanotubes grown from silicon monoxide. Adv Mater 17(5):564–567
Cheng HM, Li F, Su G, Pan HY, He LL, Sun X, Dresselhaus MS (1998) Large-scale and low-cost synthesis of single-walled carbon nanotubes by the catalytic pyrolysis of hydrocarbons. Appl Phys Lett 72(25):3282–3284
Cheng H, Cooper AC, Pez GP, Kostov MK, Piotrowski P, Stuart SJ (2005) Molecular dynamics simulations on the effects of diameter and chirality on hydrogen adsorption in single walled carbon nanotubes. J Phys Chem B 109(9):3780–3786. doi:10.1021/jp045358m
Cruz FJAL, Esteves IAAC, Mota JPB (2010) Adsorption of light alkanes and alkenes onto single-walled carbon nanotube bundles: Langmuirian analysis and molecular simulations. Colloids Surf A 357(1–3):43–52. doi:10.1016/j.colsurfa.2009.09.002
Dai L (2005) Low-temperature, controlled synthesis of carbon nanotubes. Small 1(3):274–276
Darkrim F, Levesque D (1998) Monte Carlo simulations of hydrogen adsorption in single-walled carbon nanotubes. J Chem Phys 109(12):4981–4984
Darkrim FL, Malbrunot P, Tartaglia GP (2002) Review of hydrogen storage by adsorption in carbon nanotubes. Int J Hydrogen Energy 27(2):193–202. doi:10.1016/s0360-3199(01)00103-3
De Crescenzi M, Castrucci P, Scarselli M, Diociaiuti M, Chaudhari PS, Balasubramanian C, Bhave TM, Bhoraskar SV (2005) Experimental imaging of silicon nanotubes. Appl Phys Lett 86(23):231901–231903
Dillon AC, Jones KM, Bekkedahl TA, Kiang CH, Bethune DS, Heben MJ (1997) Storage of hydrogen in single-walled carbon nanotubes. Nature 386(6623):377–379
Du Z, Manos G, Vlugt TJH, Smit B (1998) Molecular simulation of adsorption of short linear alkanes and their mixtures in silicalite. AIChE J 44(8):1756–1764
Düren T, Sarkisov L, Yaghi OM, Snurr RQ (2004) Design of new materials for methane storage. Langmuir 20(7):2683–2689. doi:10.1021/la0355500
Ellison MD, Crotty MJ, Koh D, Spray RL, Tate KE (2004) Adsorption of NH3 and NO2 on single-walled carbon nanotubes. J Phys Chem B 108(23):7938–7943. doi:10.1021/jp049356d
Fagan SB, Mota R, Baierle RJ, Paiva G, da Silva AJR, Fazzio A (2001) Stability investigation and thermal behavior of a hypothetical silicon nanotube. J Mol Struct (Thoechem) 539(1–3):101–106. doi:10.1016/s0166-1280(00)00777-6
Frenkel D, Smit B (2002) Understanding molecular simulations, from algorithms to applications, 2nd edn. Academic Press, San Diego
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision B.01. Wallingford. citeulike-article-id:9096580
Ganji MD, Seyed-aghaei N, Taghavi MM, Rezvani M, Kazempour F (2011) Ammonia adsorption on SiC nanotubes: a density functional theory investigation. Fullerenes, Nanotubes, Carbon Nanostruct 19(4):289–299. doi:10.1080/15363831003721740
Gao G, Park SH, Kang HS (2009) A first principles study of NO2 chemisorption on silicon carbide nanotubes. Chem Phys 355(1):50–54. doi:10.1016/j.chemphys.2008.10.049
George EF (2002) Hydrogen interaction with carbon nanotubes: a review of ab initio studies. J Phys: Condens Matter 14(17):R453
Ghoufi A, Gaberova L, Rouquerol J, Vincent D, Llewellyn PL, Maurin G (2009) Adsorption of CO2, CH4 and their binary mixture in Faujasite NaY: a combination of molecular simulations with gravimetry–manometry and microcalorimetry measurements. Microporous Mesoporous Mater 119(1–3):117–128. doi:10.1016/j.micromeso.2008.10.014
Gordillo MC, Brualla L, Fantoni S (2004) Neon adsorbed in carbon nanotube bundles. Phys Rev B 70(24):245420
Gottschalk F, Sonderer T, Scholz RW, Nowack B (2009) Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ Sci Technol 43(24):9216–9222. doi:10.1021/es9015553
Gottschalk F, Sonderer T, Scholz RW, Nowack B (2010) Possibilities and limitations of modeling environmental exposure to engineered nanomaterials by probabilistic material flow analysis. Environ Toxicol Chem 29(5):1036–1048
Guo C, Tang Y-h, Pei L-z, Zhagn Y (2011) Silicon carbide nanotubes with special morphology prepared by super critical hydrothermal method and photoluminescence character. J Shanghai Univ (English Edition) 15(6):538–541. doi:10.1007/s11741-011-0782-3
Gupta A, Chempath S, Sanborn MJ, Clark LA, Snurr RQ (2003) Object-oriented programming paradigms for molecular modeling. Mol Simul 29:29–46
Habenschuss A, Johnson E, Narten AH (1981) X-ray diffraction study and models of liquid methane at 92 K. J Chem Phys 74(9):5234–5241
Heyden A, Düren T, Keil FJ (2002) Study of molecular shape and non-ideality effects on mixture adsorption isotherms of small molecules in carbon nanotubes: a grand canonical Monte Carlo simulation study. Chem Eng Sci 57(13):2439–2448. doi:10.1016/s0009-2509(02)00131-8
Hobza P, šponer J, Reschel T (1995) Density functional theory and molecular clusters. J Comput Chem 16(11):1315–1325
Holland BT, Blanford CF, Stein A (1998) Synthesis of macroporous minerals with highly ordered three-dimensional arrays of spheroidal voids. Science 281(5376):538–540
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354(6348):56–58
Jakobtorweihen S, Hansen N, Keil FJ (2006) Combining reactive and configurational-bias Monte Carlo: confinement influence on the propene metathesis reaction system in various zeolites. J Chem Phys 125(22):224709
Jeong SY, Kim JY, Yang HD, Yoon BN, Choi SH, Kang HK, Yang CW, Lee YH (2003) Synthesis of silicon nanotubes on porous alumina using molecular beam epitaxy. Adv Mater 15(14):1172–1176
Jiang J, Sandler SI (2003) Nitrogen adsorption on carbon nanotube bundles: role of the external surface. Phys Rev B 68(24):245412
Jiang J, Sandler SI, Smit B (2004a) Capillary phase transitions of n-alkanes in a carbon nanotube. Nano Lett 4(2):241–244. doi:10.1021/nl034961y
Jiang J, Wagner NJ, Sandler SI (2004b) A Monte Carlo simulation study of the effect of carbon topology on nitrogen adsorption on graphite, a nanotube bundle, C60 fullerite, C168 schwarzite, and a nanoporous carbon. Phys Chem Chem Phys 6(18):4440–4444
Jiang J, Sandler SI, Schenk M, Smit B (2005) Adsorption and separation of linear and branched alkanes on carbon nanotube bundles from configurational-bias Monte Carlo simulation. Phys Rev B 72(4):045447
Johnson ER, Mackie ID, DiLabio GA (2009) Dispersion interactions in density-functional theory. J Phys Org Chem 22(12):1127–1135
Kazachkin DV, Nishimura Y, Irle S, Feng X, Vidic R, Borguet E (2010) Temperature and pressure dependence of molecular adsorption on single wall carbon nanotubes and the existence of an “adsorption/desorption pressure gap”. Carbon 48(7):1867–1875. doi:10.1016/j.carbon.2009.11.018
Klontzas E, Mavrandonakis A, Froudakis GE, Carissan Y, Klopper W (2007) Molecular hydrogen interaction with IRMOF-1: a multiscale theoretical study. J Phys Chem C 111(36):13635–13640. doi:10.1021/jp075420q
Kondratyuk P, Wang Y, Johnson JK, Yates JT (2005) Observation of a one-dimensional adsorption site on carbon nanotubes: adsorption of alkanes of different molecular lengths. J Phys Chem B 109(44):20999–21005. doi:10.1021/jp0582078
Krasheninnikov AV, Banhart F, Li JX, Foster AS, Nieminen RM (2005) Stability of carbon nanotubes under electron irradiation: role of tube diameter and chirality. Phys Rev B 72(12):125428
Kristyán S, Pulay P (1994) Can (semi)local density functional theory account for the London dispersion forces? Chem Phys Lett 229(3):175–180. doi:10.1016/0009-2614(94)01027-7
Lan J, Cheng D, Cao D, Wang W (2008) Silicon nanotube as a promising candidate for hydrogen storage: from the first principle calculations to grand canonical Monte Carlo simulations. J Phys Chem C 112(14):5598–5604. doi:10.1021/jp711754h
Lan J, Cao D, Wang W (2009) High uptakes of methane in Li-doped 3D covalent organic frameworks. Langmuir 26(1):220–226. doi:10.1021/la9020383
Lasjaunias JC, Biljaković K, Sauvajol JL, Monceau P (2003) Evidence of 1D behavior of He4 confined within carbon-nanotube bundles. Phys Rev Lett 91(2):025901
Lee JS, Jhung SH, Yoon JW, Hwang YK, Chang J-S (2009) Adsorption of methane on porous metal carboxylates. J Ind Eng Chem 15(5):674–676. doi:10.1016/j.jiec.2009.09.043
Li L, Xing Y (2008) Electrochemical durability of carbon nanotubes at 80 °C. J Power Sources 178(1):75–79. doi:10.1016/j.jpowsour.2007.12.002
Lithoxoos GP, Samios J, Carissan Y (2008) Investigation of silicon model nanotubes as potential candidate nanomaterials for efficient hydrogen storage: a combined ab initio/grand canonical Monte Carlo simulation study. J Phys Chem C 112(43):16725–16728. doi:10.1021/jp805559a
Llewellyn PL, Maurin G (2005) Gas adsorption microcalorimetry and modelling to characterise zeolites and related materials. C R Chim 8(3–4):283–302. doi:10.1016/j.crci.2004.11.004
López MJ, Cabria I, March NH, Alonso JA (2005) Structural and thermal stability of narrow and short carbon nanotubes and nanostrips. Carbon 43(7):1371–1377. doi:10.1016/j.carbon.2005.01.006
Lyu SC, Liu BC, Lee SH, Park CY, Kang HK, Yang CW, Lee CJ (2004) Large-scale synthesis of high-quality single-walled carbon nanotubes by catalytic decomposition of ethylene. J Phys Chem B 108(5):1613–1616
Mahdizadeh S, Tayyari S (2011) Influence of temperature, pressure, nanotube’s diameter and intertube distance on methane adsorption in homogeneous armchair open-ended SWCNT triangular arrays. Theor Chem Acc Theory Comput Model (Theor Chim Acta) 128(2):231–240. doi:10.1007/s00214-010-0836-1
Mahdizadeh S, Tayyari S (2012) Methane storage in homogeneous armchair open-ended single-walled boron nitride nanotube triangular arrays: a grand canonical Monte Carlo simulation study. J Mol Model 18(6):2699–2708. doi:10.1007/s00894-011-1246-6
Makogon YF (2010) Natural gas hydrates—a promising source of energy. J Nat Gas Sci Eng 2(1):49–59
Malek K, Sahimi M (2010) Molecular dynamics simulations of adsorption and diffusion of gases in silicon-carbide nanotubes. J Chem Phys 132(1):014310
Marcone B, Orlandini E, Toigo F, Ancilotto F (2006) Condensation of helium in interstitial sites of carbon nanotubes bundles. Phys Rev B 74(8):085415
Martin MG, Siepmann JI (1998) Transferable potentials for phase equilibria. 1. United-atom description of n-alkanes. J Phys Chem B 102(14):2569–2577. doi:10.1021/jp972543+
Masoud Darvish G, Amir M, Ali N (2010) Theoretical investigation of methane adsorption onto boron nitride and carbon nanotubes. Sci Technol Adv Mater 11(4):045001
Maurin G, Bourrelly S, Llewellyn PL, Bell RG (2006) Simulation of the adsorption properties of CH4 in faujasites up to high pressure: comparison with microcalorimetry. Microporous Mesoporous Mater 89(1–3):96–102. doi:10.1016/j.micromeso.2005.09.024
Mavrandonakis A, Froudakis GE, Schnell M, Mühlhäuser M (2003) From pure carbon to silicon–carbon nanotubes: an ab-initio study. Nano Lett 3(11):1481–1484. doi:10.1021/nl0343250
Mendoza-Cortés JL, Han SS, Furukawa H, Yaghi OM, Goddard WA (2010) Adsorption mechanism and uptake of methane in covalent organic frameworks: theory and experiment. J Phys Chem A 114(40):10824–10833. doi:10.1021/jp1044139
Meng T, Wang C-Y, Wang S-Y (2007) First-principles study of a single Ti atom adsorbed on silicon carbide nanotubes and the corresponding adsorption of hydrogen molecules to the Ti atom. Chem Phys Lett 437(4–6):224–228. doi:10.1016/j.cplett.2007.02.024
Menon M, Richter E, Mavrandonakis A, Froudakis G, Andriotis AN (2004) Structure and stability of SiC nanotubes. Phys Rev B 69(11):115322
Meregalli V, Parrinello M (2001) Review of theoretical calculations of hydrogen storage in carbon-based materials. Appl Phys A 72(2):143–146. doi:10.1007/s003390100789
Mpourmpakis G, Froudakis GE, Lithoxoos GP, Samios J (2006) SiC nanotubes: a novel material for hydrogen storage. Nano Lett 6(8):1581–1583. doi:10.1021/nl0603911
Mueller NC, Nowack B (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42(12):4447–4453. doi:10.1021/es7029637
Nasibulin A, Moisala A, Jiang H, Kauppinen E (2006) Carbon nanotube synthesis from alcohols by a novel aerosol method. J Nanopart Res 8(3):465–475. doi:10.1007/s11051-005-9027-8
Oh DH, Lee YH (1998) Stability and cap formation mechanism of single-walled carbon nanotubes. Phys Rev B 58(11):7407–7411
Ohashi F, Tomura S, Akaku K, Hayashi S, Wada SI (2004) Characterization of synthetic imogolite nanotubes as gas storage. J Mater Sci 39(5):1799–1801. doi:10.1023/b:jmsc.0000016188.04444.36
Palaria A, Klimeck G, Strachan A (2008) Structures and energetics of silicon nanotubes from molecular dynamics and density functional theory. Phys Rev B 78(20):205315
Paredes JI, Suárez-García F, Villar-Rodil S, Martínez-Alonso A, Tascón JMD, Bottani EJ (2003) N2 physisorption on carbon nanotubes: computer simulation and experimental results. J Phys Chem B 107(34):8905–8916. doi:10.1021/jp026662n
Pascoli SD, Femia A, Luzzati T (2001) Natural gas, cars and the environment. A (relatively) ‘clean’ and cheap fuel looking for users. Ecol Econ 38(2):179–189. doi:10.1016/s0921-8009(01)00174-4
Pei LZ, Tang YH, Chen YW, Guo C, Li XX, Yuan Y, Zhang Y (2006) Preparation of silicon carbide nanotubes by hydrothermal method. J Appl Phys 99(11):114306
Peigney A, Laurent C, Flahaut E, Bacsa RR, Rousset A (2001) Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 39(4):507–514. doi:10.1016/s0008-6223(00)00155-x
Pérez-Jordá J, Becke AD (1995) A density-functional study of van der Waals forces: rare gas diatomics. Chem Phys Lett 233(1–2):134–137. doi:10.1016/0009-2614(94)01402-h
Pham-Huu C, Keller N, Ehret G, Ledoux MJ (2001) The first preparation of silicon carbide nanotubes by shape memory synthesis and their catalytic potential. J Catal 200(2):400–410. doi:10.1006/jcat.2001.3216
Pokropivnyi V, Silenko P (2006) Silicon carbide nanotubes and nanotubular fibers: synthesis, stability, structure, and classification. Theoret Exp Chem 42(1):3–15. doi:10.1007/s11237-006-0010-y
Poling BE, Prausnitz JM, O'Connell J (2000) The properties of gases and liquids, 5th edn. McGraw-Hill, New York
Popov VN (2004) Carbon nanotubes: properties and application. Mater Sci Eng, R 43(3):61–102. doi:10.1016/j.mser.2003.10.001
Romero-Vargas Castrillón S, Giovambattista Ns, Aksay IA, Debenedetti PG (2009) Effect of surface polarity on the structure and dynamics of water in nanoscale confinement. J Phys Chem B 113(5):1438–1446. doi:10.1021/jp809032n
Ruester S, Neumann A (2008) The prospects for liquefied natural gas development in the US. Energy Policy 36(8):3160–3168. doi:10.1016/j.enpol.2008.04.030
Sabo K, Scitovski R, Vazler I, Zekić-Sušac M (2011) Mathematical models of natural gas consumption. Energy Convers Manage 52(3):1721–1727. doi:10.1016/j.enconman.2010.10.037
Salehi E, Taghikhani V, Ghotbi C, Nemati Lay E, Shojaei A (2007) Theoretical and experimental study on the adsorption and desorption of methane by granular activated carbon at 25°C. J Nat Gas Chem 16(4):415–422. doi:10.1016/s1003-9953(08)60014-6
Sanborn MJ, Snurr RQ (2001) Predicting membrane flux of CH4 and CF4 mixtures in Faujasite from molecular simulations. AIChE J 47(9):2032–2041
Sha J, Niu J, Ma X, Xu J, Zhang X, Yang Q, Yang D (2002) Silicon nanotubes. Adv Mater 14(17):1219–1221
Shao X, Wang W, Zhang X (2007) Experimental measurements and computer simulation of methane adsorption on activated carbon fibers. Carbon 45(1):188–195. doi:10.1016/j.carbon.2006.07.006
Simonyan VV, Johnson JK, Kuznetsova A, Yates JJT (2001) Molecular simulation of xenon adsorption on single-walled carbon nanotubes. J Chem Phys 114(9):4180–4185
Snurr RQ, Hupp JT, Nguyen ST (2004) Prospects for nanoporous metal–organic materials in advanced separations processes. AIChE J 50(6):1090–1095
Tanaka H, El-Merraoui M, Steele WA, Kaneko K (2002) Methane adsorption on single-walled carbon nanotube: a density functional theory model. Chem Phys Lett 352(5–6):334–341. doi:10.1016/s0009-2614(01)01486-5
Tang YH, Pei LZ, Chen YW, Guo C (2005) Self-assembled silicon nanotubes under supercritically hydrothermal conditions. Phys Rev Lett 95(11):116102
Tchouar N, Benyettou M, Kadour F (2003) Thermodynamic, structural and transport properties of Lennard–Jones liquid systems. A molecular dynamics simulations of liquid helium, neon, methane and nitrogen. Int J Mol Sci 4(12):595–606
Vlugt TJH, Schenk M (2002) Influence of framework flexibility on the adsorption properties of hydrocarbons in the zeolite silicalite. J Phys Chem B 106(49):12757–12763. doi:10.1021/jp0263931
Wang Q, Johnson JK (1999) Optimization of carbon nanotube arrays for hydrogen adsorption. J Phys Chem B 103(23):4809–4813. doi:10.1021/jp9900032
Wang X, Liew KM (2011a) Hydrogen storage in silicon carbide nanotubes by lithium doping. J Phys Chem C 115(8):3491–3496. doi:10.1021/jp106509g
Wang X, Liew KM (2011b) Silicon carbide nanotubes serving as a highly sensitive gas chemical sensor for formaldehyde. J Phys Chem C 115(21):10388–10393. doi:10.1021/jp2005937
Wijnhoven JEGJ, Vos WL (1998) Preparation of photonic crystals made of air spheres in titania. Science 281(5378):802–804
Wu H, Chan G, Choi JW, Ryu I, Yao Y, McDowell MT, Lee SW, Jackson A, Yang Y, Hu L, Cui Y (2012) Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat Nano 7(5):310–315
Xianlong W, Ming-Sheng W, Yoshio B, Dmitri G (2011) Thermal stability of carbon nanotubes probed by anchored tungsten nanoparticles. Sci Technol Adv Mater 12(4):044605
Xue C, Zhou Ze, Yang Q, Zhong C (2009) Enhanced methane adsorption in catenated metal–organic frameworks: a molecular simulation study. Chin J Chem Eng 17(4):580–584. doi:10.1016/s1004-9541(08)60247-5
Yulong W, Fei W, Guohua L, Guoqing N, Mingde Y (2008) Methane storage in multi-walled carbon nanotubes at the quantity of 80 g. Mater Res Bull 43(6):1431–1439. doi:10.1016/j.materresbull.2007.06.046
Zhang RQ, Lee ST, Law C-K, Li W-K, Teo BK (2002) Silicon nanotubes: why not? Chem Phys Lett 364(3–4):251–258. doi:10.1016/s0009-2614(02)01334-9
Zhang M, Kan YH, Zang QJ, Su ZM, Wang RS (2003) Why silicon nanotubes stably exist in armchair structure? Chem Phys Lett 379(1–2):81–86. doi:10.1016/j.cplett.2003.08.030
Zhang RQ, Lee H-L, Li W-K, Teo BK (2005) Investigation of possible structures of silicon nanotubes via density-functional tight-binding molecular dynamics simulations and ab initio calculations. J Phys Chem B 109(18):8605–8612. doi:10.1021/jp045682h
Zhou W, Wu H, Hartman MR, Yildirim T (2007) Hydrogen and methane adsorption in metal–organic frameworks: a high-pressure volumetric study. J Phys Chem C 111(44):16131–16137. doi:10.1021/jp074889i
Acknowledgments
We gratefully acknowledge the support of this research by Ferdowsi University of Mashhad (Grant No. 3/18509).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 4.
Rights and permissions
About this article
Cite this article
Mahdizadeh, S.J., Goharshadi, E.K. Natural gas storage on silicon, carbon, and silicon carbide nanotubes: a combined quantum mechanics and grand canonical Monte Carlo simulation study. J Nanopart Res 15, 1393 (2013). https://doi.org/10.1007/s11051-012-1393-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-1393-4