Abstract
There has been a growing appreciation of the importance of respiratory fungal diseases in recent years, with better understanding of their prevalence as well as their global distribution. In step with the greater awareness of these complex infections, we are currently poised to make major advances in the characterization and treatment of these fungal diseases, which in itself is largely a consequence of post-genomic technologies which have enabled rational drug development and a path towards personalized medicines. These advances are set against a backdrop of globalization and anthropogenic change, which have impacted the world-wide distribution of fungi and antifungal resistance, as well as our built environment. The current revolution in immunomodulatory therapies has led to a rapidly evolving population at-risk for respiratory fungal disease. Whilst challenges are considerable, perhaps the tools we now have to manage these infections are up to this challenge. There has been a welcome acceleration of the antifungal pipeline in recent years, with a number of new drug classes in clinical or pre-clinical development, as well as new focus on inhaled antifungal drug delivery. The “post-genomic” revolution has opened up metagenomic diagnostic approaches spanning host immunogenetics to the fungal mycobiome that have allowed better characterization of respiratory fungal disease endotypes. When these advances are considered together the key challenge is clear: to develop a personalized medicine framework to enable a rational therapeutic approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Respiratory fungal diseases have risen in prominence in recent years, as a consequence of improved diagnostics, advocacy, research and greater awareness [1,2,3,4,5,6]. However, our understanding of whether there has been a genuine increase in the prevalence of respiratory fungal diseases is less clear. Current advances across a range of different areas, including immunophenotyping, metagenomics, antifungal therapies and immunotherapies have opened up exciting opportunities to revolutionize our clinical approach to these complex respiratory infections.
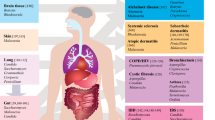
Opportunistic Respiratory Mycoses
Fungal opportunism of the respiratory tract has been dominated by the aspergilli, and in particular Aspergillus fumigatus [7]. Aspergillosis was first described in humans by Dieulafoy in the 1890s as a primary pulmonary infection, and as fungal rhinitis in 1915 [8, 9]. The ubiquitous global nature of this saprophytic mould, with small, highly dispersible conidia which are inhaled on a daily basis, and its thermophilic nature make it ideally suited as a pulmonary pathogen [7, 10]. Other species within the genus have played a prominent role as respiratory mycoses [11, 12]. Within the broader context of allergic fungal airway disease, fungal sensitization may be mediated by thermophilic fungi such as Aspergillus spp., and Candida spp. as well as thermo-intolerant fungi such as the Cladosporium and Alternaria genera [13, 14]. In the immunocompromised host, a much broader range of opportunistic fungi, such as Pneumocystis jirovecii, the mucoromycotina, and Cryptococcus spp. may cause invasive infection [15,16,17]. Fungi are also prominent causative agents of hypersensitivity pneumonitis, implicated in farmer’s lung disease (Aspergillus fumigatus, Lichtheimia corymbifera), and peat moss exposure (Penicillium spp.) amongst others [18, 19]. Thus, fungi are remarkable in their ability to induce both invasive infections as well as allergic sensitization and hypersensitivity responses.
Endemic Respiratory Mycoses
Endemic respiratory mycoses are characterized as primary pathogens that can also disseminate, often in the context of immunocompromised [20]. Histoplasmosis, an endemic mycosis of the Americas and opportunistic mycosis globally (var. duboisii), was first described as a human pathogen by Darling (Darling’s disease) in 1909 [21]. Talaromycosis, an East Asian endemic mycosis due to Talaromyces marneffei (formerly Penicillium marneffei),, was first described in 1959 by Segretain [22], and coccidioidomycosis, due to Coccidioides immitis, a mycoses of the Americas, was first described in 1948 [23, 24]. Blastomycosis, an American endemic mycosis due to Blastomyces dermatitidis was first described in 1914 [25]. Endemic mycosis gained increasing prominence, mainly as a consequence of their association with the AIDS epidemic, where they are major opportunistic pathogens, from the 1980s onwards [26]. The current revolution in immunomodulatory therapies has led to new patient groups becoming at risk of endemic mycoses [27].
Epidemiology
The epidemiological drivers of respiratory fungal disease are complex and contingent on a range of factors, many of which may have an anthropogenic basis [28]. For instance, global warming and the global trade in plants may have rapidly accelerated the propensity for new and ecologically invasive species, as well as the rapid global emergence of triazole resistance, recently characterized in A. fumigatus [29]. Changes in building construction and in particular ventilation may have led to fundamental shifts in the composition and diversity of the aerial mycobiota in the built environment [30]. Susceptibility to fungal infection is increasingly complex, where novel agents such as ibrutinib and IL-5 modulators have been shown to have potential to predispose individuals to respiratory fungal infection [31]. The widespread use of steroids is thought to have played a major role in the apparent increasing incidence of chronic respiratory fungal diseases in the context of chronic diseases of the lung [32, 33].
How Can We Use Available and Emerging Antifungals to Improve Outcomes from Respiratory Fungal Disease?
Much of our current understanding around the optimal use of antifungals for respiratory fungal diseases has been driven by well-funded, commercial, randomized controlled studies in the context of invasive pulmonary aspergillosis in the immunocompromised host [34, 35]. This has led to the licensing of voriconazole, ambisome, posaconazole and isavuconazole in this setting [34,35,36,37]. However, our understanding of how these agents can be used in the context of chronic respiratory fungal disease is less well defined [38, 39]. Most clinical trial data are centred around itraconazole, a historical azole with poor oral absorption, major drug interactions and significant side effects [40]. Furthermore, historical studies in invasive pulmonary aspergillosis have already established that itraconazole has poor efficacy for invasive aspergillosis, and therefore likely to be inferior to newer mould-active triazoles [41]. It is therefore not surprising that studies of itraconazole therapy in the context of chronic pulmonary aspergillosis, allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization have by and large showed modest or no effect. In addition, the importance of triazole therapeutic drug monitoring, which has not been addressed in clinical trials, but is now de facto standard of care in the real world, is a major confounder for these studies that requires further exploration. It is notable that those studies that failed did not undertake therapeutic drug monitoring, which is not currently a requirement under the licensing of any triazole antifungal [42]. The only study to address voriconazole in the context of allergic fungal airway disease, EVITA3, also did not involve therapeutic drug monitoring [43]. Furthermore, whilst therapy was stopped at 3 months, clinical endpoint measurements were undertaken at 12 months, on the basis that any antifungal effect would be long-lived. However, unlike itraconazole, voriconazole is aquaphilic and does not persist in tissues in the long-term. Thus, further studies of newer triazoles such as voriconazole, posaconazole and isavuconazole in the context of chronic respiratory fungal disease are urgently needed.
There is currently a lack of clarity around the use of antifungals for allergic fungal airway diseases such as severe asthma with fungal sensitization and allergic bronchopulmonary aspergillosis, where historically it was thought that these fungal diseases are purely driven by sensitization to the aerial mycobiota rather than any element of airway infection. This theory seems reasonable in the context of thermointolerant fungi such as Alternaria spp.; however for allergic bronchopulmonary aspergillosis the presence of hyphae in the mucous and evidence of mucosal inflammation with a positive Aspergillus IgG response in serum are suggestive of airway mycosis [44]. Moreover, recent careful mycological studies suggest the involvement of airway mycosis in allergic airway disease could be much more extensive than is currently believed [45]. Better understanding of these relationships and the role that antifungals could play are urgently needed.
We are currently in the midst of a revolution in the antifungal armamentorium, with posaconazole and isavuconazole representing a step change for triazole usage clinically, as a consequence of their improved side effect profiles, absorbance and spectrum of action [34, 46,47,48,49]. A number of exciting new drug classes are now in late phase clinical studies, and there has been renewed interest in inhaled antifungal development. Olorofim (F901318; F2G Ltd., Manchester, UK) is the first of a new class of drugs, the orotomides, that inhibit dihydroorotate dehydrogenase, a key enzyme in pyrimidine synthesis [50]. The drug is available orally and intravenously with wide tissue distribution. Notably the drug has a wide spectrum of activity against Aspergillus spp., including triazole-resistant strains, as well as more difficult to treat opportunistic pulmonary fungal pathogens such as Lomentospora prolificans and Scedosporium spp. as well as the causative agents of endemic mycoses [51,52,53,54]. However, there is a lack of activity against Candida spp., mucoralean fungi, and Cryptococcus spp. There is an open label study ongoing to evaluate the utility of olorofim in individuals with limited treatment options (FORMULA; NTC03583164).
Fosmanogepix (APX001; Amplyx, San Diego, Ca.), is a prodrug metabolized to manogepix, its active form [55]. It disrupts glycosylphosphatidylinositol (GPI)-anchor biosynthesis by inhibiting the enzyme Gwt1 and has good activity in vitro against Aspergillus spp., Cryptococcus neoformans, Scedosporium spp., and Fusarium spp. [56, 57]. There is currently a phase 2, multicentre study to evaluate Fosmanogepix for the treatment of invasive fungal infections caused by Aspergillus spp. or rare moulds (e.g. Scedosporium spp., Fusarium spp., and mucoralean fungi).
A number of other systemic agents are in various stages of development that could be useful in the setting of respiratory fungal disease such as orally available amphotericin B Cochleate (CAMB/MAT2203; Matinas Biopharma, Bedminster, NJ) [58, 59], MGCD290 (Mirati Therapeutics, San Diego, Ca.) [60, 61], tetrazoles (VT-1129, VT-1161, and VT-1598; Viamet Pharmaceuticals, Durham, NC) [62,63,64,65,66,67], VL-2397/ASP2397 (Vical inc.; San Diego, Ca.) [68], and T-2307 (Toyama Chemical, Tokyo, Japan) [69].
Inhaled antifungals represent a particularly interesting area for development in the context of respiratory fungal disease, where there is potential to achieve increased concentrations of drug in the respiratory mucosa compared to the systemic route, and the possibility for synergies with systemic agents. Historically amphotericin B has been used both as deoxycholate as well as in lipid forms, primarily as nebulized prophylaxis against pulmonary mould infection in haematological malignancies, and lung transplantation [70,71,72,73]. Another setting is as therapy for allergic bronchopulmonary aspergillosis or Aspergillus tracheobronchitis [70, 74]. There have been case reports around the use of nebulized triazoles for the treatment of airway mycoses with varying success. More recently there has been a concerted effort to systematically develop specifically formulated nebulized antifungals with good airway distribution and retention. PC945 (Pulmocide Ltd.) is a novel triazole specifically designed to achieve high concentrations in the airway mucosa with limited systemic exposure [75]. It has potent activity against Aspergillus and accumulates in the lung on repeat dosing [76]. Interestingly animal studies indicate improved efficacy when combined with systemic antifungals in murine pulmonary aspergillosis [77, 78]. It was well tolerated in healthy individuals and asthmatics. Initial case reports for nebulized PC945 as salvage therapy in refractory lung transplant Aspergillus tracheobronchitis showed complete response [75, 79, 80]. Phase 2 study data in asthma and cystic fibrosis patients with pulmonary aspergillosis are currently being evaluated. Pulmazole (Pulmatrix Inc) is a new dry powder itraconazole formulation that was being evaluated in adult asthmatics with allergic bronchopulmonary aspergillosis in Phase 2 studies (NCT03960606). Pulmazole is engineered using propriety technology that allows particles to be formulated as small, dense and dispersible particles for deep lung penetration. This allows for delivery as a dry powder by inhalation. There are also two formulations of voriconazole in development for inhalation, ZP-059 (Zambon Company S.P.A., Milano, Italy), and TFF-VORI (TFF pharmaceuticals, Austin, TX) that have completed Phase 1 of development.
Taken together, the likely availability of novel systemic antifungal drug classes as well as the option for inhalational antifungals has the potential to dramatically change the clinical landscape for therapeutic options for respiratory fungal diseases. A particularly exciting challenge will be to work out if combination therapies are superior and in particular whether the combination of systemic and inhaled antifungals is superior to conventional systemic therapies that are currently prevalent. This is particularly important in the context of airway mycoses where it seems likely that current systemic antifungal treatments are sup-optimal. Another unanswered question is around what utility adjunctive inhaled antifungals could have in the context of invasive pulmonary mycoses such as invasive aspergillosis. Finally, there are major unanswered questions around duration of therapy, with most trials of invasive aspergillosis using 6–12 weeks therapy but very little data to guide where shorter courses may be appropriate.
How Can We Improve the Diagnosis of Respiratory Fungal Infection?
Current diagnosis of respiratory fungal diseases revolves around three central pillars, the mycological evidence of infection, the clinical status of the host and the evidence that there is an immune response to a fungus in the host.
Detection of Fungal Infection
Classically mycological criteria have typically been from fungal cultures from either the airway or on tissue biopsy; however these would not necessarily be diagnostic on their own (unless for an endemic mycoses) as the airway has always been considered non-sterile from a microbiological perspective and fungi are ubiquitous components of the aerial microbiota [81]. Fungal polymerase chain reaction has been available for several decades, has long been established for the diagnosis of Pneumocystis pneumonia and has recently been approved for the diagnosis of invasive pulmonary aspergillosis [81]. There has been limited work on the utility of fungal species multiplex PCRs, which would be highly attractive for airway samples across a range of settings such as haematological immunocompromised, lung transplantation and cystic fibrosis where a specific, limited group of fungal pathogens account for the vast majority of infections [82, 83]. Further progress has been made with respect to more systematic use of both β-1,3 glucan and galactomannan as markers of respiratory fungal disease where β-1,3 glucan is used in serum primarily as a screening assay and galactomannan has utility both in serum and airway samples for specific diagnosis of aspergillosis. There has been significant advance in the availability of lateral flow device assays for point-of-care testing across aspergillosis as well as endemic mycoses such as histoplasmosis [84, 85]. A further area of ongoing development is whether urinary antigens have utility for the diagnosis of respiratory mycoses [86,87,88]. In general terms, it seems clear that the combination of two different assays such as PCR and antigen for identification of fungal disease leads to a much more robust diagnostic performance profile. The elephant in the room in terms of mycological diagnosis of respiratory mycoses is the airway mycobiome. There has been a significant body of work to describe this across a range of settings from the immunocompromised host, where it has been shown the mycobiome or even the microbiome could predict pulmonary fungal disease [89,90,91]. However, this work is still at a very early phase [92]. Further detailed studies have been undertaken in chronic respiratory fungal diseases as well as in chronic respiratory disease more generally, to determine either the role that fungi play in the pathogenesis of diseases such as asthma, COPD and bronchiectasis, or to delineate the composition of the mycobiome and microbiome underlying conditions such as allergic bronchopulmonary aspergillosis or cystic fibrosis-related aspergillosis [93,94,95]. Bigger multicentre studies using the latest metagenomic approaches are required, encompassing the interface between the virome, bacteriome and mycobiome [96].
By extension, for fungal infections there is a critical question around the broader environment and in particular the aerial mycobiome as a driver for fungal infection. This has high relevance whether it be in the context of the neutropenic host with acute myeloid leukaemia, where acquisition of A. fumigatus from the hospital environment has been clearly documented to cause infection [97,98,99,100], or allergic fungal airway diseases where further work is required to understand the relationship between population-level sensitization to allergenic fungi and exposure to these fungi in the environment [101, 102]. Groundbreaking metagenomic approaches have been developed to characterize the aerial mycobiome that hold great promise to allow better understanding as well as prediction of the environmental factors driving respiratory fungal disease [30].
Within the context of the immunocompromised host a key issue is around how to use diagnostics to guide treatment. In this regard, the primary questions revolve around whether universal prophylaxis (for instance posaconazole in neutropenic acute myeloid leukaemia) versus pre-emptive mycological biomarker-driven therapy or directed therapy for confirmed respiratory fungal disease is most appropriate [103]. Whilst universal prophylaxis appears an expedient solution the rising incidence of fungal resistance to antimicrobials argues against such an approach [104, 105]. In contrast, directed therapy, which is useful to minimize unnecessary antifungal usage, runs the risk of late diagnosis and consequently poorer outcomes. However, these approaches have rarely been systematically compared in randomized controlled trials [106, 107].
The emergence of antifungal resistance as a major clinical issue is an area of great concern [29]. This has been a huge problem for Candida spp. with replacement of C. albicans as the dominant pathogen with other species such as C. glabrata and C. krusei in high-risk azole-exposed populations [108], as well as the global emergence of C. auris as a high-transmission, multidrug-resistant human opportunistic pathogen [109,110,111]. More recently our understanding of emergent triazole resistance in A. fumigatus as a consequence of both in-host adaptation to selective drug pressure as well as fungicide use in the environment [104, 105], and the observation of in-host evolution of fluconazole resistance in C. neoformans [112] means that we urgently need clinically robust molecular and phenotypic diagnostics to define the epidemiology and extent of resistance in clinical settings for all three major human fungal pathogens. Significant progress has been made with population-level next generation sequencing; integration of these approaches into clinical laboratory workflows as soon as possible is now a major goal.
Immunodiagnosis of Fungal Inflammation
Immunodiagnosis is a cornerstone for the diagnosis of respiratory fungal disease such as chronic pulmonary aspergillosis, allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization, as well as having utility for the identification of fungal drivers of hypersensitivity pneumonitis [113]. The primary modality is by detection of antibody responses to immunodominant fungal antigens and allergens [114,115,116]. However, the field is vastly complex, because fungi have one of the highest numbers of antigenic molecules when compared to other allergens [117, 118]. In the context of aspergillosis this is of particular interest because whilst some antigens are immunodominant and therefore have utility as components of sensitive screening assays, other antigens may have higher predictive value for disease severity and progression [119,120,121]. For instance there are currently 23 WHO-defined fungal allergens for A. fumigatus (http://allergen.org). There has been some recent progress in the development of multiplex assays that can identify broad antigen repertoires for these diseases [93, 122]. Such an approach is likely to have utility for precision diagnostic medicine across chronic respiratory fungal infection, allergic fungal airway disease and hypersensitivity pneumonitis. Further progress has been made through the development of cellular response assays with a particular area of focus being fungal-reactive T cells [123,124,125]. These have been shown to have utility as assays to identify active infection both in the context of invasive aspergillosis as well as cystic fibrosis-related aspergillosis [126]. Large multicentre studies are required to further validate their utility for the early and accurate identification of individuals with respiratory fungal diseases. Such T cell response assays ought to be applicable to a wider range of respiratory fungal pathogens; utility has been shown already for Aspergillus spp. as well as Mucor spp [127]. Basophil activation assays, which are used to confirm the functional ability of allergens to induce effector cell degranulation, have also been shown to be useful to assess the response of patients with allergic fungal airway disease to immunotherapies such as the IgE-depleting monoclonal omalizumab [128].
Identification of Fungal Immunogenetic Risk
Immunogenetic risk prediction for respiratory fungal disease has great promise as a key diagnostic tool in the at-risk host. Important work in this area has been undertaken within the context of primary immunodeficiencies, for instance Dectin-1/Card-9/JakStat mutations and risk of chronic mucocutaneous candidiasis [129,130,131,132]. However, there has been tremendous progress in identifying immunogenetic alleles that confer risk for invasive fungal disease in the context of transplantation, where it has been shown that either donor or recipient alleles may be implicated. In the context of haematological stem cell transplant, current data suggest that where the recipient immunogenotype is predictive of risk, this is due to defects of the recipient respiratory epithelial deficiencies, whereas where the donor immunogenotype is predictive, this maps to the donor stem cell myeloid compartment [133,134,135,136]. Extension of such approaches to other at-risk groups for respiratory fungal diseases would be of great interest, with some studies already undertaken for allergic bronchopulmonary aspergillosis for instance [137, 138]. Furthermore, most immunogenetic studies thus far have been focused on selected groups of alleles. Large-scale and multinational GWAS studies would give greater resolution to which alleles are dominant and whether they penetrate in all populations.
Personalized Medicine to Identify Clinically Relevant Endotypes
The identification of specific clinically relevant disease endotypes has been greatly accelerated in respiratory medicine through the advent of systematic immunophenotyping studies [95, 139,140,141,142]. Given the current revolution in monoclonal antibody therapies in clinical medicine, with many agents now licensed for therapy of asthma and allergic rhinitis, repurposing of these monoclonals for allergic fungal airway diseases in particular, and better understanding of which may be more efficacious in this setting, is a current high priority [143, 144]. We are already using omalizumab routinely for IgE depletion in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization, with accumulating but mainly case series-based clinical data to support this approach [145,146,147]. Mepolizumab-based targeting of eosinophilic responses is also now commonplace, although there are some safety concerns more generally as eosinophils may have a protective role against invasive aspergillosis [148, 149]. Better understanding is therefore required of which immune pathways are most contributory to progressive decline in allergic fungal airway diseases in order to enable the precise targeting of monoclonal therapeutics. In order to do this, large multicentre prospective immunoprofiling studies are required adopting a systematic approach to identify those targetable immune pathways that are most predictive of severe endotypes of allergic fungal airway disease. Optimal resolution of endotypes is achieved when systemic immune signatures (i.e. peripheral blood multiparameter flow sorting), local airway signatures (i.e. sputum transcriptome, sputum mycobiome) data and clinical data (i.e. radiological scoring, respiratory physiology and clinical questionnaires) are integrated to provide multidimensional data linked to clinical longitudinal outcomes. Such an approach has been used to identify new asthma endotypes and would be of great utility for chronic respiratory fungal diseases, where we still do not fully understand for instance why only some patients with allergic bronchopulmonary aspergillosis respond to steroids, whereas others may show a response to antifungals [150]. The emergence of artificial intelligence, or at least machine learning, has opened the door for novel approaches to identification of respiratory disease radiological endotypes, where a range of fungal-specific or at least predictive features such as nodules or cavitation exist, but could be further refined and automatically identified using non-partisan image analysis approaches [151].
Conclusions
Over the last decade there has been greater awareness of the prevalence of respiratory fungal diseases globally. The advent of the 4th industrial revolution leaves us poised to exploit molecular engineering and post-genomic technologies and advances in combination with big data science and artificial intelligence to revolution our understanding of the pathogenesis of these complex infections. Substantial progress in drug discovery and development, as well as the rational design of novel immunotherapeutics, has greatly broadened the therapeutic armamentarium with which to combat respiratory fungal disease. The major challenge we face to translate these advances for the benefit of patients will be to ensure that these advances in the understanding of disease pathogenesis and novel therapeutic options are integrated within a personalized medicine framework to ensure the right patient gets the right treatment at the right time.
References
Armstrong-James D, Youngs J, Bicanic T, et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur Respir J. 2020;56(4).
Vanderbeke L, Spriet I, Breynaert C, Rijnders BJA, Verweij PE, Wauters J. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis. 2018;31(6):471–80.
Azar MM, Loyd JL, Relich RF, Wheat LJ, Hage CA. Current Concepts in the epidemiology, diagnosis, and management of histoplasmosis syndromes. Semin Respir Crit Care Med. 2020;41(1):13–30.
Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013;51(4):361–70.
Ying RS, Le T, Cai WP, et al. Clinical epidemiology and outcome of HIV-associated talaromycosis in Guangdong, China, during 2011–2017. HIV Med. 2020;21(11):729–38.
Steinbach WJ, Marr KA, Anaissie EJ, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect. 2012;65(5):453–64.
Latge JP, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev. 2019;33(1).
Holden GW. Case of Pulmonary and Glandular Aspergillosis. Trans Am Climatol Clin Assoc. 1915;31:97–105.
Tilley H. Aspergillosis of the Maxillary Antrum: (With Histological Report by S. G. Shattock, F.R.C.S.). Proc R Soc Med. 1915;8(Laryngol Sect):14–23.
Zanganeh E, Zarrinfar H, Rezaeetalab F, et al. Predominance of non-fumigatus Aspergillus species among patients suspected to pulmonary aspergillosis in a tropical and subtropical region of the Middle East. Microb Pathog. 2018;116:296–300.
Lass-Florl C, Griff K, Mayr A, et al. Epidemiology and outcome of infections due to Aspergillus terreus: 10-year single centre experience. Br J Haematol. 2005;131(2):201–7.
Rudramurthy SM, Paul RA, Chakrabarti A, Mouton JW, Meis JF. Invasive Aspergillosis by Aspergillus flavus: Epidemiology, Diagnosis, Antifungal Resistance, and Management. J Fungi (Basel). 2019;5(3).
Asano K, Hebisawa A, Ishiguro T, et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J Allergy Clin Immunol. 2020.
Chowdhary A, Agarwal K, Kathuria S, Gaur SN, Randhawa HS, Meis JF. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: a global overview. Crit Rev Microbiol. 2014;40(1):30–48.
Eades CP, Armstrong-James DPH. Invasive fungal infections in the immunocompromised host: Mechanistic insights in an era of changing immunotherapeutics. Med Mycol. 2019;57(Supplement_3):S307–S317.
Bartlett AW, Cann MP, Yeoh DK, et al. Epidemiology of invasive fungal infections in immunocompromised children; an Australian national 10-year review. Pediatr Blood Cancer. 2019;66(4):e27564.
Boch T, Reinwald M, Postina P, et al. Identification of invasive fungal diseases in immunocompromised patients by combining an Aspergillus specific PCR with a multifungal DNA-microarray from primary clinical samples. Mycoses. 2015;58(12):735–45.
Davidson J, McErlane J, Aljboor K, et al. Musical instruments, fungal spores and hypersensitivity pneumonitis. QJM. 2019;112(4):287–9.
Bellanger AP, Lignon T, Godet Y, et al. Fungal peptides from pneumonitis hypersensitivity etiologic agents are able to induce specific cellular immune response. J Immunol Methods. 2017;440:67–73.
Tirado-Sanchez A, Gonzalez GM, Bonifaz A. Endemic mycoses: epidemiology and diagnostic strategies. Expert Rev Anti Infect Ther. 2020;18(11):1105–17.
Darling ST. The morphology of the parasite (Histoplasma Capsulatum) and the Lesions of Histoplasmosis, a Fatal Disease of Tropical America. J Exp Med. 1909;11(4):515–31.
Segretain G. [Penicillium marneffei n.sp., agent of a mycosis of the reticuloendothelial system]. Mycopathol Mycol Appl. 1959;11:327–353.
Jacobson HP. Coccidioidomycosis. Arch Derm Syphilol. 1948;57(3 Pt. 2):561.
Nabarro JD. Primary pulmonary coccidioidomycosis; case of laboratory infection in England. Lancet. 1948;1(6513):982–4.
Whitman RC. Giant Cell Endothelioma of the Gums with Observations on the Formation of Giant Cells in the same, and in Tuberculosis and Blastomycosis. J Med Res. 1914;29(3):465–472 461.
Ashraf N, Kubat RC, Poplin V, et al. Re-drawing the maps for endemic mycoses. Mycopathologia. 2020;185(5):843–65.
Malcolm TR, Chin-Hong PV. Endemic mycoses in immunocompromised hosts. Curr Infect Dis Rep. 2013;15(6):536–43.
Fones HN, Fisher MC, Gurr SJ. Emerging Fungal Threats to Plants and Animals Challenge Agriculture and Ecosystem Resilience. Microbiol Spectr. 2017;5(2).
Fisher MC, Hawkins NJ, Sanglard D, Gurr SJ. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science. 2018;360(6390):739–42.
Tiew PY, Ko FWS, Pang SL, et al. Environmental fungal sensitisation associates with poorer clinical outcomes in COPD. Eur Respir J. 2020;56(2).
Bercusson A, Colley T, Shah A, Warris A, Armstrong-James D. Ibrutinib blocks Btk-dependent NF-kB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood. 2018;132(18):1985–8.
Barouky R, Badet M, Denis MS, Soubirou JL, Philit F, Guerin C. Inhaled corticosteroids in chronic obstructive pulmonary disease and disseminated aspergillosis. Eur J Intern Med. 2003;14(6):380–2.
Fraczek MG, Chishimba L, Niven RM, et al. Corticosteroid treatment is associated with increased filamentous fungal burden in allergic fungal disease. J Allergy Clin Immunol. 2018;142(2):407–14.
Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387(10020):760–9.
Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348–359.
Cornely OA, Maertens J, Bresnik M, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis. 2007;44(10):1289–97.
Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–15.
Agarwal R, Vishwanath G, Aggarwal AN, Garg M, Gupta D, Chakrabarti A. Itraconazole in chronic cavitary pulmonary aspergillosis: a randomised controlled trial and systematic review of literature. Mycoses. 2013;56(5):559–70.
Agarwal R, Dhooria S, Singh Sehgal I, et al. A randomized trial of itraconazole vs prednisolone in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Chest. 2018;153(3):656–64.
Olum R, Baluku JB, Kazibwe A, Russell L, Bongomin F. Tolerability of oral itraconazole and voriconazole for the treatment of chronic pulmonary aspergillosis: a systematic review and meta-analysis. PLoS One. 2020;15(10):e0240374.
Denning DW, Lee JY, Hostetler JS, et al. NIAID mycoses study group multicenter trial of oral itraconazole therapy for invasive aspergillosis. Am J Med. 1994;97(2):135–44.
Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother. 2014;69(5):1162–76.
Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of Aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134(1):33–9.
Woolnough K, Fairs A, Pashley CH, Wardlaw AJ. Allergic fungal airway disease: pathophysiologic and diagnostic considerations. Curr Opin Pulm Med. 2015;21(1):39–47.
Li E, Tsai CL, Maskatia ZK, et al. Benefits of antifungal therapy in asthma patients with airway mycosis: a retrospective cohort analysis. Immun Inflamm Dis. 2018;6(2):264–75.
Seifert H, Aurbach U, Stefanik D, Cornely O. In vitro activities of isavuconazole and other antifungal agents against Candida bloodstream isolates. Antimicrob Agents Chemother. 2007;51(5):1818–21.
Krishna G, Ma L, Martinho M, Preston RA, O’Mara E. A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers. J Antimicrob Chemother. 2012;67(11):2725–30.
Walsh TJ, Raad I, Patterson TF, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007;44(1):2–12.
Vazquez JA, Skiest DJ, Nieto L, et al. A multicenter randomized trial evaluating posaconazole versus fluconazole for the treatment of oropharyngeal candidiasis in subjects with HIV/AIDS. Clin Infect Dis. 2006;42(8):1179–86.
Wiederhold NP. Review of the Novel Investigational Antifungal Olorofim. J Fungi (Basel). 2020;6(3).
Kirchhoff L, Dittmer S, Buer J, Rath PM, Steinmann J. In vitro activity of olorofim (F901318) against fungi of the genus, Scedosporium and Rasamsonia as well as against Lomentospora prolificans, Exophiala dermatitidis and azole-resistant Aspergillus fumigatus. Int J Antimicrob Agents. 2020;56(3):106105.
Astvad KMT, Jorgensen KM, Hare RK, Datcu R, Arendrup MC. Olorofim Susceptibility testing of 1423 Danish mold isolates obtained in 2018–2019 confirms uniform and broad-spectrum activity. Antimicrob Agents Chemother. 2020;65(1).
Jorgensen KM, Astvad KMT, Hare RK, Arendrup MC. EUCAST Determination of Olorofim (F901318) Susceptibility of Mold Species, Method Validation, and MICs. Antimicrob Agents Chemother. 2018;62(8).
du Pre S, Beckmann N, Almeida MC, et al. Effect of the Novel Antifungal Drug F901318 (Olorofim) on Growth and Viability of Aspergillus fumigatus. Antimicrob Agents Chemother. 2018;62(8).
Shaw KJ, Ibrahim AS. Fosmanogepix: A review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J Fungi (Basel). 2020;6(4).
Gebremariam T, Alkhazraji S, Alqarihi A, et al. Fosmanogepix (APX001) is effective in the treatment of pulmonary murine Mucormycosis due to Rhizopus arrhizus. Antimicrob Agents Chemother. 2020;64(6).
Alkhazraji S, Gebremariam T, Alqarihi A, et al. Fosmanogepix (APX001) Is Effective in the treatment of immunocompromised mice infected with invasive pulmonary scedosporiosis or disseminated fusariosis. Antimicrob Agents Chemother. 2020;64(3).
Batista-Duharte A, Lastre M, Romeu B, et al. Antifungal and immunomodulatory activity of a novel cochleate for amphotericin B delivery against Sporothrix schenckii. Int Immunopharmacol. 2016;40:277–87.
Santangelo R, Paderu P, Delmas G, et al. Efficacy of oral cochleate-amphotericin B in a mouse model of systemic candidiasis. Antimicrob Agents Chemother. 2000;44(9):2356–60.
Pfaller MA, Rhomberg PR, Messer SA, Castanheira M. In vitro activity of a Hos2 deacetylase inhibitor, MGCD290, in combination with echinocandins against echinocandin-resistant Candida species. Diagn Microbiol Infect Dis. 2015;81(4):259–63.
Pfaller MA, Messer SA, Georgopapadakou N, Martell LA, Besterman JM, Diekema DJ. Activity of MGCD290, a Hos2 histone deacetylase inhibitor, in combination with azole antifungals against opportunistic fungal pathogens. J Clin Microbiol. 2009;47(12):3797–804.
Wiederhold NP, Xu X, Wang A, et al. In Vivo Efficacy of VT-1129 against Experimental cryptococcal meningitis with the use of a loading dose-maintenance dose administration strategy. Antimicrob Agents Chemother. 2018;62(11).
Wiederhold NP, Najvar LK, Garvey EP, et al. The Fungal Cyp51 inhibitor VT-1129 is efficacious in an experimental model of cryptococcal meningitis. Antimicrob Agents Chemother. 2018;62(9).
Brand SR, Sobel JD, Nyirjesy P, Ghannoum MA, Schotzinger RJ, Degenhardt TP. Randomized Phase 2 study of VT-1161 for the treatment of acute vulvovaginal candidiasis. Clin Infect Dis. 2020.
Elewski B, Brand S, Degenhardt T, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of VT-1161 oral tablets in the treatment of patients with distal and lateral subungual onychomycosis of the toenail. Br J Dermatol. 2020.
Brand SR, Degenhardt TP, Person K, et al. A phase 2, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of orally administered VT-1161 in the treatment of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2018;218(6):624 e621–624 e629.
Garvey EP, Sharp AD, Warn PA, et al. The novel fungal CYP51 inhibitor VT-1598 displays classic dose-dependent antifungal activity in murine models of invasive aspergillosis. Med Mycol. 2020;58(4):505–13.
Mammen MP, Armas D, Hughes FH, et al. First-in-human phase 1 study to assess safety, tolerability, and pharmacokinetics of a novel antifungal drug, VL-2397, in healthy adults. Antimicrob Agents Chemother. 2019;63(11).
Yamashita K, Miyazaki T, Fukuda Y, et al. The Novel Arylamidine T-2307 Selectively disrupts yeast mitochondrial function by inhibiting respiratory chain complexes. Antimicrob Agents Chemother. 2019;63(8).
Purcell IF, Corris PA. Use of nebulised liposomal amphotericin B in the treatment of Aspergillus fumigatus empyema. Thorax. 1995;50(12):1321–3.
Shirkhani K, Teo I, Armstrong-James D, Shaunak S. Nebulised amphotericin B-polymethacrylic acid nanoparticle prophylaxis prevents invasive aspergillosis. Nanomedicine. 2015;11(5):1217–26.
Alissa D, AlMaghrabi R, Nizami I, et al. Nebulized amphotericin b dosing regimen for aspergillus prevention after lung transplant. Exp Clin Transpl. 2021;19(1):58–63.
Corcoran TE, Venkataramanan R, Mihelc KM, et al. Aerosol deposition of lipid complex amphotericin-B (Abelcet) in lung transplant recipients. Am J Transpl. 2006;6(11):2765–73.
Laoudi Y, Paolini JB, Grimfed A, Just J. Nebulised corticosteroid and amphotericin B: an alternative treatment for ABPA? Eur Respir J. 2008;31(4):908–9.
Murray A, Cass L, Ito K, et al. PC945, a novel inhaled antifungal agent, for the treatment of respiratory fungal infections. J Fungi (Basel). 2020;6(4).
Colley T, Alanio A, Kelly SL, et al. In vitro and in vivo antifungal profile of a novel and long-acting inhaled azole, PC945, on Aspergillus fumigatus infection. Antimicrob Agents Chemother. 2017;61(5).
Colley T, Sehra G, Daly L, et al. Antifungal synergy of a topical triazole, PC945, with a systemic triazole against respiratory Aspergillus fumigatus infection. Sci Rep. 2019;9(1):9482.
Kimura G, Nakaoki T, Colley T, et al. In vivo biomarker analysis of the effects of intranasally dosed PC945, a novel antifungal triazole, on Aspergillus fumigatus infection in immunocompromised mice. Antimicrob Agents Chemother. 2017;61(9).
Pagani N, Armstrong-James D, Reed A. Successful salvage therapy for fungal bronchial anastomotic infection after -lung transplantation with an inhaled triazole anti-fungal PC945. J Heart Lung Transplant. 2020;39(12):1505–6.
Cass L, Murray A, Davis A, et al. Safety and nonclinical and clinical pharmacokinetics of PC945, a novel inhaled triazole antifungal agent. Pharmacol Res Perspect. 2021;9(1):e00690.
Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study Group education and research consortium. Clin Infect Dis. 2020;71(6):1367–76.
Carvalho-Pereira J, Fernandes F, Araujo R, et al. Multiplex PCR based strategy for detection of fungal pathogen DNA in patients with suspected invasive fungal infections. J Fungi (Basel). 2020;6(4).
Lau A, Stanley K, Sorrell T. Multiplex-tandem PCR for fungal diagnostics. Methods Mol Biol. 2013;968:195–201.
Linder KA, Kauffman CA, Miceli MH. Performance of Aspergillus Galactomannan lateral flow assay on bronchoalveolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis. J Fungi (Basel). 2020;6(4).
White PL, Price JS, Posso R, Cutlan-Vaughan M, Vale L, Backx M. Evaluation of the performance of the IMMY sona Aspergillus galactomannan lateral flow assay when testing serum to aid in diagnosis of invasive aspergillosis. J Clin Microbiol. 2020;58(6).
Dufresne SF, Datta K, Li X, et al. Detection of urinary excreted fungal galactomannan-like antigens for diagnosis of invasive aspergillosis. PLoS One. 2012;7(8):e42736.
Srinivasan A, Kleiman MB, Debelenko L, Stokes DC, De Vincenzo J, Wheat JL. False-negative Histoplasma antigen in acute pulmonary histoplasmosis: the value of urinary concentration by ultrafiltration and heat denaturation of serum proteins in detection of Histoplasma antigen. Pediatr Infect Dis J. 2009;28(5):447–9.
Davies G, Singh O, Prattes J, Hoenigl M, Sheppard PW, Thornton CR. Aspergillus fumigatus and its allergenic Ribotoxin Asp f I: old enemies but new opportunities for urine-based detection of invasive pulmonary Aspergillosis using lateral-flow technology. J Fungi (Basel). 2020;7(1).
McTaggart LR, Copeland JK, Surendra A, et al. Mycobiome Sequencing and analysis applied to fungal community profiling of the lower respiratory tract during fungal pathogenesis. Front Microbiol. 2019;10:512.
Shelburne SA, Ajami NJ, Chibucos MC, et al. Implementation of a pan-genomic approach to investigate holobiont-infecting microbe interaction: a case report of a leukemic patient with invasive mucormycosis. PLoS One. 2015;10(11):e0139851.
Goncalves SM, Lagrou K, Duarte-Oliveira C, Maertens JA, Cunha C, Carvalho A. The microbiome-metabolome crosstalk in the pathogenesis of respiratory fungal diseases. Virulence. 2017;8(6):673–84.
Tiew PY, Mac Aogain M, Ali N, et al. The mycobiome in health and disease: emerging concepts, methodologies and challenges. Mycopathologia. 2020;185(2):207–31.
Tiew PY, Dicker AJ, Keir HR, et al. A high-risk airway mycobiome is associated with frequent exacerbation and mortality in COPD. Eur Respir J. 2020.
Soret P, Vandenborght LE, Francis F, et al. Respiratory mycobiome and suggestion of inter-kingdom network during acute pulmonary exacerbation in cystic fibrosis. Sci Rep. 2020;10(1):3589.
Mac Aogain M, Chandrasekaran R, Lim AYH, et al. Immunological corollary of the pulmonary mycobiome in bronchiectasis: the CAMEB study. Eur Respir J. 2018;52(1).
Mac Aogain M, Narayana JK, Tiew PY, et al. Integrative microbiomics in bronchiectasis exacerbations. Nat Med. 2021;27(4):688–99.
Kabbani D, Goldraich L, Ross H, Rotstein C, Husain S. Outbreak of invasive aspergillosis in heart transplant recipients: The role of screening computed tomography scans in asymptomatic patients and universal antifungal prophylaxis. Transpl Infect Dis. 2018;20(1).
Pelaez T, Munoz P, Guinea J, et al. Outbreak of invasive aspergillosis after major heart surgery caused by spores in the air of the intensive care unit. Clin Infect Dis. 2012;54(3):e24-31.
Menotti J, Porcher R, Ribaud P, et al. Monitoring of nosocomial invasive aspergillosis and early evidence of an outbreak using cumulative sum tests (CUSUM). Clin Microbiol Infect. 2010;16(9):1368–74.
Freitas DB, Piovesan AC, Szarf G, Jasinowodolinski D, Meirelles GS. Outbreak of invasive pulmonary aspergillosis among patients hospitalized in a bone marrow transplant ward: tomographic findings. J Bras Pneumol. 2009;35(9):931–6.
Cavaleiro Rufo J, Madureira J, Paciencia I, et al. Indoor fungal diversity in primary schools may differently influence allergic sensitization and asthma in children. Pediatr Allergy Immunol. 2017;28(4):332–9.
Rivera-Mariani FE, Nazario-Jimenez S, Lopez-Malpica F, Bolanos-Rosero B. Sensitization to airborne ascospores, basidiospores, and fungal fragments in allergic rhinitis and asthmatic subjects in San Juan, Puerto Rico. Int Arch Allergy Immunol. 2011;155(4):322–34.
Barnes RA. Directed therapy for fungal infections: focus on aspergillosis. J Antimicrob Chemother. 2013;68(11):2431–4.
Abdolrasouli A, Scourfield A, Rhodes J, et al. High prevalence of triazole resistance in clinical Aspergillus fumigatus isolates in a specialist cardiothoracic centre. Int J Antimicrob Agents. 2018;52(5):637–42.
Abdolrasouli A, Rhodes J, Beale MA, et al. Genomic context of azole resistance mutations in Aspergillus fumigatus determined using whole-genome sequencing. mBio. 2015;6(3):e00536.
Morrissey CO, Chen SC, Sorrell TC, et al. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: a randomised controlled trial. Lancet Infect Dis. 2013;13(6):519–28.
Aguado JM, Vazquez L, Fernandez-Ruiz M, et al. Serum galactomannan versus a combination of galactomannan and polymerase chain reaction-based Aspergillus DNA detection for early therapy of invasive aspergillosis in high-risk hematological patients: a randomized controlled trial. Clin Infect Dis. 2015;60(3):405–14.
Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol. 2016;7:2173.
Rhodes J, Abdolrasouli A, Farrer RA, et al. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg Microbes Infect. 2018;7(1):43.
Abdolrasouli A, Armstrong-James D, Ryan L, Schelenz S. In vitro efficacy of disinfectants utilised for skin decolonisation and environmental decontamination during a hospital outbreak with Candida auris. Mycoses. 2017;60(11):758–63.
Schelenz S, Hagen F, Rhodes JL, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control. 2016;5:35.
Stone NR, Rhodes J, Fisher MC, et al. Dynamic ploidy changes drive fluconazole resistance in human cryptococcal meningitis. J Clin Invest. 2019;129(3):999–1014.
Pepys J. Allergic hypersensitivity to fungi. Postgrad Med J. 1959;35:436–40.
Pepys J. The role of human precipitins to common fungal antigens in allergic reactions. Acta Allergol Suppl (Copenh). 1960;7:108–11.
Longbottom JL, Pepys J. Comparison of antigens and allergens in Aspergillus fumigatus and Epidermophyton floccosum. Int Arch Allergy Appl Immunol. 1962;20:370–1.
Malo JL, Longbottom J, Mitchell J, Hawkins R, Pepys J. Studies in chronic allergic bronchopulmonary aspergillosis. 3. Immunological findings. Thorax. 1977;32(3):269–274.
Crameri R. Molecular cloning of Aspergillus fumigatus allergens and their role in allergic bronchopulmonary aspergillosis. Chem Immunol. 2002;81:73–93.
Onami JI, Kobayashi N, Watanabe M, et al. An updated data portal for fungal allergens with curated information. Bioinformation. 2019;15(11):820–3.
Fukutomi Y, Taniguchi M. Sensitization to fungal allergens: Resolved and unresolved issues. Allergol Int. 2015;64(4):321–31.
Crameri R, Zeller S, Glaser AG, Vilhelmsson M, Rhyner C. Cross-reactivity among fungal allergens: a clinically relevant phenomenon? Mycoses. 2009;52(2):99–106.
Casaulta C, Fluckiger S, Crameri R, Blaser K, Schoeni MH. Time course of antibody response to recombinant Aspergillus fumigatus antigens in cystic fibrosis with and without ABPA. Pediatr Allergy Immunol. 2005;16(3):217–25.
Bousquet J, Anto J, Auffray C, et al. MeDALL (Mechanisms of the Development of ALLergy): an integrated approach from phenotypes to systems medicine. Allergy. 2011;66(5):596–604.
Bacher P, Heinrich F, Stervbo U, et al. Regulatory T Cell Specificity Directs Tolerance versus Allergy against Aeroantigens in Humans. Cell. 2016;167(4):1067–1078 e1016.
Bacher P, Jochheim-Richter A, Mockel-Tenbrink N, et al. Clinical-scale isolation of the total Aspergillus fumigatus-reactive T-helper cell repertoire for adoptive transfer. Cytotherapy. 2015;17(10):1396–405.
Khanna N, Stuehler C, Conrad B, et al. Generation of a multipathogen-specific T-cell product for adoptive immunotherapy based on activation-dependent expression of CD154. Blood. 2011;118(4):1121–31.
Steinbach A, Cornely OA, Wisplinghoff H, et al. Mould-reactive T cells for the diagnosis of invasive mould infection-A prospective study. Mycoses. 2019;62(7):562–9.
Weis P, Helm J, Page L, et al. Development and evaluation of a whole blood-based approach for flow cytometric quantification of CD154+ mould-reactive T cells. Med Mycol. 2020;58(2):187–96.
Voskamp AL, Gillman A, Symons K, et al. Clinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2015;3(2):192–9.
Ferwerda B, Ferwerda G, Plantinga TS, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361(18):1760–7.
Dabas A, Arora P, Kumar S, Kapoor S, Yadav S. STAT 1 mutation associated with chronic mucocutaneous candidiasis and pancytopenia. Pediatr Allergy Immunol. 2021.
Dhalla F, Fox H, Davenport EE, et al. Chronic mucocutaneous candidiasis: characterization of a family with STAT-1 gain-of-function and development of an ex-vivo assay for Th17 deficiency of diagnostic utility. Clin Exp Immunol. 2016;184(2):216–27.
Ifrim DC, Quintin J, Meerstein-Kessel L, et al. Defective trained immunity in patients with STAT-1-dependent chronic mucocutaneaous candidiasis. Clin Exp Immunol. 2015;181(3):434–40.
Tanpaibule T, Jinawath N, Taweewongsounton A, Niparuck P, Rotjanapan P. Genetic risk surveillance for invasive aspergillosis in hematology patients: a prospective observational study. Infect Dis Ther. 2020;9(4):807–21.
White PL, Parr C, Barnes RA. Predicting invasive aspergillosis in hematology patients by combining clinical and genetic risk factors with early diagnostic biomarkers. J Clin Microbiol. 2018;56(1).
Cunha C, Monteiro AA, Oliveira-Coelho A, et al. PTX3-based genetic testing for risk of aspergillosis after lung transplant. Clin Infect Dis. 2015;61(12):1893–4.
Cunha C, Carvalho A. Toward the identification of a genetic risk signature for pulmonary aspergillosis in chronic obstructive pulmonary disease. Clin Infect Dis. 2018;66(7):1153–4.
Overton NLD, Brakhage AA, Thywissen A, Denning DW, Bowyer P. Mutations in EEA1 are associated with allergic bronchopulmonary aspergillosis and affect phagocytosis of Aspergillus fumigatus by human macrophages. PLoS One. 2018;13(3):e0185706.
Overton NL, Denning DW, Bowyer P, Simpson A. Genetic susceptibility to allergic bronchopulmonary aspergillosis in asthma: a genetic association study. Allergy Asthma Clin Immunol. 2016;12:47.
Lefaudeux D, De Meulder B, Loza MJ, et al. U-BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. J Allergy Clin Immunol. 2017;139(6):1797–807.
Loza MJ, Adcock I, Auffray C, et al. Longitudinally stable, clinically defined clusters of patients with asthma independently identified in the ADEPT and U-BIOPRED asthma studies. Ann Am Thorac Soc. 2016;13(Suppl 1):S102-103.
Shaw DE, Sousa AR, Fowler SJ, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46(5):1308–21.
Tiew PY, Ko FWS, Narayana JK, et al. “High-Risk” clinical and inflammatory clusters in COPD of Chinese descent. Chest. 2020;158(1):145–56.
Eraso IC, Sangiovanni S, Morales EI, Fernandez-Trujillo L. Use of monoclonal antibodies for allergic bronchopulmonary aspergillosis in patients with asthma and cystic fibrosis: literature review. Ther Adv Respir Dis. 2020;14:1753466620961648.
Lee T, Nair P, Corrigan CJ. Review of monoclonal antibody therapies in asthma and allergic diseases: a new paradigm for precision medicine. Asian Pac J Allergy Immunol. 2020;38(2):78–90.
Altman MC, Lenington J, Bronson S, Ayars AG. Combination omalizumab and mepolizumab therapy for refractory allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract. 2017;5(4):1137–9.
Tomomatsu K, Oguma T, Baba T, et al. Effectiveness and safety of omalizumab in patients with allergic bronchopulmonary aspergillosis complicated by chronic bacterial infection in the airways. Int Arch Allergy Immunol. 2020;181(7):499–506.
Zirbes JM, Milla CE. Steroid-sparing effect of omalizumab for allergic bronchopulmonary aspergillosis and cystic fibrosis. Pediatr Pulmonol. 2008;43(6):607–10.
Soeda S, To M, Kono Y, et al. Case series of allergic bronchopulmonary aspergillosis treated successfully and safely with long-term mepolizumab. Allergol Int. 2019;68(3):377–9.
Guerra ES, Lee CK, Specht CA, et al. Central Role of IL-23 and IL-17 producing eosinophils as immunomodulatory effector cells in acute pulmonary aspergillosis and allergic asthma. PLoS Pathog. 2017;13(1):e1006175.
Silkoff PE, Moore WC, Sterk PJ. Three major efforts to phenotype asthma: severe asthma research program, asthma disease endotyping for personalized therapeutics, and unbiased biomarkers for the prediction of respiratory disease outcome. Clin Chest Med. 2019;40(1):13–28.
Kelly B, Judge C, Bollard SM, et al. Radiology artificial intelligence, a systematic evaluation of methods (RAISE): a systematic review protocol. Insights Imaging. 2020;11(1):133.
Funding
DAJ is funded by the Wellcome Trust Collaborative Award (219551/Z/19/Z), and the Cystic Fibrosis Trust (SRC015). DAJ is funded by the DHSC Centre for Antimicrobial Optimisation, at Imperial College, London. The views expressed in this publication are those of the author and not necessarily those of the Department of Health and Social Care, NHS, or the National Institute for Health Research. DAJ holds share options in Pulmocide Limited, an inhaled antifungal company.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DAJ holds founder share options in Pulmocide Ltd and has received investigator-led grants from Pulmocide Ltd and Gilead Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Sanjay Haresh Chotirmall
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Armstrong-James, D. Future Directions for Clinical Respiratory Fungal Research. Mycopathologia 186, 685–696 (2021). https://doi.org/10.1007/s11046-021-00579-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-021-00579-5