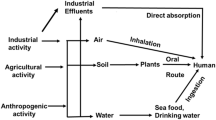
Abstract
Background
Inorganic arsenic [As(III)] and hexavalent chromium [Cr(VI)] can potentially affect metabolic functions. These heavy metal(s)/metalloids can also affect the gut microbial architecture which affects metabolic health. Here, we assessed the effects of short-term exposure of As(III) and Cr(VI) on key transcription factors in adipose tissues and on selected gut microbial abundances to understand the possible modulatory role of these toxicants on host metabolic health.
Methods and results
qRT-PCR based relative bacterial abundance studies in cecal samples, gene expression analysis for gut wall integrity in ileum and colon and adipogenesis, lipolysis, and thermogenic genes in gonadal white and brown adipose tissue (gWAT and BAT), along with tissue oxidative stress parameters have been performed. As(III) and Cr(VI) exposure reduced beneficial Lactobacilli, Bifidobacteria, Akkermansia, Lachenospiraceae, Fecalibacterium, Eubacterium, and clostridium coccoid group while increasing lipopolysaccharides producing Enterobacteriaceae abundances. It also impaired structural features and expression of key tight junction and mucin production genes in ileum and colon (Cld-2, Cld-4, ZO-1, ZO-2, MUC-2 and − 4). In gWAT it inhibited adipogenesis (PPARγ, FASN, SREBP1a), lipolysis (HSL, ACOX-1), and thermogenesis (UCP-1, PGC1a, PRDM-16, PPARa) related genes expression, whereas in BAT, it enhanced adipogenesis and reduced thermogenesis. These exposures also reduces the endogenous antioxidants levels in these tissues and promote pro-inflammatory cytokines genes expression (TLRs, IL-6, MCP-1). The combinatorial exposure appears to have more deleterious effects.
Conclusion
These effects of As(III) and Cr(VI) may not directly be linked to their known toxicological effects, instead, more intriguing crosstalk with gut microbial ecosystem hold the key.




Similar content being viewed by others
Data availability
All the research data and available materials requests can be sent to the corresponding author. Data and materials would be available on reasonable requests.
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- ACOX-1:
-
Acyl-CoA Oxidase 1
- AKK:
-
Akkermansia sp.
- ANERO:
-
Anaerostipes sp.
- As(III):
-
Inorganic arsenic
- BACT:
-
Bacteroidetes
- BACT sp:
-
Bacteroides sp.
- BAT:
-
Brown adipose tissue
- BCA:
-
Bicinchoninic acid
- BIF:
-
Bifidobacteria
- BPULL:
-
Butyricicoccus pullicaecorum
- BVIB:
-
Butyrivibrio sp.
- CEBPa:
-
CCAAT/enhancer-binding protein alpha
- CITRO:
-
Citrobacter sp.
- Cld:
-
Claudin
- CLEP:
-
Clostridium sp.
- CPCSEA:
-
Committee for the purpose of control and supervision of experiments on animals
- CPROP:
-
Clostridium propionicum
- Cr(VI):
-
Hexavalent chromium
- CRONO:
-
Cronobacter sp.
- DIO2:
-
Type II iodothyronine deiodinase
- ECOL:
-
Escherichia coli
- ENT:
-
Enterobacter sp.
- ENTB:
-
Enterobacteriaceae
- EUBACT:
-
Eubacterium sp.
- F4/80:
-
EGF-like module-containing mucin-like hormone receptor-like 1
- FASN:
-
Fatty acid synthase
- FEC:
-
Fecalibacterium sp.
- FFAR:
-
Free fatty acid receptors
- FIRM:
-
Firmicutes
- gCCOC:
-
Clostridium coccoides group
- GK:
-
Glucokinase
- GLP-1:
-
Glucagon like peptide-1
- GST:
-
Glutathione-S-Transferase
- gWAT:
-
Gonadal white adipose tissue
- HSL:
-
Hormone-sensitive lipase
- IAEC:
-
Institutional Animal Ethics Committee
- ICMR:
-
Indian Council of Medical Research
- IL-6:
-
Interleukin-6
- iNOS:
-
Inducible nitric oxide synthase
- LAB:
-
Lactobacilli
- LACH:
-
Lachnospiraceae
- Lep:
-
Leptin
- LepR:
-
Leptin receptor
- MCP-1:
-
Macrophage chemoattractant protein-1
- MDA:
-
Malondialdehyde
- MUC:
-
Mucin
- MyD88:
-
Myeloid differentiation primary response 88
- NF-kB:
-
Nuclear factor-kappa beta
- PEPCK:
-
Phosphoenolpyruvate carboxykinase
- PGC1α:
-
Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha
- PLIN-1:
-
Perilipin-1
- PPAR (α or γ):
-
Peroxisome proliferator-activated receptor (alpha or gamma)
- PRDM16:
-
PR domain containing 16
- PREVO:
-
Prevotella sp.
- ROS:
-
Roseburia sp.
- SALM:
-
Salmonella sp.
- SOD:
-
Superoxide dismutase
- SREBP1a:
-
Sterol regulatory element-binding protein 1
- TLR:
-
Toll-like receptor
- UCP-1:
-
Uncoupling protein-1
- ZO:
-
Zona occluding
References
Abdul KSM, Jayasinghe SS, Chandana EP, Jayasumana C, De Silva PMC (2015) Arsenic and human health effects: a review. Environ Toxicol Pharmacol 40(3):828–846
Shekhawat K, Chatterjee S, Joshi B (2015) Chromium toxicity and its health hazards. Int J Adv Res 3(7):167–172
Tseng C-H, Lee I-H, Chen Y-C (2019) Evaluation of hexavalent chromium concentration in water and its health risk with a system dynamics model. Sci Total Environ 669:103–111
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68(1):167–182
Kumar S, Prasad S, Yadav KK, Shrivastava M, Gupta N, Nagar S et al (2019) Hazardous heavy metals contamination of vegetables and food chain: role of sustainable remediation approaches—a review. Environ Res 179:108792
Schippa S, Conte MP (2014) Dysbiotic events in gut microbiota: impact on human health. Nutrients 6(12):5786–5805
Fan Y, Pedersen O (2021) Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 19(1):55–71
Million M, Maraninchi M, Henry M, Armougom F, Richet H, Carrieri P et al (2012) Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obes 36(6):817–825
Singh DP, Singh S, Bijalwan V, Kumar V, Khare P, Baboota RK et al (2018) Co-supplementation of isomalto-oligosaccharides potentiates metabolic health benefits of polyphenol-rich cranberry extract in high fat diet-fed mice via enhanced gut butyrate production. Eur J Nutr 57(8):2897–2911
Choiniere J, Wang L (2016) Exposure to inorganic arsenic can lead to gut microbe perturbations and hepatocellular carcinoma. Acta Pharm Sin B 6(5):426–429
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D et al (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56(7):1761–1772
Richardson JB, Dancy BCR, Horton CL, Lee YS, Madejczyk MS, Xu ZZ et al (2018) Exposure to toxic metals triggers unique responses from the rat gut microbiota. Sci Rep 8(1):6578
Zhang Z, Cao H, Song N, Zhang L, Cao Y, Tai J (2020) Long-term hexavalent chromium exposure facilitates colorectal cancer in mice associated with changes in gut microbiota composition. Food Chem Toxicol 138:111237
Chi L, Bian X, Gao B, Tu P, Ru H, Lu K (2017) The effects of an environmentally relevant level of arsenic on the gut microbiome and its functional metagenome. Toxicol Sci 160(2):193–204
Bae J, Jang Y, Kim H, Mahato K, Schaecher C, Kim IM et al (2019) Arsenite exposure suppresses adipogenesis, mitochondrial biogenesis and thermogenesis via autophagy inhibition in brown adipose tissue. Sci Rep 9(1):14464–14464
Tripathi S, Fhatima S, Parmar D, Singh DP, Mishra S, Mishra R et al (2022) Therapeutic effects of CoenzymeQ10, Biochanin A and Phloretin against arsenic and chromium induced oxidative stress in mouse (Mus musculus) brain. 3 Biotech 12(5):116
Sharma A, Kshetrimayum C, Sadhu HG, Kumar S (2018) Arsenic-induced oxidative stress, cholinesterase activity in the brain of Swiss albino mice, and its amelioration by antioxidants Vitamin E and Coenzyme Q10. Environ Sci Pollut Res 25(24):23946–23953
Zuo Z, Liu Z, Gao T, Yin Y, Wang Z, Hou Y et al (2019) Prolonged inorganic arsenic exposure via drinking water impairs brown adipose tissue function in mice. Sci Total Environ 668:310–317. https://doi.org/10.1016/j.scitotenv.2019.03.008
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Hu ML (1994) Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol 230:380–385. https://doi.org/10.1016/s0076-6879(94)33044-1
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Ertani A, Mietto A, Borin M, Nardi S (2017) Chromium in agricultural soils and crops: a review. Water Air Soil Pollut 228(5):190
Roy A, Kordas K, Lopez P, Rosado JL, Cebrian ME, Vargas GG et al (2011) Association between arsenic exposure and behavior among first-graders from Torreon, Mexico. Environ Res 111(5):670–676
Mukherjee B, Bindhani B, Saha H, Sinha D, Ray MR (2014) Platelet hyperactivity, neurobehavioral symptoms and depression among Indian women chronically exposed to low level of arsenic. Neurotoxicology 45:159–167
Frediani JK, Naioti EA, Vos MB, Figueroa J, Marsit CJ, Welsh JA (2018) Arsenic exposure and risk of nonalcoholic fatty liver disease (NAFLD) among U.S. adolescents and adults: an association modified by race/ethnicity, NHANES 2005–2014. Environ Health 17(1):6
Lin Y-C, Lian I-B, Kor C-T, Chang C-C, Su P-Y, Chang W-T et al (2017) Association between soil heavy metals and fatty liver disease in men in Taiwan: a cross sectional study. BMJ Open 7(1):e014215
Grau-Perez M, Kuo C-C, Gribble MO, Balakrishnan P, Spratlen MJ, Vaidya D et al (2017) Association of low-moderate arsenic exposure and arsenic metabolism with incident diabetes and insulin resistance in the strong heart family study. Environ Health Perspect 125(12):127004
Jia X, Qiu T, Yao X, Jiang L, Wang N, Wei S et al (2020) Arsenic induces hepatic insulin resistance via mtROS-NLRP3 inflammasome pathway. J Hazard Mater 399:123034
BIS, Drinking Water-Specification (Second Revision) B.o.I. Standards, Editor. 2012, IS 10500: 2012 BIS: New Delhi, pp. 1–11
Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G et al (2016) The gut microbiota and host health: a new clinical frontier. Gut 65(2):330–339
Brabec JL, Wright J, Ly T, Wong HT, McClimans CJ, Tokarev V et al (2020) Arsenic disturbs the gut microbiome of individuals in a disadvantaged community in Nepal. Heliyon 6(1):e03313–e03313
Crovesy L, Ostrowski M, Ferreira DMTP, Rosado EL, Soares-Mota M (2017) Effect of Lactobacillus on body weight and body fat in overweight subjects: a systematic review of randomized controlled clinical trials. Int J Obes 41(11):1607–1614
Petrilli FL, De Flora S (1977) Toxicity and mutagenicity of hexavalent chromium on Salmonella typhimurium. Appl Environ Microbiol 33(4):805–809
Bridgeman SC, Northrop W, Melton PE, Ellison GC, Newsholme P, Mamotte CDS (2020) Butyrate generated by gut microbiota and its therapeutic role in metabolic syndrome. Pharmacol Res 160:105174
Rivera-Chávez F, Zhang LF, Faber F, Lopez CA, Byndloss MX, Olsan EE et al (2016) Depletion of butyrate-producing Clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe 19(4):443–454
Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO et al (2016) Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65(3):426–436
Singh DP, Khare P, Bijalwan V, Baboota RK, Singh J, Kondepudi KK et al (2017) Coadministration of isomalto-oligosaccharides augments metabolic health benefits of cinnamaldehyde in high fat diet fed mice. BioFactors 43(6):821–835
Davey JC, Nomikos AP, Wungjiranirun M, Sherman JR, Ingram L, Batki C et al (2008) Arsenic as an endocrine disruptor: arsenic disrupts retinoic acid receptor–and thyroid hormone receptor–mediated gene regulation and thyroid hormone–mediated amphibian tail metamorphosis. Environ Health Perspect 116(2):165–172
Nascimento S, Göethel G, Gauer B, Sauer E, Nardi J, Cestonaro L et al (2018) Exposure to environment chemicals and its possible role in endocrine disruption of children from a rural area. Environ Res 167:488–498
Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E (2008) Arsenic exposure and prevalence of type 2 diabetes in US Adults. JAMA 300(7):814–822
Farkhondeh T, Samarghandian S, Azimi-Nezhad M (2019) The role of arsenic in obesity and diabetes. J Cell Physiol 234(8):12516–12529
Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K et al (2015) Developmental origins of health and disease: integrating environmental influences. Endocrinology 156(10):3416–3421
Wafer R, Tandon P, Minchin JEN (2017) The role of peroxisome proliferator-activated receptor gamma (PPARG) in adipogenesis: applying knowledge from the fish aquaculture industry to biomedical research. Front Endocrinol 8:102
Rosen ED, Hsu C-H, Wang X, Sakai S, Freeman MW, Gonzalez FJ et al (2002) C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev 16(1):22–26
Gossai A, Lesseur C, Farzan S, Marsit C, Karagas MR, Gilbert-Diamond D (2015) Association between maternal urinary arsenic species and infant cord blood leptin levels in a New Hampshire Pregnancy Cohort. Environ Res 136:180–186. https://doi.org/10.1016/j.envres.2014.10.005
Kitchin KT, Ahmad S (2003) Oxidative stress as a possible mode of action for arsenic carcinogenesis. Toxicol Lett 137(1):3–13
Flora SJS (2011) Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med 51(2):257–281
Acknowledgements
Authors would like to acknowledge the support of the Director, ICMR-NIOH for the infrastructural and institutional facilities.
Funding
No separate funding was available to declare for this study. Funders have no role in study design, data analysis, and the decision to submit it for publication.
Author information
Authors and Affiliations
Contributions
DPS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigations, Methodology, Project administration, Supervision, Visualizations, Writing-original draft, Review, and editing; SKY: Investigations, Writing-original draft; KP: Investigations, Writing-original draft; SP: Investigations, Writing-original draft; VB: Data curation, Visualization, writing original draft, review, and editing; GPP: Investigations, GS: Resources; RP: Resources, KKK: Conceptualization, Methodology; RKB: Data curation, writing original draft; MB: Methodology, Writing-original draft, Review, and editing; SD: Conceptualization, review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Suitable ethical clearances (IAEC/NIOH/2020-21/23/# 8) were obtained for the use of tissue samples at the terminal time point from the institutional animal ethics committee of the ICMR-NIOH, Ahmedabad.
Consent to participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, D.P., Yadav, S.K., Patel, K. et al. Short-term trivalent arsenic and hexavalent chromium exposures induce gut dysbiosis and transcriptional alteration in adipose tissue of mice. Mol Biol Rep 50, 1033–1044 (2023). https://doi.org/10.1007/s11033-022-07992-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07992-z