Abstract
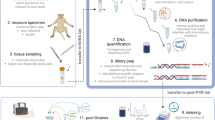
White scar oyster Crassostrea belcheri is a commercially important bivalve species in Thailand. Appropriate genetic markers are needed for effective management to elevate its production efficiency. Type II microsatellites of C. belcheri were identified and characterized using an Illumina paired-end shotgun sequencing. A total of 14,743,710 reads were generated for which 198,849 reads containing microsatellites and 217,998 microsatellite loci were found. Twenty out of 60 microsatellite loci (33.33%) were polymorphic and these microsatellites were further tested against DNA bulks (N = 10 each) originating from 7 different geographic locations in Thai waters. Results indicated that newly developed microsatellites can be used for genetic diversity analysis of C. belcheri. Genotyping of C. belcheri collected from Surat Thani (Gulf of Thailand; N = 50) were performed. The number of alleles per locus ranged from 2 to 12 (average = 4.95). Observed and expected heterozygosities ranged from 0.0000 to 0.9400 (average = 0.3419) and 0.1139 to 0.8190 (average = 0.5844), respectively. Genome information and 20 newly isolated microsatellites will facilitate further studies in population genetics, stock management, and genetic improvement of C. belcheri in Thailand.

Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Day AJ, Visootiviseth P, Hawkins AJ (2000) Genetic diversity among cultured oysters (Crassostrea spp.) throughout Thailand. Sci Asia 26:115–122
Bussarawit S, Simonsen V (2006) Genetic variation in populations of white scar (Crassostrea belcheri) and black scar oysters (C. iredalei) along the coast of Thailand by means of isozymes. Phuket Mar Biol Cent Res Bull 67:11–21
Liu ZJ (2007) Aquaculture genome technologies. Blackwell, USA
Chistiakov DA, Hellemans B, Volckaert FA (2006) Microsatellites and their genomic distribution, evolution, function and applications: a review with special reference to fish genetics. Aquaculture 255:1–29
Zane L, Bargelloni L, Patarnello T (2002) Strategies for microsatellite isolation: a review. Mol Ecol 11:1–16
Primmer C, Møller A, Ellegren H (1996) A wide-range survey of cross-species microsatellite amplification in birds. Mol Ecol 5:365–378
Hamilton MB, Pincus EL, Fiore AD, Fleischer RC (1999) Universal linker and ligation procedures for construction of genomic DNA libraries enriched for microsatellites. Biotechniques 27:500–507
Berman M, Austin CM, Miller AD (2014) Characterisation of the complete mitochondrial genome and 13 microsatellite loci through next-generation sequencing for the New Caledonian spider-ant Leptomyrmex pallens. Mol Biol Rep 41:1179–1187
Li G, Hubert S, Bucklin K, Ribes V, Hedgecock D (2003) Characterization of 79 microsatellite DNA markers in the Pacific oyster Crassostrea gigas. Mol Ecol Notes 3:228–232
Wang Y, Guo X (2007) Development and characterization of EST-SSR markers in the eastern oyster Crassostrea virginica. Mar Biotechnol 9:500–511
Phuwan N, Ninwichian P, Khemklad S, Khamnamtong B (2018) Development of polymorphic microsatellites in white scar oyster Crassostrea belcheri. Chiang Mai J Sci 45:2666–2678
Ellis J, Burke J (2007) EST-SSRs as a resource for population genetic analyses. Heredity 99:125–132
Andrews S (2010) FastQC a quality control tool for high throughput sequence data. Babraham Bioinformatics, Babraham Institute, Cambridge
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Castoe TA, Poole AW, de Koning AJ, Jones KL, Tomback DF, Oyler-McCance SJ, Fike JA, Lance SL, Streicher JW, Smith EN (2012) Rapid microsatellite identification from Illumina paired-end genomic sequencing in two birds and a snake. PLoS ONE 7:e30953
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40:e115–e115
Liewlaksaneeyanawin C, Ritland CE, El-Kassaby YA, Ritland K (2004) Single-copy, species-transferable microsatellite markers developed from loblolly pine ESTs. Theor Appl Genet 109:361–369
Bassam BJ, Caetano-Anollés G, Gresshoff PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196:80–83
Yeh F, Yang R, Boyle T, Ye Z, Xiyan JM (2000) PopGene32, Microsoft Windows-based freeware for population genetic analysis. Molecular Biology and Biotechnology Centre, University of Alberta, Edmonton
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Raymond M (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rousset F (2008) genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour 8:103–106
Van Oosterhout C, Hutchinson WF, Wills DP, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Somridhivej B, Wang S, Sha Z, Liu H, Quilang J, Xu P, Li P, Hu Z, Liu Z (2008) Characterization, polymorphism assessment, and database construction for microsatellites from BAC end sequences of channel catfish (Ictalurus punctatus): a resource for integration of linkage and physical maps. Aquaculture 275:76–80
Kang J, Kim Y, Park J, Noh E, Jeong J, Lee Y, Choi T (2013) Development of microsatellite markers for a hard-shelled mussel, Mytilus coruscus, and cross-species transfer. Genet Mol Res 12:4009–4017
Rodrigues MDN, Moreira CGÁ, Gutierrez HJP, Almeida DB, Junoir D, Moreira HLM (2015) Development of microsatellite markers for use in breeding catfish, Rhamdia sp. Afr J Biotechnol 14:400–411
Liu K, Li Q, Li Q (2020) Multiplex PCR sets of novel microsatellite loci for iwagaki oyster Crassostrea nippona and their application in parentage assignment. J Ocean Univ China 19:191–198
Luo W, Nie Z, Zhan F, Wei J, Wang W, Gao Z (2012) Rapid development of microsatellite markers for the endangered fish Schizothorax biddulphi (Günther) using next generation sequencing and cross-species amplification. Int J Mol Sci 13:14946–14955
Peng J, Lapitan NL (2005) Characterization of EST-derived microsatellites in the wheat genome and development of eSSR markers. Funct Integr Genom 5:80–96
Perez F, Ortiz J, Zhinaula M, Gonzabay C, Calderon J, Volckaert FA (2005) Development of EST-SSR markers by data mining in three species of shrimp: Litopenaeus vannamei, Litopenaeus stylirostris, and Trachypenaeus birdy. Mar Biotechnol 7:554–569
Hou R, Bao Z, Wang S, Su H, Li Y, Du H, Hu J, Wang S, Hu X (2011) Transcriptome sequencing and de novo analysis for Yesso scallop (Patinopecten yessoensis) using 454 GS FLX. PLoS ONE 6:e21560
Meglécz E, Nève G, Biffin E, Gardner MG (2012) Breakdown of phylogenetic signal: a survey of microsatellite densities in 454 shotgun sequences from 154 non model eukaryote species. PLoS ONE 7:e40861
Deng Y, Lei Q, Tian Q, Xie S, Du X, Li J, Wang L, Xiong Y (2014) De novo assembly, gene annotation, and simple sequence repeat marker development using Illumina paired-end transcriptome sequences in the pearl oyster Pinctada maxima. Biosci Biotechnol Biochem 78:1685–1692
Gardner MG, Fitch AJ, Bertozzi T, Lowe AJ (2011) Rise of the machines–recommendations for ecologists when using next generation sequencing for microsatellite development. Mol Ecol Resour 11:1093–1101
Fernández-Pérez J, Nantón A, Arias-Pérez A, Insua A, Méndez J (2019) Fifteen novel microsatellite loci, developed using next-generation sequencing, reveal the lack of genetic structure in Donax vittatus from Iberian Peninsula. Estuar Coast Shelf Sci 217:218–225
Nunziata SO, Lance SL, Jones KL, Nerkowski SA, Metcalf AE (2013) Development and characterization of twenty-three microsatellite markers for the freshwater minnow Santa Ana speckled dace (Rhinichthys osculus spp., Cyprinidae) using paired-end Illumina shotgun sequencing. Conserv Genet Res 5:145–148
Norrell AE, Crawley D, Jones KL, Saillant EA (2014) Development and characterization of eighty-four microsatellite markers for the red snapper (Lutjanus campechanus) using Illumina paired-end sequencing. Aquaculture 430:128–132
Fan S, Wang J, Huang G, Liu B, Yu D (2015) Identification of twenty novel polymorphic microsatellite DNA markers from transcripts of the pearl oyster Pinctada fucata using next-generation sequencing approach. J Genet 94:82–85
Thai BT, Tan MH, Lee YP, Gan HM, Tran TT, Austin CM (2016) Characterisation of 12 microsatellite loci in the Vietnamese commercial clam Lutraria rhynchaena Jonas 1844 (Heterodonta: Bivalvia: Mactridae) through next-generation sequencing. Mol Biol Rep 43:391–396
Berg KD, Glaser CL, Thompson RE, Hamilton SR, Griffin CA, Eshleman JR (2000) Detection of microsatellite instability by fluorescence multiplex polymerase chain reaction. J Mol Diagn 2:20–28
Zhao X, Shi H, Yu H, Li Q (2015) Characterization of polymorphic microsatellite markers and genetic diversity in the Hong Kong oyster Crassostrea hongkongensis using paired-end Illumina shotgun sequencing. Genes Genom 37:615–620
McLean J, Taylor E (2001) Resolution of population structure in a species with high gene flow: microsatellite variation in the eulachon (Osmeridae: Thaleichthys pacificus). Mar Biol 139:411–420
Launey S, Ledu C, Boudry P, Bonhomme F, Naciri-Graven Y (2002) Geographic structure in the European flat oyster (Ostrea edulis L.) as revealed by microsatellite polymorphism. J Hered 93:331–351
Li Q, Liu S, Kong L (2009) Microsatellites within genes and ESTs of the Pacific oyster Crassostrea gigas and their transferability in five other Crassostrea species. Electron J Biotechnol 12:15–16
Brown BL, Franklin DE, Gaffney PM, Hong M, Dendanto D, Kornfield I (2000) Characterization of microsatellite loci in the eastern oyster, Crassostrea virginica. Mol Ecol 9:2216–2218
McGoldrick D (2000) The transmission of microsatellite alleles in Australian and North American stocks of the Pacific oyster (Crassostrea gigas): selection and null alleles. J Shellfish Res 19:779–788
Li R, Qi L, Yu R (2009) Parentage determination and effective population size estimation in mass spawning Pacific oyster, Crassostrea gigas, based on microsatellite analysis. J World Aquac Soc 40:667–677
Yu Z, Wang Y, Fu D (2010) Development of fifty-one novel EST-SSR loci in the Pacific oyster, Crassostrea gigas by data mining from the public EST database. Conserv Genet Resour 2:13–18
Fitch AJ, Leeworthy G, Li X, Bowman W, Turner L, Gardner MG (2013) Isolation and characterisation of eighteen microsatellite markers from the sea cucumber Holothuria scabra (Echinodermata: Holothuriidae). Aust J Zool 60:368–371
Li Q, Park C, Kijima A (2002) Isolation and characterization of microsatellite loci in the Pacific abalone, Haliotis discus hannai. J Shellfish Res 21:811–815
Patel A, Das P, Barat A, Meher PK, Jayasankar P (2010) Utility of cross-species amplification of 34 rohu microsatellite loci in Labeo bata, and their transferability in six other species of the cyprinidae family. Aquac Res 41:590–593
Carlsson J (2008) Effects of microsatellite null alleles on assignment testing. J Hered 99:616–623
Galindo-Sánchez CE, Gaffney PM, Pérez-Rostro CI, De la Rosa-Vélez J, Candela J, Cruz P (2008) Assessment of genetic diversity of the eastern oyster Crassostrea virginica in Veracruz, Mexico using microsatellite markers. J Shellfish Res 27:721–727
Prakoon W, Tunkijjanukij S, Nguyen TT, Na-Nakorn U (2010) Spatial and temporal genetic variation of green mussel, Perna viridis in the Gulf of Thailand and implication for aquaculture. Mar Biotechnol 12:506–515
Peyran C, Planes S, Tolou N, Iwankow G, Boissin E (2020) Development of 26 highly polymorphic microsatellite markers for the highly endangered fan mussel Pinna nobilis and cross-species amplification. Mol Biol Rep 4:2551–2559
Funding
This research was financially supported by the Prince of Songkla University, Surat Thani Campus Collaborative Research Fund contract no. 003/2562, Coordinating Center for Thai Government Science and Technology Scholarship Students (CSTS) National Science and Technology Development Agency (NSTDA) contract no. SCH-NR2016-181 and Discipline of Excellence in Sustainable Aquaculture, Prince of Songkla University.
Author information
Authors and Affiliations
Contributions
All authors have contributed to and agreed on the content of the manuscript, and the respective roles of each author are listed as follows. PN: funding acquisition, sample collection, experimental design, conducted the experiments, data analysis, drafting of the research article, and final approval of the research article. JR: funding acquisition, sample collection, and final approval of the research article. NP: sample collection, conducted the experiments, data analysis, and final approval of the research article. BK: experimental design and final approval of the research article. SK: experimental design, critical revision of the research article for important intellectual content, and final approval of the research article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All experimental procedures were performed in accordance with guidelines for the care and use of laboratory animals approved by the Institutional Animal Care and Use Committee of the Prince of Songkla University.
Consent for publication
All authors approved the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ninwichian, P., Ruangsri, J., Phuwan, N. et al. Development of polymorphic microsatellites for genetic studies of white scar oyster (Crassostrea belcheri) using paired-end shotgun sequencing. Mol Biol Rep 48, 4273–4283 (2021). https://doi.org/10.1007/s11033-021-06442-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06442-6