Abstract
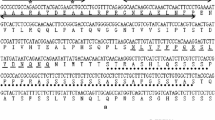
Transcription factors play vital roles in stress signal transduction and gene expression modulation during plant growth and development. Sequence analysis showed that OsAP21 contained an AP2/ERF domain of 57 amino acids. By comparison of deduced amino acid sequences of AP2/ERF-related proteins, we deduced that OsAP21 is a transcription factor gene, which belonging to rice AP2/ERF family CBF/DREB subfamily. Further, we report that transgenic Arabidopsis thaliana plants expressing the OsAP21 gene exhibited stronger growth than wild type plants under salt/drought stress. Analysis of RT-PCR for RD29B gene implied that OsAP21 over-expressed plants had a higher expression level of RD29B gene than wild type plants, and drought and salt treatments could enlarge these differences. Collectively, our results indicate that OsAP21 may play an important role in the response of transgenic Arabidopsis plants to salt/drought stresses.







Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- AP2/ERF:
-
APETALA2/Ethylene responsive factor
- GM:
-
Germination medium
- LEA:
-
Late embryogenesis-abundant
- TBARS:
-
Thiobarbituric acid reactive substances
References
Epstein E, Norlyn JD, Rush DW, Kingsbury RW, Kelley DB et al (1980) Saline culture of crops: a genetic approach. Science 210:399–404
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47:377–403
Jakab G, Ton J, Flors V, Zimmerli L, Metraux JP et al (2005) Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiol 139:267–274
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Tang W, Charles TM, Newton RJ (2005) Overexpression of the pepper transcription factor CaPF1 in transgenic Virginia pine (Pinus Virginiana Mill.) confers multiple stress tolerance and enhances organ growth. Plant Mol Biol 59:603–617
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K et al (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290:998–1009
Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQ et al (1996) AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8:155–168
Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T et al (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737–1749
Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Hu YX, Wang YX, Liu XF, Li JY (2004) Arabidopsis RAV1 is down-regulated by brassinosteroid and may act as a negative regulator during plant development. Cell Res 14:8–15
Sohn KH, Lee SC, Jung HW, Hong JK, Hwang BK (2006) Expression and functional roles of the pepper pathogen-induced transcription factor RAV1 in bacterial disease resistance, and drought and salt stress tolerance. Plant Mol Biol 61:897–915
Onate-Sanchez L, Singh KB (2002) Identification of Arabidopsis ethylene-responsive element binding factors with distinct induction kinetics after pathogen infection. Plant Physiol 128:1313–1322
Hao D, Ohme-Takagi M, Sarai A (1998) Unique mode of GCC box recognition by the DNA-binding domain of ethylene-responsive element-binding factor (ERF domain) in plant. J Biol Chem 273:26857–26861
Lee JH, Hong JP, Oh SK, Lee S, Choi D et al (2004) The ethylene-responsive factor like protein 1 (CaERFLP1) of hot pepper (Capsicum annuum L.) interacts in vitro with both GCC and DRE/CRT sequences with different binding affinities: possible biological roles of CaERFLP1 in response to pathogen infection and high salinity conditions in transgenic tobacco plants. Plant Mol Biol 55:61–81
Xu ZS, Xia LQ, Chen M, Cheng XG, Zhang RY et al (2007) Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol Biol 65:719–732
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6:251–264
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140:411–432
Fischer U, Droge-Laser W (2004) Overexpression of NtERF5, a new member of the tobacco ethylene response transcription factor family enhances resistance to tobacco mosaic virus. Mol Plant Microbe Interact 17:1162–1171
Tournier B, Sanchez-Ballesta MT, Jones B, Pesquet E, Regad F et al (2003) New members of the tomato ERF family show specific expression pattern and diverse DNA-binding capacity to the GCC box element. FEBS Lett 550:149–154
Zhang X, Henriques R, Lin SS, Niu QW, Chua NH (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1:641–646
Havaux M, Lutz C, Grimm B (2003) Chloroplast membrane photostability in chlP transgenic tobacco plants deficient in tocopherols. Plant Physiol 132:300–310
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline in water-stress studies. Plant Soil 39:205–207
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Delta Delta C(T)) Method. Methods 25:402–408
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
Wanner LA, Junttila O (1999) Cold-induced freezing tolerance in Arabidopsis. Plant Physiol 120:391–400
Kishor P, Hong Z, Miao GH, Hu C, Verma D (1995) Overexpression of [delta]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108:1387–1394
Zhang G, Chen M, Li L, Xu Z, Chen X et al (2009) Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J Exp Bot 60:3781–3796
Zhu SY, Yu XC, Wang XJ, Zhao R, Li Y et al (2007) Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19:3019–3036
Xianjun P, Xingyong M, Weihong F, Man S, Liqin C et al (2011) Improved drought and salt tolerance of Arabidopsis thaliana by transgenic expression of a novel DREB gene from Leymus chinensis. Plant Cell Rep 30:1493–1502
Acknowledgments
This research was supported by National Natural Science Foundation of China (30800602), and was sponsored by Shanghai Pujiang Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, X., Xue, Y., Wang, R. et al. Transcription factor OsAP21 gene increases salt/drought tolerance in transgenic Arabidopsis thaliana . Mol Biol Rep 40, 1743–1752 (2013). https://doi.org/10.1007/s11033-012-2228-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-2228-1