Abstract
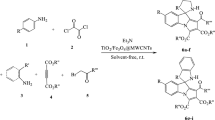
In this study, we synthesized schiff base of pyrimidoazepine derivatives in high yields using multicomponent reactions of isatins, alkyl bromides, activated acetylenic compounds, guanidine and aldehydes in the presence of Fe3O4/CuO/ZnO@ Multi Walled Carbon Nanotubes (MWCNT) as a high performance catalyst in water at room temperature. The Fe3O4/CuO/ZnO@MWCNT synthesizes using Petasites hybridus rhizome water extract as a green media and moderate base. As well Fe3O4/CuO/ZnO@MWCNT magnetic nanocomposites show a good improvement in the yield of the product and displayed significant reusable activity. Investigation of antioxidant ability of synthesized compounds using radical trapping of diphenyl-picrylhydrazine and ferric reduction power experiment is another purpose in this research. Also, the antimicrobial activity of some synthesized compounds proved by employing the disk diffusion test on Gram-positive and Gram-negative bacteria. This procedure has some benefits such as short reaction time, product with excellent yields, simple catalyst and products separation.
Graphical abstract











Similar content being viewed by others
References
Cimerman Z, Miljanic S, Galic N (2000) Schiff bases derived from aminopyridines as spectrofluorimetric analytical reagents. Croat Chem Acta 73:81–95
Singh P, Goel RL, Singh BP (1975) Synthesis, characterization and biological activity of schiff base analogues of indole-3-carboxaldehyde. J Indian Chem Soc 52:958–959
Perry BF, Beezer AE, Miles RJ, Smith BW, Miller J, Nascimento MG (1988) Synthesis and characterization of three novel Schiff base compounds: experimental and theoretical study. Microbois 45:181
Elmali A, Kabak M, Elerman Y (1999) Keto–enol tautomerism, conformations and structure of N-(2-hydroxy-5-methylphenyl), 2-hydroxybenzaldehydeimine. J Mol Struct 477:151
Patel PR, Thaker BT, Zele S (1999) Preparation and characterisation of some lanthanide complexes involving a heterocyclic β- diketone. Indian J Chem 38A:563–566
Patai S (ed) (1970) The chemistry of the carbon-nitrogen double bond. J Wiley & Sons, London
Jungreis E, Thabet S (1969) Analytical applications of schiff bases. Marcell Dekker, New York
Michael JP (2005) Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep 22:627–646
Joule JA, Mills K (2000) In heterocyclic chemistry, 4th edn. Blackwell, Cambridge, pp 194–232
Joffe AM, Farley JD, Linden D, Goldsand G (1989) Trimethoprim-sulfamethoxazole-associated aseptic meningitis: case reports and review of the literature. Am J Med 87:332–338
Petersen E, Schmidt DR (2003) Sulfadiazine and pyrimethamine in the postnatal treatment of congenital toxoplasmosis: what are the options? Expert Rev Anti-Infect Therapy 1:175–182
Nadal E, Olavarria E (2004) Imatinib mesylate (Gleevec/Glivec) a molecular-targeted therapy for chronic myeloid leukaemia and other malignancies. Int J Clin Pract 58:511–516
Blum JL (2001) The role of capecitabine, an oral, enzymatically activated fluoropyrimidine, in the treatment of metastatic breast cancer. Oncologist 6:56–64
Kçytepe S, Pasahan A, Ekinci E, SeÅkin T (2005) Synthesis, characterization and H2O2-sensing properties of pyrimidine-based hyperbranched polyimides. Eur Polym J 41:121–127
Kanbara T, Kushida T, Saito N, Kuwajima I, Kubota K, Yamamoto T (1992) Preparation and properties of highly electron-accepting poly(pyrimidine-2,5-diyl). Chem Lett 21(4):583–586
Lagoja IM (2005) Pyrimidine as constituent of natural biologically active compounds. Chem Biodiv 2:1–50
Domling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106:17
Tietze LF, Rackelmann NN (1967) Domino reactions in the synthesis of heterocyclic natural products and analogs. Pure Appl Chem 2004:11
Domling A, Ugi I (2000) Multicomponent reactions with isocyanides. Angew Chem Int Ed 39:3168
Kolb J, Beck B, Almstetter M, Heck S, Herdtweck E, Domling A (2003) New MCRs: the first 4-component reaction leading to 2,4-disubstituted thiazoles. Mol Divers 6:297
Domling A, Ugi I, Werner B (2003) The chemistry of isocyanides, their multicomponent reactions and their libraries. Molecules 8:53
Bon RS, Vliet BV, Sprenkels NE, Schmitz RF, Kanter FJJ, Stevens CV, Swart M, Bickelhaupt FM, Groen MB, Orru RV (2005) Multicomponent synthesis of 2-imidazolines. J Org Chem 70:3542
Banfi L, Basso A, Guanti G, Kielland N, Repeto C, Riva R (2007) Ugi ugi multicomponent reaction followed by an intramolecular nucleophilic substitution: convergent multicomponent synthesis of 1-Sulfonyl 1,4-Diazepan-5-ones and of Their Benzo-Fused Derivatives. J Org Chem 72:2151
Galliford CV, Scheidt KA (1811) Catalytic multicomponent reactions for the synthesis of N-Aryl Trisubstituted Pyrroles. J Org Chem 2007:72
Barbero A, Diez-Varga A, Pulido FJ, González-Ortega A (2016) Synthesis of azepane derivatives by silyl-aza-prins cyclization of allylsilyl amines: influence of the catalyst in the outcome of the reaction. Org Lett 18:1972–1975
Cini E, Bifulco G, Menchi G, Rodriquez M, Taddei M (2012) Synthesis of enantiopure 7-substituted azepane-2-carboxylic acids as templates for conformationally constrained peptidomimetics. Eur J Org Chem 2012:2133–2141
René O, Stepek IA, Gobbi A, Fauber BP, Gaines S (2015) Palladium-catalyzed ring expansion of spirocyclopropanes to form caprolactams and azepanes. J Org Chem 80:10218–10225
Drouillat B, Dorogan IV, Kletskii M, Burov ON, Couty F (2016) Competitive Ring expansion of azetidines into pyrrolidines and/or azepanes. J Org Chem 81:6677–6685
Choi J, Yadav NN, Ha HJ (2017) Preparation of a stable bicyclic aziridinium ion and its ring expansion toward piperidines and azepanes. Asian J Org Chem 6:1292–1307
Yarmoliuk DV, Serhiichuk D, Smyrnov V, Tymtsunik AV, Hryshchuk OV, Kuchkovska Y, Grygorenko OO (2018) Synthesis of azabicyclo[n.1.0]alkane-derived bifunctional building blocks via the Corey-Chaykovsky cyclopropanation. Tetrahedron Lett 59:4611–4615
Zhou J, Yeung YY (2014) N-Bromosuccinimide-induced Aminocyclization-Aziridine ring-expansion cascade: an asymmetric and highly stereoselective approach toward the synthesis of azepane. Org Lett 16:2134–2137
Sahay R, Sundaramurthy J, Suresh Kumar P, Thavasi V, Mhaisalkar SG, Ramakrishna S (2012) Synthesis and characterization of CuO nanofibers, and investigation for its suitability as blocking layer in ZnO NPs based dye sensitized solar cell and as photocatalyst in organic dye degradation. J Solid State Chem 186:261–267
Djurišić AB, Chen X, Leung YH, Man A (2012) ZnO nanostructures: growth, properties and applications. J Mater Chem 22:6526–6535
Riente P, Mendoza C, Pericás MA (2011) Functionalization of Fe3O4magnetic nanoparticles for organocatalytic Michael reactions. J Mater Chem 21:7350–7355
Zhang B-T, Zheng X, Li H-F, Lin JM (2013) Application of carbon-based nanomaterials in sample preparation: a review. Anal Chim Acta 784:1–17
Xin T, Ma M, Zhang H, Gu J, Wang S, Liu M, Zhang Q (2014) A facile approach for the synthesis of magnetic separable Fe3O4@TiO2, core–shell nanocomposites as highly recyclable photocatalysts. Appl Surf Sci 288:51–59
Thombal RS, Jadhav VH (2016) Facile O–glycosylation of glycals using Glu-Fe3O4-SO3H, a magnetic solid acid catalyst. RSC Adv 6:30846–30851
Thombal PR, Thombal RS, Han SS (2020) Chitosan-derived N-doped carbon catalysts with a metallic core for the oxidative dehydrogenation of NH–NH bonds. RSC Adv 10:474–481
Thombal PR, Han SS (2018) Novel synthesis of Lewis and Bronsted acid sites incorporated CS-Fe3O4@SO3H catalyst and its application in one-pot synthesis of tri(furyl)methane under aqueous media. Biofuel Res J 20:886–893
Mandel K, Hutter F, Gellermann C, Sextl G (2013) Reusable superparamagnetic nanocomposite particles for magnetic separation of iron hydroxide precipitates to remove and recover heavy metal ions from aqueous solutions. Sep Purif Technol 109:144–147
Wachs IE (2005) Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials. Catal Today 100:79–94
Guo Z, Liu B, Zhang Q, Deng W, Wang Y, Yang Y (2014) Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem Soc Rev 43:3480–3524
Dastan A, Kulkarnia A, Torok B (2012) Environmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approaches. Green Chem 14:17–37
Jabłonska M, Palkovits R (2016) Nitrogen oxide removal over hydrotalcite-derived mixed metal oxides. Catal Sci Technol 6:49–72
Shi J (2013) On the synergetic catalytic effect in heterogeneous nanocomposite catalysts. Chem Rev 113:2139–2181
Lin-Bing S, Xiao-Qin L, Hong-Cai Z (2015) Design and fabrication of mesoporous heterogeneous basic catalysts. Chem Soc Rev 44:5092–5147
Zhang Q, Vigier KDV, Royer S, Jerome F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41:7108–7146
Climent MJ, Corma A, Borra SI (2012) Homogeneous and heterogeneous catalysts for multicomponent reactions. RSC Adv 2:16–58
Wachs IE, Routray K (2012) Catalysis science of bulk mixed oxides. ACS Catal 2:1235–1246
Halliwell B (1999) Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radical Res 31:261–272
Ahmadi F, Kadivar M, Shahedi M (2007) Antioxidant activity of Kelussia odoratissima Mozaff. in model and food systems. Food Chem 105:57–64
Bidchol AM (2011) Free radical scavenging activity of aqueous and ethanolic. Food Bioprocess Tech 4:1137
Liu L, Meydani M (2002) “Combined vitamin C and E supplementation retards early progression of arteriosclerosis in heart transplant patients. Nutr Rev 60:368–371
Ezzatzadeh E, Hossaini ZS (2018) Green synthesis and antioxidant activity of novel series of benzofurans from euparin extracted of Petasites hybridus. Nat Prod Res 33(11):1–7
Ezzatzadeh E, Hossaini ZS (2018) A novel one-pot three-component synthesis of benzofuran derivatives via Strecker reaction: study of antioxidant activity. Nat Prod Res 34(28):1–7
Ezzatzadeh E, Hossaini ZS (2020) Four-component green synthesis of benzochromene derivatives using nano-KF/clinoptilolite as basic catalyst: study of antioxidant activity. Mol Divers 24:81–91
Yavari I, Sabbaghan M, Hossaini ZS (2008) Efficient synthesis of functionalized 2,5-dihydrofurans and 1,5-dihydro-2 H -pyrrol-2-ones by reaction of isocyanides with activated acetylenes in the presence of hexachloroacetone. Chem Mon 139:625–628
Yavari I, Sabbaghan M, Hossaini ZS (2008) Proline-promoted efficient synthesis of 4-Aryl-3,4-dihydro-2H,5H-pyrano[3,2-c]chromene-2,5-diones in aqueous media. Synlett 2008:1153–1154
Yavari I, Hossaini ZS, Sabbaghan M, Ghazanfarpour-Darjani M (2007) Efficient synthesis of functionalized spiro-2,5-dihydro-1,2-λ5-oxaphospholes. Tetrahedron 63:9423–9428
Yavari I, Sabbaghan M, Hossaini ZS, Ghazanfarpour-Darjani M (2008) Surprising formation of chlorinated butenolides from dialkyl acetylenedicarboxylates and hexachloroacetone in the presence of triphenyl phosphate. Helv Chim Acta 91:1144–1147
Rajabi M, Hossaini ZS, Khalilzadeh MA, Datta Sh, Halder M, Mousa ShA (2015) Synthesis of a new class of furo[3,2-c]coumarins and its anticancer activity. J Photochem Photobiol, B 148:66–72
Hossaini ZS, Zareyee D, Sheikholeslami-Farahani F, Vaseghi S, Zamani A (2017) ZnO–NR as the efficient catalyst for the synthesis of new thiazole and cyclopentadienone phosphonate derivatives in water. Heteroat Chemi 28:e21362
Rostami-charati F, Hossaini ZS, Zareyee D, Afrashteh S, Hosseinzadeh M (2017) ZnO-Nanorods as an efficient catalyst for the synthesis of 1,3-thiazolidine derivatives by aqueous multicomponent reactions of isothiocyanates. J Heterocycl Chem 54:1937–1942
Rostami-Charati F, Hossaini ZS, Khalilzadeh MA, Jafaryan H (2012) Solvent-free synthesis of pyrrole derivatives. J Heterocycl Chem 49:217–220
Rostami-Charati F, Hossaini ZS (2012) Facile synthesis of phosphonates via catalyst-free multicomponent reactions in Water. Synlett 23:2397–2399
Ezzatzadeh E (2018) Green synthesis of α-aminophosphonates using ZnO nanoparticles as an efficient catalyst. Zeitschrift für Naturforschung B 73:179–184
Rostami-Charati F, Hossaini ZS, Rostamian R, Zamani A, Abdoli M (2017) Green synthesis of indol-2-one derivatives from N-alkylisatins in the presence of KF/clinoptilolite nanoparticles. Chem Heterocycl Compd 53:480–483
Yavari I, Seyfi S, Hossaini ZS, Sabbaghan M, Shirgahi-Talari F (2008) Efficient synthesis of 2-thioxo-1,3-thiazolanes from primary amines, CS2, and ethyl bromopyruvate. Monatshefte für Chem-Chem Mon 139:1479–1482
Rezayati S, Sheikholeslami-Farahani F, Hossaini ZS, Hajinasiri R, Afshari Sharif Abad S (2016) Regioselctive thiocyanation of aromatic and heteroaromatic compounds using a novel bronsted acidic ionic liquid. Comb Chem High Throughput Screen 9:720–727
Tavakolinia F, Baghipour T, Hossaini ZS, Zareyee D, Khalilzadeh MA (2012) Free antiproliferative activity of novel thiopyran analogs on MCF-7 breast and HCT-15 colon cancer cells: synthesis, cytotoxicity, cell cycle analysis, and DNA-binding. Nucleic Acid Ther 22:265–270
Rostami-Charati F, Hossaini ZS, Sheikholeslami-Farahani F, Azizi Z, Siadati SA (2015) Synthesis of 9H-furo [2, 3-f] chromene derivatives by promoting ZnO nanoparticles. Comb Chem High Troughput Screen 18:872–880
SajjadiGhotbabadi H, Javanshir Sh, Rostami-Charati F (2016) Nano KF/Clinoptilolite: an effective heterogeneous base nanocatalyst for synthesis of substituted quinolines in water. Catal Lett 146:338–344
Yavari I, Ghazanfarpour-Darjani M, Hossaini ZS, Sabbaghan M, Hosseini N (2008) Methoxide ion promoted efficient synthesis of 1,3-Oxathiolane-2-thiones by reaction of oxiranes and carbon disulfide. Synlett 06:889–891
Yavari I, Hossaini ZS, Souri S, Seyfi S (2009) Diastereoselective synthesis of fused [1,3]thiazolo[1,3]oxazins and [1,3]oxazino[2,3-b][1,3]benzothiazoles. Mol Divers 13:439–443
Hajinasiri R, Hossaini ZS, Rostami-Charati F (2011) Efficient synthesis of α-aminophosphonatesvia one-pot reactions of aldehydes, amines, and phosphates in ionic liquid. Heteroat Chem 22:625–629
Rostami Charati F, Hossaini ZS, Hosseini-Tabatabaei MR (2011) A simple synthesis of oxaphospholes. Phosphorus Sulfur Silicon Relat Elem 186:1443–1448
Yavari I, Nematpour M, Hossaini ZS (2010) Ph3P-mediated one-pot synthesis of functionalized 3, 4-dihydro-2 H-1, 3-thiazines from N, N′-dialkylthioureas and activated acetylenes in water. Monatshefte für Chemie-Chem Mon 141:229–232
Rezayati S, Hajinasiri R, Hossaini ZS, Abbaspour S (2018) Chemoselective synthesis of 1,1-diacetates (acylals) using 1,1’-butylenebispyridinium hydrogen sulfate as a new, halogen-free and environmental-friendly catalyst under solvent-free conditions. Asian J Green Chem 2:268–280
Hossaini ZS, Sheikholeslami-Farahani F, Rostami-Charati F (2015) Green synthesis of phosphoryl-2-Oxo-2H-Pyran via three component reaction of Trialkyl Phosphites. Comb Chem High Throughput Screen 17:804–8078
Sheikholeslami-Farahani F, Hossaini ZS, Rostami-Charati F (2014) Solvent-free synthesis of substituted thiopyrans via multicomponent reactions of α-haloketones. Chin Chem Lett 25:152–154
Ghazvini M, Sheikholeslami-Farahani F, Soleimani-Amiri S, Salimifard M (2018) Green synthesis of Pyrido [2, 1-a] isoquinolines and Pyrido [1, 2-a] quinolines by Using ZnO nanoparticles. Synlett 29:493–496
Hossaini ZS, Rostami-Charati F, Sheikholeslami-Farahani F, Ghasemian M (2015) Synthesis of functionalized benzene using Diels-Alder reaction of activated acetylenes with synthesized phosphoryl-2-oxo-2H-pyran. Zeitschrift für Naturforschung B 70:355–360
Rostami-Charati F, Hossaini ZS, Rostamian R, Ghambarian M, Zamani A (2017) Green synthesis of indol-2-one derivatives from N-alkylisatins in the presence of KF/clinoptilolite nanoparticles. Chem Heterocycl Compd 53:480–483
Soleimani-Amiri S, Arabkhazaeli M, Hossaini Z (2018) Synthesis of chromene derivatives via three-component reaction of 4-hydroxycumarin catalyzed by magnetic Fe3O4 nanoparticles in water. J Heterocycl Chem 55:209
Koohi M, Amiri SS, Shariati M (2017) Silicon impacts on structure, stability and aromaticity of C20-nSin heterofullerenes (n= 1–10): a density functional perspective. J Mol Struct 1127:522–531
Kassaee MZ, Aref Rad H, Soleimani Amiri S (2010) Carbon–nitrogen nanorings and nanoribbons: a theoretical approach for altering the ground states of cyclacenes and polyacenes. Monatshefte fuer Chemie/Chem Mon 141(12):1313–1319
Oskooie HA, Solemani Amiri S, Heravi MM, Ghassemzadeh M (2005) Reductive amination of aldehydes and ketones with sodium borohydride supported Onto HZSM-5 zeolite under microwave irradiation in a solvent free system. Phosphorus Sulfur Silicon Relat Elem Sulf 9:2047–2050
Ghavidel H, Mirza B, Soleimani-Amiri S (2021) A novel, efficient, and recoverable basic Fe3O4@C nano-catalyst for green synthesis of 4H-chromenes in water via one-pot three component reactions. Polycycl Aromat Compd 41(3):604–625
Chen M-N, Mo L-P, Cui Z-S, Zhang ZH (2019) Current opinion in green and sustainable. Chemistry 15:27–37
Zhang Mo, Liua Y-H, Shang Z-R, Hai-Chuan Hu, Zhang Z-H (2017) Supported molybdenum on graphene oxide/Fe3O4: an efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal Commun 88:39–44
Yildirim A, Mavi A, Kara AA (2001) Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem 49(8):4083–4089
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948
Yen GC, Duh PD (1994) Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J Agric Food Chem 42:629–632
Acknowledgements
We thank from Islamic Azad University of Gorgan for their spiritual support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This manuscript has not any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Experimental part
General
All materials employed in this work were purchased from Fluka and Merck with no further purification. The structure of Fe3O4/CuO/ZnO@MWCNT MNCs was confirmed by XRD, SEM, EDX and. X-ray diffraction patterns (XRD) was performed for calculating of the size of prepared Fe3O4/CuO/ZnO@MWCNT MNCs. The Scherrer’s formula; D = 0.9λ/β cosθ used for calculating the average crystallite size of Fe3O4/CuO/ZnO@MWCNT MNCs, where D is the diameter of the nanoparticles, λ (CuKα) = 1.5406 Å and β is the full-width at half-maximum of the diffraction lines. FT-IR spectra were recorded by a Shimadzu IR-460 spectrometer for synthesized compounds. Also, the 1H and 13C NMR spectra are used for the structure confirmation of synthesized compounds by BRUKER DRX-500 AVANCE spectrometer at 500.1 and 125.8 MHz respectively using TMS as the internal standard or 85% H3PO4 as the external standard for solution in CDCl3. A FINNIGAN-MAT 8430 spectrometer with an ionization potential of 70 eV utilized for Mass spectra. The elemental analysis C, H, and N for synthesized compounds was carried out by a Heraeus CHN–O-Rapid analyzer. Energy-dispersive X-ray Spectroscopy (EDX) was performed by Mira 3-XMU FESEM (Tescan Co, Brno, Czech Republic).
Green synthesis of Fe 3 O 4 /CuO/ZnO@MWCNT
Synthesis of Fe3O4/CuO/ZnO@MWCNT MNCs was performed by adding CuCl2 (1 g), Zn(OAC)2 (1 g) and FeCl2.4H2O ( 1.5 g) to 50 mL of Petasites hybridus rhizome water extract to obtain mixed Cu(OH)2, Zn(OH)2 and Fe (OH)2 colloids at room temperature. The colloid was separated utilizing centrifuge, dried and calcined at 350 °C for 2 h for preparation of a gray brown solid ZnO/CuO/Fe3O4 magnetic nanocomposite. Then, prepared Fe3O4/CuO/ZnO MNCs and 0.1 g multi walled carbon nanotubes (MWCNTs) was dispersed in 100 mL Petasites hybridus rhizome water extract at 100 °C. The mixture was sonicated for 30 min and the colloid was separated using centrifuge, dried and calcined at 500 °C for 45 min. After reaching the temperature to r.t., the solid was cleaned several times using water and ethanol. The Fe3O4/CuO/ZnO@MWCNT MNCs magnetic nanoparticles separated with external magnetic field.
General procedure for preparation of compounds 6a–k
The mixture of isatins 1 (2 mmol), electron deficient acetylenic compounds 2 (2 mmol) and bio- Fe3O4/CuO/ZnO@MWCNT MNCs (0.02 g) and Et3N was stirred in water (3 mL) at room temperature for 30 min. After this time \(\alpha\) haloketones 3 (2 mmol) was added to the mixture and new mixture stirred for 15 min. After this time guanidine 4 (2 mmol) was added to previous mixture. Finally, aldehydes were added to latest mixture and new mixture was stirred for 4 h in water at room temperature. The reaction is completed after 2 h and development of the reaction is proved by TLC. The catalyst was separated by external magnet and the solid residue was collected by filtration and washed with EtOH and Et2O to afforded purified compounds 6.
Dimethyl 2-((4-methoxybenzylidene)amino)-4-(4-methoxyphenyl)-7H-benzo[b]pyrimido[4,5-d]azepine-5,6-dicarboxylate (6a): Yellow powder, mp 157–159˚C, Yield: 0.81 g (95%). IR (KBr) (νmax/cm−1): 3193, 1703, 1672, 1531, 1380, 1261 cm−1. 1H NMR (500 MHz, CDCl3): 3.76 (3 H, s, MeO), 3.85 (3 H, s, MeO), 3.89 (3 H, s, MeO), 3.96 (3 H, S, MeO), 7.16 (2 H, d, 3 J = 7.7 Hz, 2 CH), 7.34 (2 H, d, 3 J = 7.7 Hz, 2 CH), 7.49 (1 H, t, 3 J = 7.7 Hz, CH), 7.58 (1 H, t, 3 J = 7.7 Hz, CH), 7.67 (2 H, d, 3 J = 7.7 Hz, 2 CH), 7.86 (2 H, d, 3 J = 7.7 Hz, 2 CH), 7.96 (1 H, d, 3 J = 7.8 Hz, CH), 8.06 (1 H, d, 3 J = 7.8 Hz, CH), 8.39 (1 H, s, CH), 10.25 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): 51.6 (MeO), 52.5 (MeO), 55.6 (MeO), 56.0 (MeO), 110.5 (CH), 113.4 (C), 114.3 (C), 114.9 (2 CH), 115.8 (2 CH), 116.7 (CH), 121.2 (CH), 127.2 (C), 128.6 (CH), 129.3 (C), 132.5 (2 CH), 134.2 (2 CH), 140.2 (C), 142.5 (C), 143.8 (C), 148.3 (C), 149.6 (C), 151.3 (C), 153.6 (C), 158.3 (CH), 159.2 (C), 161.7 (C = O), 162.6 (C = O) ppm. EI-MS: 550 (M+, 15), 396 (84), 31(100). Anal. Calcd for C31H26N4O6 (550.56): C 67.63, H 4.76, N 10.18; Found: C 67.78, H 4.85, N 10.26.
Diethyl 2-((4-methoxybenzylidene)amino)-4-(4-methoxyphenyl)-7H-benzo[b]pyrimido[4,5-d]azepine-5,6-dicarboxylate (6b): Yellow powder, mp 162–164˚C, Yield: 0.84 g (93%). IR (KBr) (νmax/cm−1): 3174, 1708, 1673, 1434, 1381, 1272 cm−1. 1H NMR (500 MHz, CDCl3): 1.14 (3 H, t, 3 J = 7.4 Hz, CH3), 1.28 (3 H, t, 3 J = 7.4 Hz, CH3), 3.85 (3 H, s, MeO), 3.92 (3 H, s, MeO), 4.18 (2 H, q, 3 J = 7.4 Hz, CH2O), 4.26 (2 H, q, 3 J = 7.4 Hz, CH2O), 7.18 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.32 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.47 (1 H, t, 3 J = 7.6 Hz, CH), 7.53 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.62 (1 H, t, 3 J = 7.6 Hz, CH), 7.69 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.77 (1 H, d, 3 J = 7.6 Hz, CH), 7.98 (1 H, d, 3 J = 7.6 Hz, CH), 8.56 (1 H, s, CH), 10.27 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): 13.7 (Me), 14.2 (Me), 55.6 (MeO), 56.2 (MeO), 61.5 (CH2O), 62.7 (CH2O), 110.8 (CH), 113.6 (C), 114.5 (C), 115.2 (2 CH), 116.2 (2 CH), 117.3 (CH), 121.5 (CH), 127.6 (C), 128.5 (C), 129.3 (CH), 132.3 (2 CH), 134.5 (2 CH), 140.7 (C), 143.4 (C), 144.2 (C), 149.5 (C), 150.7 (C), 151.4 (C), 153.5 (C), 157.3 (CH), 158.6 (C), 161.7 (C = O), 163.4 (C = O) ppm. EI-MS: 578 (M+, 15), 412 (68), 45(100). Anal. Calcd for C33H30N4O6 (578.62): C 68.50, H 5.23, N 9.68; Found: C 68.62, H 5.38, N 9.83.
Methyl 2-((4-cyanobenzylidene)amino)-4-(p-tolyl)-7H-benzo[b]pyrimido[4,5-d]azepine-5-carboxylate (6c): Yellow powder, mp 128–130˚C, Yield: 0.65 g (92%). IR (KBr) (νmax/cm−1): 3182, 2198, 1710, 1675, 1492, 1378, 1257 cm−1. 1H NMR (500 MHz, CDCl3): 2.32 (3 H, s, Me), 3.83 (3 H, s, MeO), 7.08 (2 H, d, 3 J = 7.8 Hz, CH), 7.28 (1 H, t, 3 J = 7.6 Hz, CH), 7.38 (1 H, t, 3 J = 7.6 Hz, CH), 7.46 (2 H, d, 3 J = 7.8 Hz, CH), 7.55 (2 H, d, 3 J = 7.8 Hz, CH), 7.67 (1 H, d, 3 J = 7.6 Hz, CH), 7.73 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.93 (1 H, d, 3 J = 7.6 Hz, CH), 8.46 (1 H, s, CH), 8.68 (1 H, s, CH), 10.34 (1 H, s, NH), ppm. 13C NMR (125.7 MHz, CDCl3): 21.3 (Me), 51.7 (MeO), 108.3 (C), 110.5 (CH), 115.3 (C), 116.2 (CN), 117.4 (CH), 118.2 (C), 121.3 (CH), 127.3 (C), 128.2 (2 CH), 129.5 (CH), 130.4 (2 CH), 131.3 (2 CH), 133.4 (2 CH), 138.2 (C), 139.2 (C), 142.3 (C), 144.7 (C), 145.6 (CH), 148.6 (C), 149.6 (C), 151.6 (C), 158.4 (C), 161.7 (C = O) ppm. EI-MS: 471 (M+, 10), 440 (86), 31(100). Anal. Calcd for C29H21N5O2 (471.51): C 73.87, H 4.49, N 14.85; Found: C 73.96, H 4.63, N 14.96.
Methyl 2-((4-cyanobenzylidene)amino)-4-(4-methoxyphenyl)-10-methyl-7H-benzo[b]pyrimido [4,5-d]azepine-5-carboxylate (6d): Yellow powder, mp 133–135 °C, Yield: 0.73 g (95%). IR (KBr) (νmax/cm−1): 3195, 2195, 1718, 1676, 1493, 1374, 1272 cm−1. 1H NMR (500 MHz, CDCl3): 2.25 (3 H, s, Me), 3.78 (3 H, s, MeO), 3.83 (3 H, s, MeO), 7.25 (2 H, d, 3 J = 7.7 Hz, 2 CH), 7.34 (1 H, d, 3 J = 7.5 Hz, CH), 7.45 (1 H, d, 3 J = 7.5 Hz, CH), 7.58 (2 H, d, 3 J = 7.7 Hz, 2 CH), 7.69 (2 H, d, 3 J = 7.7 Hz, 2 CH), 7.79 (1 H, s, CH), 7.86 (2 H, d, 3 J = 7.7 Hz, 2 CH), 8.53 (1 H, s, CH), 8.62 (1 H, s, CH), 10.42 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): 21.2 (Me), 51.5 (MeO), 55.8 (MeO), 108.2 (C), 114.3 (C), 115.3 (2 CH), 115.9 (CN), 116.5 (CH), 118.6 (C), 125.4 (CH), 130.3 (C), 131.6 (2 CH), 132.3 (2 CH), 133.4 (CH), 134.2 (2 CH), 137.6 (C), 138.2 (C), 139.8 (C), 145.3 (CH), 146.2 (C), 148.3 (C), 149.5 (C), 151.3 (C), 152.6 (C), 157.6 (CH), 161.6 (C = O) ppm. EI-MS: 501 (M+, 15), 354 (68), 31(100). Anal. Calcd for C30H23N5O3 (501.54): C 71.84, H 4.62, N 13.96; Found: C 71.93, H 4.78, N 14.12.
Dimethyl 2-((4-chlorobenzylidene)amino)-4-(4-methoxyphenyl)-10-methyl-7H-benzo[b] pyrimido[4,5-d]azepine-5,6-dicarboxylate (6e): Yellow powder, mp 161–163 °C, Yield: 0.84 g (95%). IR (KBr) (νmax/cm−1): 3371, 1718, 1673, 1374, 1210 cm−1. 1H NMR (500 MHz, CDCl3): 2.27 (3 H, s, Me), 3.76 (3 H, s, MeO), 3.85 (3 H, s, MeO), 3.98 (3 H, s, MeO), 7.25 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.38 (1 H, d, 3 J = 7.5 Hz, CH), 7.49 (1 H, d, 3 J = 7.5 Hz, CH), 7.56 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.68 (2 H, d, 3 J = 7.6 Hz, 2 CH), 7.75 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.83 (1 H, s, CH), 8.46 (1 H, s, CH), 10.36 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): 21.5 (Me), 51.3 (MeO), 52.5 (MeO), 55.7 (MeO), 112.3 (C), 114.5 (C), 115.4 (2 CH), 116.7 (CH), 125.5 (CH), 128.6 (2 CH), 130.5 (C), 131.2 (2 CH), 132.7 (CH), 133.6 (C), 134.6 (2 CH), 136.2 (C), 138.3 (C), 139.5 (C), 143.6 (C), 144.8 (C), 148.5 (C), 149.7 (C), 151.6 (C), 152.7 (C), 158.3 (CH), 161.3 (C = O), 162.6 (C = O) ppm. EI-MS: 569 (M+, 10), 410 (64), 31(100). Anal. Calcd for C31H25N4ClO5 (569.00): C 65.44, H 4.43, N 9.85; Found: C 65.63, H 4.62, N 9.98.
Dimethyl -2-((4-cyanobenzylidene)amino)-4-(p-tolyl)-7H-benzo[b]pyrimido[4,5-d]azepine-5,6-dicarboxylate (6f): Yellow powder, mp 141–143 °C, Yield: 0.74 g (90%). IR (KBr) (νmax/cm−1): 3322, 2202, 1703, 1672, 1531, 1380, 1261 cm−1. 1H NMR (500 MHz, CDCl3): 2.25 (3 H, s, Me), 3.75 (3 H, s, MeO), 3.87 (3 H, s, MeO), 7.32 (1 H, t, 3 J = 7.6 Hz, CH), 7.43 (1 H, t, 3 J = 7.6 Hz, CH), 7.54 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.65 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.73 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.83 (1 H, d, 3 J = 7.6 Hz, CH), 7.92 (2 H, d, 3 J = 7.8 Hz, 2 CH), 8.02 (1 H, d, 3 J = 7.8 Hz, CH), 8.53 (1 H, s, CH), 10.52 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): 20.5 (Me), 51.3 (MeO), 52.6 (MeO), 111.3 (CH), 113.2 (C), 114.5 (C), 116.3 (CH), 118.3 (CN), 121.4 (CH), 127.3 (2 CH), 128.2 (C), 129.4 (CH), 131.2 (2 CH), 132.4 (2 CH), 133.5 (2 CH), 137.4 (C), 138.2 (C), 142.3 (C), 143.8 (C), 144.5 (C), 149.2 (C), 150.2 (C), 151.3 (C), 158.3 (CH), 161.5 (C = O), 162.7 (C = O) ppm. EI-MS: 529 (M+, 10), 380 (48), 31(100). Anal. Calcd for C31H23N5O4 (529.55): C 70.31, H 4.38, N 13.23; Found: C 70.52, H 4.54, N 13.38.
Dimethyl 10-nitro-2-((4-nitrobenzylidene)amino)-4-(p-tolyl)-7H-benzo[b]pyrimido[4,5-d] azepine-5,6-dicarboxylate (6 g): Yellow powder, mp 171–173 °C, Yield: 0.79 g (87%). IR (KBr) (νmax/cm−1): 3189, 1711, 1677, 1488, 1377, 1211 cm−1. 1H NMR (500 MHz, CDCl3): 2.32 (3 H, s, Me), 3.78 (3 H, s, MeO), 3.85 (3 H, s, MeO), 7.56 (2H, d, 3 J = 7.8 Hz, 2 CH), 7.63 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.78 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.86 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.97 (1 H, d, 3 J = 7.6 Hz, CH), 8.07 (1 H, d, 3 J = 7.6 Hz, CH), 8.36 (1 H, s, CH), 8.68 (1 H, s, CH), 10.32 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): 21.6 (Me), 51.7 (MeO), 52.4 (MeO), 113.5 (CH), 114.3 (C), 115.6 (C), 124.8 (2 CH), 125.2 (CH), 126.7 (CH), 127.3 (2 CH), 128.3 (C), 131.5 (2 CH), 132.2 (2 CH), 137.6 (C), 138.5 (C), 140.2 (C), 141.4 (C), 143.6 (C), 144.8 (C), 148.3 (C), 153.2 (C), 154.8 (C), 158.4 (C), 159.3 (CH), 160.5 (C = O), 162.8 (C = O) ppm. EI-MS: 594 (M+, 15), 563 (56), 31(100). Anal. Calcd for C30H22N6O8 (594.53): C 60.61, H 3.73, N 14.14; Found: C 60.72, H 3.87, N 14.28.
Dimethyl 10-methyl-2-((4-nitrobenzylidene)amino)-4-(4-nitrophenyl)-7H-benzo[b]pyrimido [4,5-d]azepine-5,6-dicarboxylate (6 h): Yellow powder, mp 169–171 °C, Yield: 0.79 g (87%). IR (KBr) (νmax/cm−1): 3321, 1712, 1674, 1488, 1384, 1273 cm−1. 1H NMR (500 MHz, CDCl3): 2.32 (3 H, s, Me), 3.75 (3 H, s, MeO), 3.85 (3 H, s, MeO), 7.26 (1 H, d, 3 J = 7.6 Hz, CH), 7.38 (1 H, d, 3 J = 7.6 Hz, CH), 7.68 (1 H, s, CH), 7.75 (2 H, d, 3 J = 7.8 Hz, 2 CH), 8.05 (2 H, d, 3 J = 7.8 Hz, 2 CH), 8.23 (2 H, d, 3 J = 7.8 Hz, 2 CH), 8.43 (2 H, d, 3 J = 7.8 Hz, 2 CH), 8.74 (1 H, s, CH), 10.43 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): 20.8 (Me), 51.3 (MeO), 52.7 (MeO), 109.2 (C), 114.5 (C), 117.2 (CH), 124.6 (2 CH), 125.4 (CH), 126.8 (2 CH), 130.6 (C), 131.8 (2 CH), 132.4 (CH), 137.6 (C), 138.2 (2 CH), 139.2 (C), 142.3 (C), 144.2 (C), 145.3 (C), 147.3 (C), 148.6 (C), 154.2 (C), 156.4 (C), 158.4 (C), 159.4 (CH), 162.5 (C = O), 163.8 (C = O) ppm. EI-MS: 594 (M+, 15), 563 (64), 31(100). Anal. Calcd for C30H22N6O8 (594.53): C 60.61, H 3.73, N 14.14; Found: C 60.76, H 3.92, N 14.32.
Ethyl 10-chloro-2-((4-chlorobenzylidene)amino)-4-(p-tolyl)-7H-benzo[b]pyrimido[4,5-d]azepine -5-carboxylate (6i): Yellow powder, mp 153–155 °C, Yield: 0.64 g (85%). IR (KBr) (νmax/cm−1): 3278, 1708, 1673, 1434, 1381, 1272 cm−1. 1H NMR (500 MHz, CDCl3): 1.25 (3 H, t, 3 J = 7.4 Hz, CH3), 2.34 (3 H, s, Me), 4.23 (2 H, q, 3 J = 7.4 Hz, CH2O), 7.24 (2 H, d, 3 J = 7.8 Hz, 2 CH),7.32 (1 H, d, 3 J = 7.7 Hz, CH), 7.48 (1 H, d, 3 J = 7.7 Hz, CH), 7.56 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.65 (2 H, d, 3 J = 7.7 Hz, 2 CH), 7.72 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.83 (1 H, s, CH), 7.92 (1 H, s, CH), 8.38 (1 H, s, CH), 10.52 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): 13.8 (Me), 23.4 (Me), 62.5 (CH2O), 108.5 (C), 115.2 (C), 117.6 (CH), 124.2 (CH), 125.3 (C), 126.0 (C), 126.8 (2 CH), 127.8 (2 CH), 128.6 (CH), 129.6 (2 CH), 130.8 (2 CH), 133.2 (C), 136.4 (C), 138.3 (C), 142.5 (C), 144.5 (C), 145.7 (CH), 149.2 (C), 150.4 (C), 151.3 (C), 158.2 (CH), 162.3 (C = O) ppm. EI-MS: 529 (M+, 15), 356 (58), 45(100). Anal. Calcd for C29H22Cl2N4O2 (529.42): C 65.79, H 4.19, N 10.58; Found: C 65.93, H 4.32, N 10.72.
4-Ethyl-5,6-dimethyl 2-((4-cyanobenzylidene)amino)-10-methyl-7H-benzo[b]pyrimido[4,5-d] azepine-4,5,6-tricarboxylate (6j): Pale yellow powder, mp 117–119 °C, Yield: 0.74 g (97%). IR (KBr) (νmax/cm−1): 3174, 2198, 1708, 1673, 1434, 1381, 1272 cm−1. 1H NMR (500 MHz, CDCl3): 1.28 (3 H, t, 3 J = 7.4 Hz, CH3), 2.25 (3 H, s, Me), 3.78 (3 H, s, MeO), 3.85 (3 H, s, MeO), 4.18 (2 H, q, 3 J = 7.4 Hz, CH2O), 7.34 (1 H, d, 3 J = 7.7 Hz, CH), 7.46 (1 H, d, 3 J = 7.7 Hz, CH), 7.63 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.75 (2 H, d, 3 J = 7.8 Hz, 2 CH), 7.83 (1 H, s, CH), 8.64 (1 H, s, CH), 10.64 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): 14.2 (Me), 21.7 (Me), 51.6 (MeO), 52.5 (MeO), 62.3 (CH2O), 113.4 (C), 114.8 (C), 115.6 (CH), 116.3 (C), 118.6 (CN), 125.3 (CH), 131.6 (2 CH), 132.6 (2 CH), 133.2 (CH), 134.2 (C), 135.6 (C), 136.8 (C), 142.3 (C), 144.3 (C), 148.2 (C), 156.3 (C), 157.2 (C), 158.6 (CH), 160.4 (C = O), 161.5 (C = O), 162.8 (C = O) ppm. EI-MS: 525 (M+, 15), 376 (86), 31(100). Anal. Calcd for C28H23N5O6 (525.51): C 63.99, H 4.41, N 13.33; Found: C 64.12, H 4.58, N 13.52.
4-Ethyl 5-methyl 2-((4-nitrobenzylidene)amino)-7H-benzo[b]pyrimido[4,5-d]azepine-4,5-dicarboxylate (6 k): Yellow powder, mp 128–132 °C, Yield: 0.57 g (85%). IR (KBr) (νmax/cm−1): 3182, 1710, 1675, 1492, 1378, 1257 cm−1. 1H NMR (500 MHz, CDCl3): 1.32 (3 H, t, 3 J = 7.4 Hz, CH3), 3.85 (3 H, s, MeO), 4.26 (2 H, q, 3 J = 7.4 Hz, CH2O), 7.34 (1 H, t, 3 J = 7.6 Hz, CH), 7.42 (1 H, t, 3 J = 7.6 Hz, CH), 7.58 (1 H, d, 3 J = 7.6 Hz, CH), 7.72 (1 H, s, CH),7.86 (1 H, d, 3 J = 7.6 Hz, CH), 7.95 (2 H, d, 3 J = 7.8 Hz, 2 CH), 8.12 (2 H, d, 3 J = 7.8 Hz, 2 CH), 8.57 (1H, s, CH), 10.62 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): 14.3 (Me), 51.8 (MeO), 62.3 (CH2O), 107.6 (C), 108.6 (CH), 115.2 (CH), 116.3 (C), 121.5 (CH), 125.6 (2 CH), 129.2 (CH), 131.3 (2 CH), 133.5 (C), 135.7 (C), 144.2 (C), 145.3 (CH), 146.2 (C), 149.2 (C), 156.2 (C), 157.3 (C), 158.3 (CH), 162.3 (C = O), 165.6 (C = O) ppm. EI-MS: 473 (M+, 15), 304 (68), 31(100). Anal. Calcd for C24H19N5O6 (473.44): C 60.89, H 4.05, N 14.79; Found: C 61.06, H 4.18, N 14.93.
4-ethyl 5-methyl -2-((thiophen-2-ylmethylene)amino)-7H-benzo[b]pyrimido[4,5-d]azepine-4,5-dicarboxylate (6 l): Yellow powder, mp 145–147 °C, Yield: 0.74 g (85%). IR (KBr) (νmax/cm−1): 3387, 1735, 1712, 1695, 1587, 1496, 1287 cm−1. 1H NMR (500 MHz, CDCl3): 1.26 (3 H, t, 3 J = 7.4 Hz, CH3), 3.87 (3 H, s, MeO), 4.23 (2 H, q, 3 J = 7.4 Hz, CH2O), 6.86 (1 H, t, 3 J = 7.6 Hz, CH), 7.12 (1 H, d, 3 J = 7.6 Hz, CH), 7.25 (1 H, d, 3 J = 7.6 Hz, CH), 7.34 (1 H, t, 3 J = 7.6 Hz, CH), 7.46 (1 H, d, 3 J = 7.7 Hz, CH), 7.53 (1 H, d, 3 J = 7.7 Hz, CH), 7.64 (1 H, t, 3 J = 7.7 Hz, CH), 7.49 (1H, s, CH), 8.35 (1 H, s, CH), 10.78 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): 14.2 (Me), 52.3 (MeO), 62.5 (CH2O), 107.8 (C), 108.5 (CH), 115.3 (CH), 119.2 (C), 120.5 (CH), 121.7 (CH), 126.2 (CH), 127.5 (C), 128.6 (CH), 130.2 (CH), 143.2 (C), 144.7 (CH), 145.7 (C), 146.4 (C), 151.2 (C), 153.4 (CH), 158.2 (C), 165.4 (C = O), 168.2 (C = O) ppm. EI-MS: 434 (M+, 15), 403 (78), 31(100). Anal. Calcd for C22H18N4O4S (434.47): C 60.82, H 4.18, N 12.90; Found: C 60.93, H 4.27, N 13.06.
4-Ethyl 5-methyl -2-((furan-2-ylmethylene)amino)-7H-benzo[b]pyrimido[4,5-d]azepine-4,5-dicarboxylate (6 m): Yellow powder, mp 137–139˚C, Yield: 0.71 g (85%). IR (KBr) (νmax/cm−1): 3374, 1742, 1724, 1696, 1576, 1487, 1295 cm−1. 1H NMR (500 MHz, CDCl3): 1.25 (3 H, t, 3 J = 7.4 Hz, CH3), 3.83 (3 H, s, MeO), 4.18 (2 H, q, 3 J = 7.4 Hz, CH2O), 6.92 (1 H, t, 3 J = 7.6 Hz, CH), 7.16 (1 H, d, 3 J = 7.6 Hz, CH), 7.22 (1 H, d, 3 J = 7.6 Hz, CH), 7.35 (1 H, t, 3 J = 7.6 Hz, CH), 7.43 (1 H, d, 3 J = 7.7 Hz, CH), 7.52 (1 H, t 3 J = 7.7 Hz, CH), 7.74 (1 H, d, 3 J = 7.7 Hz, CH), 7.63 (1H, s, CH), 8.42 (1 H, s, CH), 10.84 (1 H, s, NH) ppm. 13C NMR (125.7 MHz, CDCl3): 14.5 (Me), 51.6 (MeO), 62.3 (CH2O), 107.6 (CH), 108.7 (C), 109.6 (CH), 110.3 (CH), 114.6 (CH), 119.2 (C), 121.4 (CH), 127.2 (C), 129.2 (CH), 143.2 (C), 144.6 (C), 145.7 (CH), 146.8 (CH), 151.3 (C), 158.6 (C), 159.4 (CH), 160.2 (C), 165.8 (C = O), 169.3 (C = O) ppm. EI-MS: 418 (M+, 15), 387 (78), 31(100). Anal. Calcd for C22H18N4O5 (418.40): C 63.15, H 4.34, N 13.39; Found: C 63.26, H 4.47, N 13.48.
Study of schiff base of Pyrimidoazepines antioxidant activity utilizing DPPH radical trapping test.
The DPPH radical scavenging experiment was utilized for investigation of some generated schiff base of pyrimidoazepines antioxidant ability such as 6b–6e like the method reported by Shimada et. al. [92]. For obtaining to this object, various concentrations (200–1000 ppm) of schiff base of Pyrimidoazepines 6b–6e were added to equal volume of DPPH methanolic solution (1 mmol/L). The mixture was stirred for 30 min at environmental temperature and putted in a dark room after this time and the absorbance of mixture was recorded at 517 nm. The schiff base of pyrimidoazepines 6b–6e was replaced with standard type of methanol (3 mL). In this procedure, Butylated hydroxytoluene (BHT) and 2-tertbutylhydroquinone (TBHQ) are standard antioxidants. The percentage of inhibition for the radical of DPPH calculated by utilizing Yen and Duh [93] formula.
Study of reducing ability for synthesized schiff base of pyrimidoazepines
The ability of iron (III) reducing by the schiff base of pyrimidoazepines 6b-6e was investigated utilizing Yildirim et al. method [91]. For this object, the samples (1 mL), potassium ferricyanide (K3Fe(CN)6; 2.5 mL, 10 g/L) and buffer of phosphate (2.5 mL, 0.2 mol/L, pH 6.6) were combined together and maintained for 30 min at 50 °C. Then, trichloroacetic acid (2.5 mL, 10% w/v) was added to the previous solution and centrifuged for 10 min. Finally, the supernatant (2.5 mL), distilled water (2.5 mL) and FeCl3 (0.5 mL, 1 g/L) mixed together and the absorbance of samples was measured at 700 nm. The higher absorbance of sample show higher reducing power of it. For accuracy of calculating, each measuring was carried out in three times. One way study of variance (ANOVA) that was used for data analyzing of compounds is running the SPSS software version 18.0 that proved difference of samples and control. Duncan multiple range experiments was employed for separation mean with the importance quantity of 95% (P < 0.05).
Study of prepared schiff base of pyrimidoazepines antibacterial ability
Gram-positive and Gram-negative bacteria were utilized for investigation of antibacterial effect of synthesized schiff base of pyrimidoazepines against two standards such as Streptomycin and Gentamicin with a concentration 40 μg/mL by employing the disk diffusion procedure. Two types of bacteria that are used in this experiment were produced from the Persian type culture collection (PTCC), Tehran, Iran. The bacteria were cultured for 16 to 24 h at 37 °C for preparing the turbidity equivalent bacteria to McFarland Standard No. 0.5. The bacterial suspension was produced according to the turbidity of the 0.5 McFarland (About 1.5 × 108 CFU/mL) standards and cultured with a sterile swab on Mueller Hinton agar. For valuation of their antibacterial activity of schiff base of pyrimidoazepines with concentration of 25 µg/ml were poured on sterile blank disks. The plates were incubated in an incubator at 37 °C for 24 h. The inhibition zone diameter was measured and compared to with the standard.
Rights and permissions
About this article
Cite this article
Shirangi, H.S., Moradi, A.V., Golsefidi, M.A. et al. Green synthesis and investigation of antioxidant and antimicrobial activity of new schiff base of pyrimidoazepine derivatives: application of Fe3O4/CuO/ZnO@MWCNT MNCs as an efficient organometallic nanocatalyst. Mol Divers 26, 3003–3019 (2022). https://doi.org/10.1007/s11030-021-10349-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10349-6