Abstract
Schizophrenia (SZ) is a devastating neurodevelopmental disease with an accelerated ageing feature. The criteria of metabolic disease firmly fit with those of schizophrenia. Disturbances in energy and mitochondria are at the core of complex pathology. Genetic and environmental interaction creates changes in redox, inflammation, and apoptosis. All the factors behind schizophrenia interact in a cycle where it is difficult to discriminate between the cause and the effect. New technology and advances in the multi-dispensary fields could break this cycle in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Schizophrenia is a devastating developmental and chronic disease with the onset starting in late childhood (Jaaro-Peled and Sawa 2020). This complicated disorder is an outcome of gene-environment interactions (Wahbeh and Avramopoulos 2021). Nongenetic components include drug abuse, parasitic infestations and stress, are apparent risk factors for developing schizophrenia in young adults (Janoutová et al. 2016). This disease represents 1% of the population in most countries and, unfortunately, is represented in both sexes. It is well-known that schizophrenic parents are more susceptible to having schizophrenic children than others. Other than the common environmental risk factors, genetic and epigenetic mechanisms can explain this trend (van de Leemput et al. 2016).
The current review studies cell danger markers as a potential mechanism behind schizophrenia (SZ). It is proposed, in this review, that schizophrenia may result from known or hidden factors that induce neuronal danger responses. Hopefully, we are tracking different cell danger markers related to SZ here.
Immune injury-inducing cell danger in Schizophrenia
There is strong evidence from postmortem brain examination of schizophrenic candidates, that neuroinflammation response is a component of schizophrenia pathology with evident increased microglial activity (van Kesteren et al. 2017; Trépanier et al. 2016). To tack this hypothesis in more depth, schizophrenia is associated with an inflammatory response in the white matter, representing aging criteria in the white matter with more activity in astrocytes and microglia (Najjar and Pearlman 2015; Wright et al. 2014). Here, it is suggested that schizophrenia has a double pathology pattern with an inflammatory senescence component and a neurodevelopmental component. The cognitive impairment is caused by a mismatch between chronological age and biological component (Wang et al. 2021). However, it is not well known whether white matter ageing induces inflammation in the brains of people with schizophrenia or quite the opposite. Studies conclude that white matter ageing is associated with blood–brain barrier defects. Such an imbalance creates disharmony between the central and peripheral immune machinery (Müller 2019). Such split may be associated with cell danger response to the genetic and environmental phenomenon.
Infection risk factor as a possible cause of Schizophrenia
As mentioned before, the immunological response has been associated with psychotic pathology. Studies suggest that perinatal viral infections may be implicated in SC pathology. Aftab et al. (2016) claimed human endogenous retroviral infections to be a risk factor for SC's neurodevelopmental and neuroinflammatory components. Other research work demonstrated the comorbidity between hepatitis C and SZ. A recent study created a relationship between the Zika virus and SZ. Furthermore, there is strong evidence that maternal herpes simplex is another risk factor (Šprah et al. 2017; Davies et al. 2020; Pierson et al. 2020). The polymorphism in the immunoglobulin genes explains the interaction between environmental and genetic etiologies of schizophrenia. Genetic surveillance demonstrated the correlation between immunoglobulin genes and the risk of developing SC due to an incorrect response to viral infections (Pandey et al. 2016).
Toxoplasmosis has been extensively studied as a risk factor for neuropsychiatric disorders, especially SZ. Possible mechanisms include immunopathology and blood–brain barrier disruption (Xiao et al. 2018). The parasitic infestation has been considered a risk factor for SZ.
Studies demonstrated that unlucky mothers exposed to infections showed increased maternal blood levels of immune antibodies that are considered a potential cause of neuropathology. The scope of developmental disorders includes SZ, autism, and other psychiatry profile. The evidence of this hypothesis is based on experimental animal work supported by clinical statistical data (Estes and McAllister 2016). Many viruses are claimed for this chronic brain insult including flu, measles, mumps, and others (Reisinger et al. 2015). The incidence of SZ increases from 1 to 20% on maternal infectious exposure. The rates of autistic insults raise 13 times because of maternal infection. The strong association between infection and developmental injury was recorded not only by high maternal blood levels of antibodies but also by high CSF levels of cytokines (Patterson 2009). It seems that the immune system produces a variable response to pathogens in combination with other multifactorial risk factors including chronic intoxications (Knuesel et al. 2014). Indeed, it is not clear why 80% of infected mothers deliver healthy individuals (Selten and Morgan 2010). The scientists proposed autoimmunity as a model of schizophrenia to explain how diverse pathogens produce the same pathology (Endres et al. 2020). Another hypothesis concluded that SZ is a heterogeneous disorder of different pathogenesis factors (Winship et al. 2019).
Recently, during the COVID-19 pandemic, many cases infected with the coronavirus developed psychosis. The viral infection is known to induce an aberrant immune response that ends the state of neuroinflammation explaining a potential mechanism explaining psychosis (Watson et al. 2021). Individual cases in the 40 s developed new-onset psychotic episodes without any history and responded to classic antipsychotics (Kozato et al. 2021). It is important to notice that mild neuroinflammatory findings were recorded in toxoplasmosis and proposed to be part of schizophrenia pathogenesis. However, Toxoplasmosis was reported in a third of the community with no explanation of individual risk factors of specific induction of psychosis by parasitic infestation (Fuglewicz et al. 2017).
On the other hand, it was reported that SZ patients were considered a vulnerable group and more susceptible to catching corona infection. It can be said that infection increases the risk of psychosis and schizophrenic candidates are more susceptible to infection (Barlati et al. 2021).
Mitochondrial Disorders as a risk factor for SZ
Mitochondrial proteins, either nuclear transcribed or mitochondrial coded, have been implicated in the pathogenesis of SZ. These polymorphisms affect metabolic pathways and synaptic functions in SZ and bipolar disorders (Schulmann et al. 2019). Dysfunctional mitochondrial function, either inherited or induced, creates oxidative stress load and accelerates the ageing process, which has been seen in degenerative diseases, and is a component of the pathogenesis of SZ. The defective mitochondrial adaptation was associated with the inflammatory and apoptotic endpoints, suggesting that loss of function is part of SZ (Wu et al. 2019). Studies showed impaired mitochondrial activity with disrupted redox status, induced endothelial dysfunction and atherosclerosis (Morris et al. 2020). Hypo-functioning mitochondria impair synaptic trafficking, calcium metabolism, and activity of action potentials, resulting in dysfunctional neuronal activity. It can be said that schizophrenia is impaired dynamics of neurons that are essentially dependent on energy (Vanden Berghe et al. 2002; Montalvo et al. 2006).
Purigenic signaling as a mechanism is Schizophrenia
ATP and adenosine work as neurotransmitters. ATP plays the role of the excitatory transmitter, while adenosine plays the opposite. This machinery regulates energy from one side and neurons' activity, including synaptic trafficking and plasticity (Lindberg et al. 2015). ATP helps release excitatory glutamic acid, and later ATP is metabolised to adenosine as a regulatory mechanism (Burnstock 2008). The receptors affected by purines and pyrimidines are P1, P2, and P2Y. These receptors are essential for ischemia, aging, and neuroinflammation (Burnstock 2008 Optimal mitochondrial function is essential for keeping an ATP/Adenosine balance during activity and rest (Barraco et al. 1993). Disrupted mitochondrial control of pyrogenic metabolism induces increasing intracellular calcium, ending in apoptosis, hindering synaptic plasticity, a feature of schizophrenia and other psychiatric disorders (Mattson et al. 2008; Cheng et al. 2010; Manji et al. 2012). Excess dopamine activity, an essential parameter in schizophrenia, impairs mitochondrial activity. Experiment results showed that dopamine has a negative effect on cellular metabolism, particularly complex activity (Brenner-Lavie et al. 2009).
Oxygen consumption
As mentioned before, impaired mitochondrial function, either degenerative or developmental in the case of excess dopamine, is associated with low oxygen consumption. This defective machinery is a cause of increased dissolved oxygen with increased oxidative stress creating a cycle of cell destruction, inflammation, or aging (Lu 2013).
An experimental study on cerebral organoids derived from schizophrenic candidates showed disturbed mitochondrial oxidation detected by transcriptomic analysis (Kathuria et al. 2020). Complex 1 deformities have been reported in schizophrenic patients, unlike bipolar disease. It is possible to consider reduced oxygen consumption as a marker of schizophrenia (Rosenfeld et al. 2011). Experimental transplantation of mitochondria to the forebrain to animals showed an improved psychotic profile at the level of proteomic level. The transplantation of the healthy mitochondrial was considered a therapeutic test of the schizophrenic animal model (Ene et al. 2022). An extensive study on a family suffering from SZ showed higher mitochondrial bulk, increased lactate level in the blood, and low mitochondrial DNA. The mitochondrial enzymes were defective and the oxygen consumption was low (Torrell et al. 2017).
The NMDA receptors are downregulated in SZ candidates and these findings in conjunction with decreased antioxidant capacity are associated with disturbed redox balance, oxidative stress, and neuroinflammation (Beeraka et al. 2022).
Studies showed that exercise improved the performance of SZ candidates and recommended strength exercise as adjuvant therapy (Keller-Varady et al. 2016). MRI examination of sports-practicing SZ patients showed evidence of improved structural changes in the hippocampus (Malchow et al. 2016).
Cysteine and sulfur metabolism
Glutathione synthesis depends on cysteine as a substrate. This amino acid is considered a semi-essential amino acid, produced endogenously and supplied by food. The endogenous source comes from the essential amino acid methionine. The process of glutathione synthesis is essential for both the antioxidant capacity and methylation of the DNA, which is a crucial factor in epigenetic risk factors in many diseases, including SZ. (Matsuzawa et al. 2008; Aldini et al. 2018).
Cases suffering from homocystinuria manifested with lens dislocation and mental retardation. These patients developed schizophrenic criteria in adolescence. The mechanism behind psychosis can be explained by the fact that methionine and homocysteine with the oxidation product as agonists for the excitatory glutamate receptors (Eschweiler et al. 1997). These psychotic episodes do not respond to conventional antipsychotics. Vitamin supply of folic acid and pyridoxine improves the delusional spectrum (Colafrancesco et al. 2015).
Vitamin D metabolism
Studies raised concern about the role of Vitamin D deficiency in the pathogenesis of SZ. Normal levels of vitamin D are essential for brain development in neonates. Experimental animal depletion of neonatal vitamin D altered the dopaminergic system in a manner comparable to SZ pathology (Cui et al. 2021).
Vitamin D3 is generated by the effects of skin sun exposure in addition to dietary sources. This vitamin is subjected to hydroxylation into active vitamin D that can pass through the blood–brain barrier and bind the vitamin D receptors (Eyles et al. 2005; Cui et al. 2013). On exposure to cell stress, the activity of the mitochondrial 24 hydroxylase increases, contributing to vitamin D deficiency. This emergency condition may explain the inflammatory status associated with SZ (Kivity et al. 2011; Shanmugasundaram and Selvaraj 2012).
Folic acid and B12 metabolism
Early-onset psychotic patients have been examined for folic acid, vitamin B12, and cortisol level assays. Those untreated candidates suffered from low folate and vitamin B12, in contrast to higher cortisol and homocysteine levels, suggesting cellular stress as part of the neuropathology. To complete the picture, SZ is associated with dietary insufficiency with lower levels of B12 and folate, creating a cycle of vitamin depletion (Kale et al. 2010; Yazici et al. 2019). The mitochondrial Krebs cycle, methylation of DNA or histones, and methionine synthesis are all linked to folic acid and B12 (Naviaux 2008; Smiraglia et al. 2008).
Genetic polymorphism of genes involved in vitamin B12 absorption and metabolism has been claimed to cause cognitive decline in psychiatric disorders like SZ. Further studies are needed to explain the possible mechanisms (Mitchell et al. 2014).
Metabolomics features in Schizophrenia
The metabolic profile of SZ showed accelerated ageing and inflammatory response. These data are supported by proteomic studies (Campeau et al. 2022). An experimental study examined the metabolic profiles of chronically hospitalised SZ candidates and showed lower glutamate and urea cycle metabolism (Okamoto et al. 2021). On the other hand, a research study showed increased glutamate metabolism in SZ and, at the same time, lower polyunsaturated fatty acids, vitamin E, and creatinine. The differences in glutamate metabolism suggest heterogeneity of SZ, or the presence of different phases of the pathology over time (Davison et al. 2018. Even with antipsychotic treatment, the amino acid metabolome was altered, suggesting redox, inflammation, lipid peroxidation, DNA damage, mitochondrial injury, and apoptosis pathways. The metabolomics profile showed decreased plasma levels of valine, aspartate, citrulline, glycine, arginine, and ornithine in schizophrenic patients (Davison et al. 2018).
Microbiota and Schizophrenia
Experimental fecal transplantation of microbiota from schizophrenic patients to mice, showed significant alterations in the animal behavior corresponding to SZ. The animal brain had abnormal tryptophan and dopamine activity (Zhu et al. 2020). Gut flora was a critical factor in oxidative inflammation and the permeability of the intestine, making a model for what happened distantly in the brain (Konjevod et al. 2021). The gut-brain axis is strongly linked to SZ. The process of the pathogenesis of psychosis was associated with changes in the gut microbiome. Adjuvant therapy with probiotic therapy has not been proven for SZ. Such a line of treatment partially modifies the pathology. The activity of the intestinal flora was associated with a concurrent modification of the inflammatory status of the brain. It is not clear what the causal relationship of this axis is (Mangiola et al. 2016; Helaly et al. 2019; Samochowiec and Misiak 2021). Enterobacteriaceae and Enterobacteriods strains were associated with high-risk SZ in contrast to Gammaproteobacteria. These results could be related to Flora's production of serotonin. Excess serotonin-producing strains were more related to increased rates of SZ (Zhuang et al. 2020).
Experimental fecal transplantation of microbiota from schizophrenic patients to mice showed significant alterations in the animal behavior corresponding to SZ. The animal brain had abnormal tryptophan and dopamine activity (Zhu et al. 2020). At the same time, gut flora was a critical factor in oxidative inflammation and the permeability of the intestine, providing a model for what was happening distantly in the brain (Konjevod et al. 2021).
Metals and Schizophrenia
Exposure to heavy metals for a long time, like lead and arsenic, is blamed for being associated with an increased risk of SZ. In utero and young, exposure to these oxidative pressures may induce neurodevelopmental-aging combinations of the SZ pathology (Opler and Susser 2005, Ma et al. 2019). Furthermore, sustained metal toxicity creates chronic inflammatory status in the brain and impaired dopamine receptor function. Neuroinflammation and disrupted dopamine function are core mechanisms in SZ, arsenic has extra hyperphosphorylation of the cytoskeletal proteins, contributing to degenerative diseases in the brain (Finefrock et al. 2003; Vahidnia et al. 2007; Jomova and Valko 2011).
Nutritional metals like iron are essential for dopamine signaling, synaptogenesis, and myelination, energy metabolism. Several studies have found a link between iron deficiency during pregnancy and an increased risk of SZ later in life (Bastian et al. 2020; Maxwell and Rao 2021).
The causality of Schizophrenia
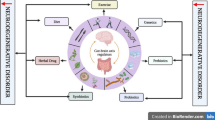
In summary, schizophrenic patients have cell stress for many interacting causes, whether genetic or environmental. Indeed, it is part of a metabolic disease with an energy disorder. However, the cause-effect is challenging to analyze. Many pathological elements can be both a cause and an effect at the same time, making the etiology of this profound disorder and other chronic diseases difficult to determine. Here, we hope for advances in physics to provide a new perspective. The discovery of gravitation waves in 2015 can explain or add a new horizon to understanding space–time at the molecular level (Vitale 2021). It is postulated that a strong gravitational field can modify the dynamics and energy functions of the cells. It is possible to track the medical records of psychiatric patients, the visits to the clinics, and the relapse in a timetable in correlation to the activity of LIGO records, hoping to create an association. This hypothesis can explain the dual pathology of SZ as the candidates suffer from biological ageing and developmental components at the same time. The activity of Wnt signalling in SZ supports the trophic component (developmental) (Inestrosa et al. 2012) (Tables 1 and 2, Fig. 1).
Conclusion
Schizophrenia is a developmental disorder with associated cell danger factors. It is not known what is the starting point of that response like any multifactorial disease. Multiple factors like an immune response, infection and mitochondrial dysfunction have been proposed to explain SZ. Metabolic abnormalities of cysteine, Sulphur were suggested as a theory of psychosis besides vitamin deficiency. New tools in physics could add potential explanation to solve the such dilemma. Gravity risk factor may open new scope to overcome such chronic psychiatric diseases.
Data availability
Not applicable
Code availability (software application or custom code)
Not applicable
References
Aftab A, Shah AA, Hashmi AM (2016) Pathophysiological role of HERV-W in Schizophrenia. J Neuropsychiatry Clin Neurosci 28(1):17–25. https://doi.org/10.1176/appi.neuropsych.15030059
Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, Sergio F (2018) N-Acetylcysteine as an antioxidant and disulfide breaking agent: the reasons why. Free Radical Res 52(7):751–762. https://doi.org/10.1080/10715762.2018.1468564
Amiri S, Dizaji R, Momeny M, Gauvin E, Hosseini MJ (2021) Clozapine attenuates mitochondrial dysfunction, inflammatory gene expression, and behavioral abnormalities in an animal model of schizophrenia. Neuropharmacology 187:108503. https://doi.org/10.1016/j.neuropharm.2021.108503
Arabska J, Wysokiński A, Brzezińska-Błaszczyk E, Kozłowska E (2022) Serum Levels and in vitro CX3CL1 (Fractalkine), CXCL8, and IL-10 Synthesis in Phytohemaglutinin-Stimulated and Non-stimulated Peripheral Blood Mononuclear Cells in Subjects With Schizophrenia. Front Psych 13:845136. https://doi.org/10.3389/fpsyt.2022.845136
Arai M, Miyashita M, Kobori A, Toriumi K, Horiuchi Y, Itokawa M (2014) Carbonyl stress and schizophrenia. Psychiatry Clin Neurosci 68(9):655–665. https://doi.org/10.1111/pcn.12216
Arons MH, Lee K, Thynne CJ, Kim SA, Schob C, Kindler S, Montgomery JM, Garner CC (2016) Shank3 Is part of a zinc-sensitive signaling system that regulates excitatory synaptic strength. J Neurosci 36(35):9124–9134. https://doi.org/10.1523/JNEUROSCI.0116-16.2016
Barlati S, Nibbio G, Vita A (2021) Schizophrenia during the COVID-19 pandemic. Curr Opin Psychiatry 34(3):203–210. https://doi.org/10.1097/YCO.0000000000000702
Barraco RA, Martens KA, Parizon M, Normile HJ (1993) Adenosine A2a receptors in the nucleus accumbens mediate locomotor depression. Brain Res Bull 31(3–4):397–404. https://doi.org/10.1016/0361-9230(93)90233-2
Bastian TW, Rao R, Tran PV, Georgieff MK (2020) The effects of early-life iron deficiency on brain energy metabolism. Neurosci Insights 15:2633105520935104. https://doi.org/10.1177/2633105520935104
Beeraka NM, Avila-Rodriguez MF, Aliev G (2022) Recent reports on redox stress-induced mitochondrial DNA variations, neuroglial interactions, and NMDA receptor system in pathophysiology of Schizophrenia. Mol Neurobiol 59(4):2472–2496. https://doi.org/10.1007/s12035-021-02703-4
Berber B, Doluca O (2021) A comprehensive drug repurposing study for COVID19 treatment: novel putative dihydroorotate dehydrogenase inhibitors show association to serotonin-dopamine receptors. Brief Bioinform 22(2):1023–1037. https://doi.org/10.1093/bib/bbaa379
Brenner-Lavie H, Klein E, Ben-Shachar D (2009) Mitochondrial complex I as a novel target for intraneuronal DA: modulation of respiration in intact cells. Biochem Pharmacol 78(1):85–95. https://doi.org/10.1016/j.bcp.2009.03.024
Burnstock G (2008) Purinergic signaling and disorders of the central nervous system. Nat Rev Drug Discovery 7(7):575–590. https://doi.org/10.1038/nrd2605
Camacho-Abrego I, González-Cano SI, Aguilar-Alonso P, Brambila E, la Cruz F, Flores G (2021) Changes in nitric oxide, zinc and metallothionein levels in limbic regions at pre-pubertal and post-pubertal ages presented in an animal model of schizophrenia. J Chem Neuroanat 111:101889. https://doi.org/10.1016/j.jchemneu.2020.101889
Campeau A, Mills RH, Stevens T, Rossitto LA, Meehan M, Dorrestein P, Daly R, Nguyen TT, Gonzalez DJ, Jeste DV, Hook V (2022) Multi-omics of human plasma reveals molecular features of dysregulated inflammation and accelerated aging in Schizophrenia. Mol Psychiatry 27(2):1217–1225. https://doi.org/10.1038/s41380-021-01339-z
Cao B, Chen Y, Rosenbalt JD, McIntyre RS, Wang D, Yan L (2020) Association of alkali metals and Alkaline-earth metals with the risk of schizophrenia in a Chinese population: a case-control study. J Trace Elem Med Biol 60:126478. https://doi.org/10.1016/j.jtemb.2020.126478
Cheng A, Hou Y, Mattson MP (2010) Mitochondria and neuroplasticity. ASN Neuro 2(5):e00045. https://doi.org/10.1042/AN20100019
Colafrancesco G, Di Marzio GM, Abbracciavento G, Stoppioni V, Leuzzi V, Ferrara M (2015) Acute psychosis in an adolescent with undiagnosed homocystinuria. Eur J Pediatr 174(9):1263–1266. https://doi.org/10.1007/s00431-015-2552-2
Corsi-Zuelli F, Deakin B, de Lima MHF, Qureshi O, Barnes NM, Upthegrove R, Louzada-Junior P, Del-Ben CM (2021) T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun - Health 17:100330. https://doi.org/10.1016/j.bbih.2021.100330
Couch ACM, Berger T, Hanger B, Matuleviciute R, Srivastava DP, Thuret S, Vernon AC (2021) Maternal immune activation primes deficiencies in adult hippocampal neurogenesis. Brain Behav Immun 97:410–422. https://doi.org/10.1016/j.bbi.2021.07.021
Cui X, McGrath JJ, Burne T, Eyles DW (2021) Vitamin D and Schizophrenia: 20 years on. Mol Psychiatry 26(7):2708–2720. https://doi.org/10.1038/s41380-021-01025-0
Cui X, Pelekanos M, Liu PY, Burne TH, McGrath JJ, Eyles DW (2013) The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience 236:77–87. https://doi.org/10.1016/j.neuroscience.2013.01.035
Davies C, Segre G, Estradé A, Radua J, De Micheli A, Provenzani U, Oliver D, Salazar de Pablo G, Ramella-Cravaro V, Besozzi M, Dazzan P, Miele M, Caputo G, Spallarossa C, Crossland G, Ilyas A, Spada G, Politi P, Murray RM, McGuire P, …, Fusar-Poli P (2020) Prenatal and perinatal risk and protective factors for psychosis: a systematic review and meta-analysis. Lancet. Psychiatry 7(5): 399–410. https://doi.org/10.1016/S2215-0366(20)30057-2
Davison J, O’Gorman A, Brennan L, Cotter DR (2018) A systematic review of metabolite biomarkers of Schizophrenia. Schizophr Res 195:32–50. https://doi.org/10.1016/j.schres.2017.09.021
Endres D, Leypoldt F, Bechter K, Hasan A, Steiner J, Domschke K, Wandinger KP, Falkai P, Arolt V, Stich O, Rauer S, Prüss H, van Elst LT (2020) Autoimmune encephalitis as a differential diagnosis of schizophreniform psychosis: clinical symptomatology, pathophysiology, diagnostic approach, and therapeutic considerations. Eur Arch Psychiatry Clin Neurosci 270(7):803–818. https://doi.org/10.1007/s00406-020-01113-2
Ene HM, Karry R, Farfara D, Ben-Shachar D (2022) Mitochondria play an essential role in the trajectory of adolescent neurodevelopment and behavior in adulthood: evidence from a schizophrenia rat model. Mol Psychiatry. https://doi.org/10.1038/s41380-022-01865-4.Advanceonlinepublication
Eschweiler G, Rosin R, Thier P, Giedke H (1997) Postoperative psychosis in homocystinuria. Eur Psychiatry 12(2):98–101. https://doi.org/10.1016/S0924-9338(97)89648-4
Estes ML, McAllister AK (2016) Maternal immune activation: Implications for neuropsychiatric disorders. Science (New York, N.Y.) 353(6301):772–777. https://doi.org/10.1126/science.aag3194
Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ (2005) Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 29(1):21–30. https://doi.org/10.1016/j.jchemneu.2004.08.006
Finefrock AE, Bush AI, Doraiswamy PM (2003) Current status of metals as therapeutic targets in Alzheimer’s disease. J Am Geriatr Soc 51(8):1143–1148. https://doi.org/10.1046/j.1532-5415.2003.51368.x
Forero DA, González-Giraldo Y (2020) Integrative in silico analysis of genome-wide dna methylation profiles in Schizophrenia. J Mol Neurosci 70(11):1887–1893. https://doi.org/10.1007/s12031-020-01585-w
Franklin F, Rajamanikam A, Raju CS, Gill JS, Francis B, Sy-Cherng LW, Kumar S (2022) Higher amoebic and metronidazole resistant forms of Blastocystis sp. seen in schizophrenic patients. Parasit Vectors 15(1):313. https://doi.org/10.1186/s13071-022-05418-0
Fuglewicz AJ, Piotrowski P, Stodolak A (2017) Relationship between toxoplasmosis and schizophrenia: a review. Adv Clin Exp Med 26(6):1031–1036. https://doi.org/10.17219/acem/61435
Guo Z, Tse YC, Zhang Y, Sun Q, Vecchiarelli HA, Aukema R, Hill MN, Wong TP, Boksa P (2018) Prenatal immune activation potentiates endocannabinoid-related plasticity of inhibitory synapses in the hippocampus of adolescent rat offspring. Eur Neuropsychopharmacol 28(12):1405–1417. https://doi.org/10.1016/j.euroneuro.2018.09.003
Helaly A, El-Attar YA, Khalil M, Ahmed Ghorab D, El-Mansoury AM (2019) Antibiotic abuse induced histopathological and neurobehavioral disorders in mice. Curr Drug Saf 14(3):199–208. https://doi.org/10.2174/1574886314666190612130921
Inestrosa NC, Montecinos-Oliva C, Fuenzalida M (2012) Wnt signaling: role in Alzheimer disease and Schizophrenia. Neuroimmune Pharmacol 7(4):788–807. https://doi.org/10.1007/s11481-012-9417-5
Jaaro-Peled H, Sawa A (2020) Neurodevelopmental factors in Schizophrenia. Psychiatr Clin North Am 43(2):263–274. https://doi.org/10.1016/j.psc.2020.02.010
Janoutová J, Janácková P, Serý O, Zeman T, Ambroz P, Kovalová M, Varechová K, Hosák L, Jirík V, Janout V (2016) Epidemiology and risk factors of schizophrenia. Neuro Endocrinol Lett 37(1):1–8
Jmii H, Fisson S, Aouni M, Jaidane H (2021) Type B coxsackieviruses and central nervous system disorders: critical review of reported associations. Rev Med Virol 31(4):e2191. https://doi.org/10.1002/rmv.2191
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283(2–3):65–87. https://doi.org/10.1016/j.tox.2011.03.001
Kale A, Naphade N, Sapkale S, Kamaraju M, Pillai A, Joshi S, Mahadik S (2010) Reduced folic acid, vitamin B12, and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res 175(1–2):47–53. https://doi.org/10.1016/j.psychres.2009.01.013
Kathuria A, Lopez-Lengowski K, Jagtap SS, McPhie D, Perlis RH, Cohen BM, Karmacharya R (2020) Transcriptomic landscape and functional characterization of induced pluripotent stem cell-derived cerebral organoids in Schizophrenia. JAMA Psychiat 77(7):745–754. https://doi.org/10.1001/jamapsychiatry.2020.0196
Kecel-Gunduz S, Budama-Kilinc Y, Cakir-Koc R, Zorlu T, Bicak B, Kokcu Y, Kaya Z, Ozel AE, Akyuz S (2020) In silico analysis of sulpiride, synthesis, characterization and in vitro studies of its nanoparticle for the treatment of Schizophrenia. Curr Comput Aided Drug Des 16(2):104–121. https://doi.org/10.2174/1573409915666190627125643
Keller-Varady K, Hasan A, Schneider-Axmann T, Hillmer-Vogel U, Adomßent B, Wobrock T, Schmitt A, Niklas A, Falkai P, Malchow B (2016) Endurance training in patients with schizophrenia and healthy controls: differences and similarities. Eur Arch Psychiatry Clin Neurosci 266(5):461–473. https://doi.org/10.1007/s00406-015-0651-8
Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB (2015) Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2(3):258–270. https://doi.org/10.1016/S2215-0366(14)00122-9
Kivity S, Agmon-Levin N, Zisappl M, Shapira Y, Nagy EV, Dankó K, Szekanecz Z, Langevitz P, Shoenfeld Y (2011) Vitamin D and autoimmune thyroid diseases. Cell Mol Immunol 8(3):243–247. https://doi.org/10.1038/cmi.2010.73
Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP (2014) Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 10(11):643–660. https://doi.org/10.1038/nrneurol.2014.187
Konjevod M, Nikolac Perkovic M, Sáiz J, Svob Strac D, Barbas C, Rojo D (2021) Metabolomics analysis of microbiota-gut-brain axis in neurodegenerative and psychiatric diseases. J Pharm Biomed Anal 194:113681. https://doi.org/10.1016/j.jpba.2020.113681
Kozato N, Mishra M, Firdosi M (2021) New-onset psychosis due to COVID-19. BMJ Case Rep 14(4):e242538. https://doi.org/10.1136/bcr-2021-242538
Kumar A, Gupta S, Sharma P, Prasad R, Pal A (2019) In silico method for identification of novel copper and iron metabolism proteins in various neurodegenerative disorders. Neurotoxicology 73:50–57. https://doi.org/10.1016/j.neuro.2019.02.020
Li S, Song J, Ke P, Kong L, Lei B, Zhou J, Huang Y, Li H, Li G, Chen J, Li X, Xiang Z, Ning Y, Wu F, Wu K (2021) The gut microbiome is associated with brain structure and function in schizophrenia. Scientific Reports 11(1):9743. https://doi.org/10.1038/s41598-021-89166-8
Lindberg D, Shan D, Ayers-Ringler J, Oliveros A, Benitez J, Prieto M, McCullumsmith R, Choi DS (2015) Purinergic signaling and energy homeostasis in psychiatric disorders. Curr Mol Med 15(3):275–295. https://doi.org/10.2174/1566524015666150330163724
Lu SC (2013) Glutathione synthesis. Biochem Biophys Acta 1830(5):3143–3153. https://doi.org/10.1016/j.bbagen.2012.09.008
Luczynski P, McVey Neufeld KA, Oriach CS, Clarke G, Dinan TG, Cryan JF (2016) Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol 19(8):pyw020. https://doi.org/10.1093/ijnp/pyw020
Ma J, Yan L, Guo T, Yang S, Guo C, Liu Y, Xie Q, Wang J (2019) Association of typical toxic heavy metals with Schizophrenia. Int J Environ Res Public Health 16(21):4200. https://doi.org/10.3390/ijerph16214200
Malchow B, Keeser D, Keller K, Hasan A, Rauchmann BS, Kimura H, Schneider-Axmann T, Dechent P, Gruber O, Ertl-Wagner B, Honer WG, Hillmer-Vogel U, Schmitt A, Wobrock T, Niklas A, Falkai P (2016) Effects of endurance training on brain structures in chronic schizophrenia patients and healthy controls. Schizophr Res 173(3):182–191. https://doi.org/10.1016/j.schres.2015.01.005
Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A (2016) Gut microbiota in autism and mood disorders. World J Gastroenterol 22(1):361–368. https://doi.org/10.3748/wjg.v22.i1.361
Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, Chen G (2012) Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci 13(5):293–307. https://doi.org/10.1038/nrn3229
Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E, Takanashi J, Matsuda T, Shimizu E, Ikehira H, Iyo M, Hashimoto K (2008) Negative correlation between brain glutathione level and negative symptoms in Schizophrenia: a 3T 1H-MRS study. PLoS ONE 3(4):e1944. https://doi.org/10.1371/journal.pone.0001944
Mattson MP, Gleichmann M, Cheng A (2008) Mitochondria in neuroplasticity and neurological disorders. Neuron 60(5):748–766. https://doi.org/10.1016/j.neuron.2008.10.010
Maxwell AM, Rao RB (2021) Perinatal iron deficiency as an early risk factor for Schizophrenia. Nutr Neurosci: 1–10. https://doi.org/10.1080/1028415X.2021.1943996 (Advance online publication)
Mitchell ES, Conus N, Kaput J (2014) B vitamin polymorphisms and behavior: evidence of associations with neurodevelopment, depression, Schizophrenia, bipolar disorder, and cognitive decline. Neurosci Biobehav Rev 47:307–320. https://doi.org/10.1016/j.neubiorev.2014.08.006
Montalvo GB, Artalejo AR, Gilabert JA (2006) ATP from subplasmalemmal mitochondria controls Ca2+-dependent inactivation of CRAC channels. J Biol Chem 281(47):35616–35623. https://doi.org/10.1074/jbc.M603518200
Morris G, Puri BK, Olive L, Carvalho A, Berk M, Walder K, Gustad LT, Maes M (2020) Endothelial dysfunction in neuro progressive disorders-causes and suggested treatments. BMC Med 18(1):305. https://doi.org/10.1186/s12916-020-01749-w
Müller N (2019) The role of intercellular adhesion molecule-1 in the pathogenesis of psychiatric disorders. Front Pharmacol 10:1251. https://doi.org/10.3389/fphar.2019.01251
Najjar S, Pearlman DM (2015) Neuroinflammation and white matter pathology in Schizophrenia: systematic review. Schizophr Res 161(1):102–112. https://doi.org/10.1016/j.schres.2014.04.041
Naviaux RK (2008) Mitochondrial control of epigenetics. Cancer Biol Ther 7(8):1191–1193. https://doi.org/10.4161/cbt.7.8.6741
Okamoto N, Ikenouchi A, Watanabe K, Igata R, Fujii R, Yoshimura R (2021) A metabolomics study of serum in hospitalized patients with chronic Schizophrenia. Front Psych 12:763547. https://doi.org/10.3389/fpsyt.2021.763547
Opler MG, Susser ES (2005) Fetal environment and Schizophrenia. Environ Health Perspect 113(9):1239–1242. https://doi.org/10.1289/ehp.7572
Pandey JP, Namboodiri AM, Elston RC (2016) Immunoglobulin G genotypes and the risk of Schizophrenia. Hum Genet 135(10):1175–1179. https://doi.org/10.1007/s00439-016-1706-2
Patterson PH (2009) Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 204(2):313–321. https://doi.org/10.1016/j.bbr.2008.12.016
Pierson J, Yeruva RR, El-Mallakh RS (2020) Can in utero Zika virus exposure be a risk factor for Schizophrenia in the offspring? World J Biol Psychiatry 21(1):2–11. https://doi.org/10.1080/15622975.2018.1500027
Pu Z, Sun Y, Jiang H, Hou Q, Yan H, Wen H, Li G (2021) Effects of berberine on gut microbiota in patients with mild metabolic disorders induced by Olanzapine. Am J Chin Med 49(8):1949–1963. https://doi.org/10.1142/S0192415X21500920
Reisinger S, Khan D, Kong E, Berger A, Pollak A, Pollak DD (2015) The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol Ther 149:213–226. https://doi.org/10.1016/j.pharmthera.2015.01.001
Roberts RC (2017) Postmortem studies on mitochondria in schizophrenia. Schizophr Res 187:17–25. https://doi.org/10.1016/j.schres.2017.01.056
Rosenfeld M, Brenner-Lavie H, Ari SG, Kavushansky A, Ben-Shachar D (2011) Perturbation in mitochondrial network dynamics and in complex I dependent cellular respiration in schizophrenia. Biol Psychiat 69(10):980–988. https://doi.org/10.1016/j.biopsych.2011.01.010
Samochowiec J, Misiak B (2021) Gut microbiota and microbiome in Schizophrenia. Curr Opin Psychiatry 34(5):503–507. https://doi.org/10.1097/YCO.0000000000000733
Schoenrock SA, Tarantino LM (2016) Developmental vitamin D deficiency and schizophrenia: the role of animal models. Genes Brain Behav 15(1):45–61. https://doi.org/10.1111/gbb.12271
Schulmann A, Ryu E, Goncalves V, Rollins B, Christiansen M, Frye MA, Biernacka J, Vawter MP (2019) Novel complex interactions between mitochondrial and nuclear dNA in Schizophrenia and bipolar disorder. Mol Neuropsychiatry 5(1):13–27. https://doi.org/10.1159/000495658
Selten JP, Morgan VA (2010) Prenatal exposure to influenza and major affective disorder. Bipolar Disord 12(7):753–754. https://doi.org/10.1111/j.1399-5618.2010.00849.x
Shanmugasundaram R, Selvaraj RK (2012) Vitamin D-1α-hydroxylase and vitamin D-24-hydroxylase mRNA studies in chickens. Poult Sci 91(8):1819–1824. https://doi.org/10.3382/ps.2011-02129
Smiraglia DJ, Kulawiec M, Bistulfi GL, Gupta SG, Singh KK (2008) A novel role for mitochondria in regulating epigenetic modification in the nucleus. Cancer Biol Ther 7(8):1182–1190. https://doi.org/10.4161/cbt.7.8.6215
Šprah L, Dernovšek MZ, Wahlbeck K, Haaramo P (2017) Psychiatric readmissions and their association with physical comorbidity: a systematic literature review. BMC Psychiatry 17(1):2. https://doi.org/10.1186/s12888-016-1172-3
Srivastava R, Faust T, Ramos A, Ishizuka K, Sawa A (2018) Dynamic changes of the mitochondria in psychiatric illnesses: new mechanistic insights from human neuronal models. Biol Psychiat 83(9):751–760. https://doi.org/10.1016/j.biopsych.2018.01.007
Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP (2016) Postmortem evidence of cerebral inflammation in Schizophrenia: a systematic review. Mol Psychiatry 21(8):1009–1026. https://doi.org/10.1038/mp.2016.90
Torrell H, Alonso Y, Garrabou G, Mulet D, Catalán M, Valiente-Pallejà A, Carreño-Gago L, García-Arumí E, Montaña E, Vilella E, Martorell L (2017) Mitochondrial dysfunction in a family with psychosis and chronic fatigue syndrome. Mitochondrion 34:1–8. https://doi.org/10.1016/j.mito.2016.10.007
Tsavou A, Curtis D (2019) In-silico investigation of coding variants potentially affecting the functioning of the glutamatergic N-methyl-D-aspartate receptor in schizophrenia. Psychiatr Genet 29(2):44–50. https://doi.org/10.1097/YPG.0000000000000216
Vahidnia A, van der Voet GB, de Wolff FA (2007) Arsenic neurotoxicity–a review. Hum Exp Toxicol 26(10):823–832. https://doi.org/10.1177/0960327107084539
van de Leemput J, Hess JL, Glatt SJ, Tsuang MT (2016) Genetics of Schizophrenia: historical insights and prevailing evidence. Adv Genet 96:99–141. https://doi.org/10.1016/bs.adgen.2016.08.001
Vanden Berghe P, Kenyon JL, Smith TK (2002) Mitochondrial Ca2+ uptake regulates the excitability of myenteric neurons. J Neurosci 22(16):6962–6971. https://doi.org/10.1523/JNEUROSCI.22-16-06962.2002
van Kesteren CF, Gremmels H, de Witte LD, Hol EM, Van Gool AR, Falkai PG, Kahn RS, Sommer IE (2017) Immune involvement in the pathogenesis of Schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry 7(3):e1075. https://doi.org/10.1038/tp.2017.4
Venkataramaiah C (2020) Modulations in the ATPases during ketamine-induced schizophrenia and regulatory effect of “3-(3, 4-dimethoxy phenyl) -1- (4-methoxyphenyl) prop-2-en-1-one”: an in vivo and in silico studies. J Recept Signal Transduct Res 40(2):148–156. https://doi.org/10.1080/10799893.2020.1720242
Venkataramaiah C, Lakshmi Priya B, Rajendra W (2021) Perturbations in the catecholamine metabolism and protective effect of “3-(3, 4-dimethoxy phenyl)-1-4(methoxy phenyl) prop-2-en-1-one” during ketamine-induced schizophrenia: an in vivo and in silico studies. J Biomol Struct Dyn 39(10):3523–3532. https://doi.org/10.1080/07391102.2020.1765875
Vitale S (2021) The first 5 years of gravitational-wave astrophysics. Science (New York, N.Y.) 372(6546):eabc7397. https://doi.org/10.1126/science.abc7397
Wahbeh MH, Avramopoulos D (2021) Gene-environment interactions in Schizophrenia: a literature review. Genes 12(12):1850. https://doi.org/10.3390/genes12121850
Wang J, Kochunov P, Sampath H, Hatch KS, Ryan MC, Xue F, Neda J, Paul T, Hahn B, Gold J, Waltz J, Hong LE, Chen S (2021) White matter brain aging in relationship to Schizophrenia and its cognitive deficit. Schizophr Res 230:9–16. https://doi.org/10.1016/j.schres.2021.02.003
Watson CJ, Thomas RH, Solomon T, Michael BD, Nicholson TR, Pollak TA (2021) COVID-19 and psychosis risk: real or delusional concern? Neurosci Lett 741:135491. https://doi.org/10.1016/j.neulet.2020.135491
Winship IR, Dursun SM, Baker GB, Balista PA, Kandratavicius L, Maia-de-Oliveira JP, Hallak J, Howland JG (2019) An overview of animal models related to Schizophrenia. Can J Psychiatry Rev Can Psychiatri 64(1):5–17. https://doi.org/10.1177/0706743718773728
Wright S, Kochunov P, Chiappelli J, McMahon R, Muellerklein F, Wijtenburg SA, White MG, Rowland LM, Hong LE (2014) Accelerated white matter aging in Schizophrenia: role of white matter blood perfusion. Neurobiol Aging 35(10):2411–2418. https://doi.org/10.1016/j.neurobiolaging.2014.02.016
Wu Y, Chen M, Jiang J (2019) Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 49:35–45. https://doi.org/10.1016/j.mito.2019.07.003
Xiao J, Prandovszky E, Kannan G, Pletnikov MV, Dickerson F, Severance EG, Yolken RH (2018) Toxoplasma gondii: biological parameters of the connection to Schizophrenia. Schizophr Bull 44(5):983–992. https://doi.org/10.1093/schbul/sby082
Yazici AB, Akcay Ciner O, Yazici E, Cilli AS, Dogan B, Erol A (2019) Comparison of vitamin B12, vitamin D, and folic acid blood levels in patients with Schizophrenia, drug addiction, and controls. J Clin Neurosci 65:11–16. https://doi.org/10.1016/j.jocn.2019.04.031
Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q, Sun Q, Fan Y, Xie Y, Yang Z, Jie Z, Zhao B, Xiao L, Yang L, Zhang T, Liu B, Guo L, He X, Chen Y, Chen C, …, Ma X (2020) Transplantation of microbiota from drug-free patients with Schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry 25(11): 2905–2918. https://doi.org/10.1038/s41380-019-0475-4
Zhuang Z, Yang R, Wang W, Qi L, Huang T (2020) Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and Schizophrenia. J Neuroinflammation 17(1):288. https://doi.org/10.1186/s12974-020-01961-8
Author information
Authors and Affiliations
Contributions
Ahmed Healy was responsible for editing and submission. Doaa Ghorab was responsible for the idea outline.
Corresponding author
Ethics declarations
Ethics approval
(not applicable)
Consent to participate
(not applicable)
Consent for publication
(not applicable).
Conflicts of interest
The authors declared no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Helaly, A.M.N., Ghorab, D.S.E.D. Schizophrenia as metabolic disease. What are the causes?. Metab Brain Dis 38, 795–804 (2023). https://doi.org/10.1007/s11011-022-01147-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01147-6