Abstract
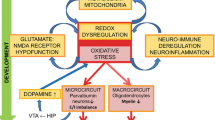
Schizophrenia (SZ) is a chronic psychiatric disorder affecting several people worldwide. Mitochondrial DNA (mtDNA) variations could invoke changes in the OXPHOS system, calcium buffering, and ROS production, which have significant implications for glial cell survival during SZ. Oxidative stress has been implicated in glial cells-mediated pathogenesis of SZ; the brain comparatively more prone to oxidative damage through NMDAR. A confluence of scientific evidence points to mtDNA alterations, Nrf2 signaling, dynamic alterations in dorsolateral prefrontal cortex (DLPFC), and provocation of oxidative stress that enhance pathophysiology of SZ. Furthermore, the alterations in excitatory signaling related to NMDAR signaling were particularly reported for SZ pathophysiology. Current review reported the recent evidence for the role of mtDNA variations and oxidative stress in relation to pathophysiology of SZ, NMDAR hypofunction, and glutathione deficiency. NMDAR system is influenced by redox dysregulation in oxidative stress, inflammation, and antioxidant mediators. Several studies have demonstrated the relationship of these variables on severity of pathophysiology in SZ. An extensive literature search was conducted using Medline, PubMed, PsycINFO, CINAHL PLUS, BIOSIS Preview, Google scholar, and Cochrane databases. We summarize consistent evidence pointing out a plausible model that may elucidate the crosstalk between mtDNA alterations in glial cells and redox dysregulation during oxidative stress and the perturbation of NMDA neurotransmitter system during current therapeutic modalities for the SZ treatment. This review can be beneficial for the development of promising novel diagnostics, and therapeutic modalities by ascertaining the mtDNA variations, redox state, and efficacy of pharmacological agents to mitigate redox dysregulation and augment NMDAR function to treat cognitive and behavioral symptoms in SZ.





Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- LARS2:
-
Leucyl-tRNA synthetase
- GAPDH:
-
Glyceralde-hyde-3-phosphate dehydrogenase
- ETC:
-
Electron transport chain
- AMPK:
-
AMP-activated protein kinase
- PGC-1α:
-
Peroxisome proliferator-activated receptor gamma coactivator-1 alpha
- DLPFC:
-
Dorsolateral prefrontal cortex
- mtSNPs:
-
Mitochondrial single nucleotide polymorphism
- PFC:
-
Prefrontal cortex
- GCLM:
-
Glutamate cysteine ligase
- GRIN2A:
-
Glutamate ionotropic receptor NMDA type subunit 2A
- Disc1:
-
Disrupted-in-schizophrenia 1
- NDUFV1:
-
NADH:ubiquinone oxidoreductase core subunit V1
- HSP:
-
Heat shock protein
- PGC-1α:
-
Peroxisome proliferator-activated receptor-gamma coactivator-1alpha
- NF-kB:
-
Nuclear factor–kappa B
- TNF-α:
-
Tumor necrotic factor–α
- ETC:
-
Electron transport chain
- GSTT-2:
-
Glutathione S-transferase theta 2
- GAD67:
-
Glutamic acid decarboxylase 67
References
Kondej M, Stępnicki P, Kaczor AA (2018) Multi-target approach for drug discovery against schizophrenia. Int J Mol Sci 19(10):3105
Bitanihirwe BK, Woo T-UW (2011) Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev 35(3):878–893
Rajasekaran A, Venkatasubramanian G, Berk M, Debnath M (2015) Mitochondrial dysfunction in schizophrenia: pathways, mechanisms and implications. Neurosci Biobehav Rev 48:10–21
Adell A (2020) Brain NMDA receptors in schizophrenia and depression. Biomolecules 10(6):947
Inan M, Petros TJ, Anderson SA (2013) Losing your inhibition: linking cortical GABAergic interneurons to schizophrenia. Neurobiol Dis 53:36–48
Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M (2009) Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol 19(2):220–230
Wood SJ, Yücel M, Pantelis C, Berk M (2009) Neurobiology of schizophrenia spectrum disorders: the role of oxidative stress. Ann Acad Med Singapore 38(5):396–401
Yao JK, Reddy RD, Van Kammen DP (2001) Oxidative damage and schizophrenia. CNS Drugs 15(4):287–310
Yao JK, Leonard S, Reddy RD (2004) Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr Bull 30(4):923–934
Yao JK, Leonard S, Reddy R (2006) Altered glutathione redox state in schizophrenia. Dis Markers 22(1–2):83–93
Yao J, Dougherty G, Reddy R, Keshavan M, Montrose D, Matson W, Rozen S, Krishnan R et al (2010) Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia. Mol Psychiatry 15(9):938–953
Matsuzawa D, Hashimoto K (2011) Magnetic resonance spectroscopy study of the antioxidant defense system in schizophrenia. Antioxid Redox Signal 15(7):2057–2065
Zhang M, Zhao Z, He L, Wan C (2010) A meta-analysis of oxidative stress markers in schizophrenia. Sci China Life Sci 53(1):112–124
Weinberger DR (1987) Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44(7):660–669
Lewis DA, Lieberman JA (2000) Catching up on schizophrenia: natural history and neurobiology. Neuron 28(2):325–334
Lewis DA, Levitt P (2002) Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 25(1):409–432
Sawa A, Snyder SH (2002) Schizophrenia: diverse approaches to a complex disease. Science 296(5568):692–695
Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A (2009) Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1–ErbB4 and DISC1. Trends Neurosci 32(9):485–495
Harrison PJ, Weinberger DR (2005) Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 10(1):40–68
Fatemi SH, Folsom TD (2009) The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull 35(3):528–548
Rampino A, Marakhovskaia A, Soares-Silva T, Torretta S, Veneziani F, Beaulieu JM (2019) Antipsychotic drug responsiveness and dopamine receptor signaling; old players and new prospects. Front Psych 9:702
Hashimoto K (2014) Targeting of NMDA receptors in new treatments for schizophrenia. Expert Opin Ther Targets 18(9):1049–1063
Goldsmith CAW, Rogers DP (2008) The case for autoimmunity in the etiology of schizophrenia. Pharmacotherapy 28(6):730–741
Laskaris L, Di Biase MA, Everall I, Chana G, Christopoulos A, Skafidas E, Cropley VL, Pantelis C (2016) Microglial activation and progressive brain changes in schizophrenia. Br J Pharmacol 173(4):666–680
Horrobin DF, Glen A, Hudson CJ (1995) Possible relevance of phospholipid abnormalities and genetic interactions in psychiatric disorders: the relationship between dyslexia and schizophrenia. Med Hypotheses 45(6):605–613
Murray AJ, Rogers JC, Katshu MZUH, Liddle PF, Upthegrove R (2021) Oxidative stress and the pathophysiology and symptom profile of schizophrenia spectrum disorders. Frontiers in Psychiatry 1235(12)
Clay HB, Sillivan S, Konradi C (2011) Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci 29(3):311–324
Amar S, Shamir A, Ovadia O, Blanaru M, Reshef A, Kremer I, Rietschel M, Schulze TG et al (2007) Mitochondrial DNA HV lineage increases the susceptibility to schizophrenia among Israeli Arabs. Schizophr Res 94(1–3):354–358
Marchbanks R, Ryan M, Day I, Owen M, McGuffin P, Whatley S (2003) A mitochondrial DNA sequence variant associated with schizophrenia and oxidative stress. Schizophr Res 65(1):33–38
Rollins B, Martin MV, Sequeira PA, Moon EA, Morgan LZ, Watson SJ, Schatzberg A, Akil H, Myers RM et al (2009) Mitochondrial variants in schizophrenia, bipolar disorder, and major depressive disorder. PloS One 4(3):e4913
Verge B, Alonso Y, Valero J, Miralles C, Vilella E, Martorell L (2011) Mitochondrial DNA (mtDNA) and schizophrenia. Eur Psychiatry 26(1):45–56
Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y, Young TA, Bullard J et al (2005) Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiat 58(2):85–96
Iwamoto K, Bundo M, Kato T (2005) Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet 14(2):241–253
Ben-Shachar D, Karry R (2008) Neuroanatomical pattern of mitochondrial complex I pathology varies between schizophrenia, bipolar disorder and major depression. PloS One 3(11):e3676
Karry R, Klein E, Shachar DB (2004) Mitochondrial complex I subunits expression is altered in schizophrenia: a postmortem study. Biol Psychiat 55(7):676–684
Da Silva T, Wu A, Laksono I, Prce I, Maheandiran M, Kiang M, Andreazza AC, Mizrahi R (2018) Mitochondrial function in individuals at clinical high risk for psychosis. Sci Rep 8(1):1–10
Chan DC (2006) Mitochondria: dynamic organelles in disease, aging, and development. Cell 125(7):1241–1252
Mattson MP, Gleichmann M, Cheng A (2008) Mitochondria in neuroplasticity and neurological disorders. Neuron 60(5):748–766
Ben-Shachar D, Laifenfeld D (2004) Mitochondria, synaptic plasticity, and schizophrenia. In: International review of neurobiology, vol 59. Elsevier, pp 273–296
Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM, Jones EG, Bunney WE et al (2008) Mitochondrial involvement in psychiatric disorders. Ann Med 40(4):281–295
Bulygin KV, Beeraka NM, Saitgareeva AR, Nikolenko VN, Gareev I, Beylerli O, Akhmadeeva LR, Mikhaleva LM et al (2020) Can miRNAs be considered as diagnostic and therapeutic molecules in ischemic stroke pathogenesis?—Current status. Int J Mol Sci 21(18):6728
Prayson RA, Wang N (1998) Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS) syndrome: an autopsy report. Arch Pathol Lab Med 122(11):978
Millar J, Christie S, Anderson S, Lawson D, Loh DH-W, Devon R, Arveiler B, Muir W et al (2001) Genomic structure and localisation within a linkage hotspot of Disrupted In Schizophrenia 1, a gene disrupted by a translocation segregating with schizophrenia. Mol Psychiatry 6(2):173–178
Sachs N, Sawa A, Holmes S, Ross C, DeLisi L, Margolis R (2005) A frameshift mutation in Disrupted in Schizophrenia 1 in an American family with schizophrenia and schizoaffective disorder. Mol Psychiatry 10(8):758–764
Porteous DJ, Thomson P, Millar JK, Evans KL, Hennah W, Soares D, McCarthy S, McCombie W et al (2014) DISC1 as a genetic risk factor for schizophrenia and related major mental illness: response to Sullivan. Mol Psychiatry 19(2):141–143
Sullivan PF (2013) Questions about DISC1 as a genetic risk factor for schizophrenia. Mol Psychiatry 18(10):1050–1052
Brandon NJ, Millar JK, Korth C, Sive H, Singh KK, Sawa A (2009) Understanding the role of DISC1 in psychiatric disease and during normal development. J Neurosci 29(41):12768–12775
Chubb J, Bradshaw NJ, Soares D, Porteous D, Millar J (2008) The DISC locus in psychiatric illness. Mol Psychiatry 13(1):36–64
Martorell L, Segués T, Folch G, Valero J, Joven J, Labad A, Vilella E (2006) New variants in the mitochondrial genomes of schizophrenic patients. Eur J Hum Genet 14(5):520–528
Whatley S, Curti D, Gupta FD, Ferrier I, Jones S, Taylor C, Marchbanks R (1998) Superoxide, neuroleptics and the ubiquinone and cytochrome b 5 reductases in brain and lymphocytes from normals and schizophrenic patients. Mol Psychiatry 3(3):227–237
Dröge W, Schipper HM (2007) Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell 6(3):361–370
Parikh V, Khan MM, Mahadik SP (2003) Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res 37(1):43–51
Kazuno A-a, Munakata K, Nagai T, Shimozono S, Tanaka M, Yoneda M, Kato N, Miyawaki A et al (2006) Identification of mitochondrial DNA polymorphisms that alter mitochondrial matrix pH and intracellular calcium dynamics. PLoS Genet 2(8):e128
Ueno H, Nishigaki Y, Kong Q-P, Fuku N, Kojima S, Iwata N, Ozaki N, Tanaka M (2009) Analysis of mitochondrial DNA variants in Japanese patients with schizophrenia. Mitochondrion 9(6):385–393
Munakata K, Iwamoto K, Bundo M, Kato T (2005) Mitochondrial DNA 3243A> G mutation and increased expression of LARS2 gene in the brains of patients with bipolar disorder and schizophrenia. Biol Psychiat 57(5):525–532
Ni P, Chung S (2020) Mitochondrial dysfunction in schizophrenia. BioEssays 42(6):1900202
Park C, Park SK (2012) Molecular links between mitochondrial dysfunctions and schizophrenia. Mol Cells 33(2):105–110
Martins-de-Souza D, Harris LW, Guest PC, Bahn S (2011) The role of energy metabolism dysfunction and oxidative stress in schizophrenia revealed by proteomics. Antioxid Redox Signal 15(7):2067–2079
Martins-de-Souza D, Gattaz WF, Dias-Neto E (2011) What does proteomics tell us about schizophrenia? In: Handbook of Schizophrenia Spectrum Disorders, Volume I. Springer, pp 345–366
Prabakaran S, Swatton J, Ryan M, Huffaker S, Huang J-J, Griffin J, Wayland M, Freeman T et al (2004) Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry 9(7):684–697
Maas D, Vallès A, Martens G (2017) Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl Psychiatry 7(7):e1171–e1171
Bonora M, De Marchi E, Patergnani S, Suski J, Celsi F, Bononi A, Giorgi C, Marchi S et al (2014) Tumor necrosis factor-α impairs oligodendroglial differentiation through a mitochondria-dependent process. Cell Death Differ 21(8):1198–1208
Zou Y, Jiang W, Wang J, Li Z, Zhang J, Bu J, Zou J, Zhou L et al (2014) Oligodendrocyte precursor cell-intrinsic effect of Rheb1 controls differentiation and mediates mTORC1-dependent myelination in brain. J Neurosci 34(47):15764–15778
Cao K, Zheng A, Xu J, Li H, Liu J, Peng Y, Long J, Zou X et al (2014) AMPK activation prevents prenatal stress-induced cognitive impairment: modulation of mitochondrial content and oxidative stress. Free Radical Biol Med 75:156–166
Chen Y, Lu R, Zheng H, Xiao R, Feng J, Wang H, Gao X, Guo L (2015) The NFKB1 polymorphism (rs4648068) is associated with the cell proliferation and motility in gastric cancer. BMC Gastroenterol 15(1):21
Glauert HP, Calfee-Mason K, Li Y, Nilakantan V, Twaroski ML, Tharappel J, Spear BT (2008) The role of NF-κB in PPARα-mediated hepatocarcinogenesis. Ppar Research 2008:286249
Li Y, Beeraka NM, Guo W, Lei Y, Hu Q, Guo L, Fan R, Liu J, et al (2021) Prognosis of patients with brainstem glioblastoma based on ‘age, surgery and radiotherapy’: a SEER database analysis
Nikolenko VN, Rizaeva NA, Beeraka NM, Oganesyan MV, Kudryashova VA, Dubovets AA, Borminskaya ID, Bulygin KV et al (2021) The mystery of claustral neural circuits and recent updates on its role in neurodegenerative pathology. Behav Brain Funct 17(1):1–10
Shen Q, Li Z, Sun Y, Wang T, Wan C, Li X, Zhao X, Feng G et al (2008) The role of pro-inflammatory factors in mediating the effects on the fetus of prenatal undernutrition: implications for schizophrenia. Schizophr Res 99(1–3):48–55
Diz-Chaves Y, Astiz M, Bellini MJ, Garcia-Segura LM (2013) Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain Behav Immun 28:196–206
Feigenson KA, Kusnecov AW, Silverstein SM (2014) Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev 38:72–93
Abi-Saab W, D’souza D, Moghaddam B, Krystal J (1998) The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry 31(S2):104–109
Rolland B, Jardri R, Amad A, Thomas P, Cottencin O, Bordet R (2014) Pharmacology of hallucinations: several mechanisms for one single symptom? BioMed Res Int
Coyle JT (2012) NMDA receptor and schizophrenia: a brief history. Schizophr Bull 38(5):920–926
Rolland B, Jardri R, Amad A, Thomas P, Cottencin O, Bordet R (2014) Pharmacology of hallucinations: several mechanisms for one single symptom? BioMed Research International 2014:307106
Goldsmith DR, Rapaport MH (2020) Inflammation and negative symptoms of schizophrenia: Implications for reward processing and motivational deficits. Frontiers in Psychiatry 11:46
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB et al (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51(3):199–214
Lahti AC, Weiler MA, Michaelidis BT, Parwani A, Tamminga CA (2001) Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 25(4):455–467
Hardingham GE, Do KQ (2016) Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci 17(2):125–134
Dogan AE, Yuksel C, Du F, Chouinard V-A, Öngür D (2018) Brain lactate and pH in schizophrenia and bipolar disorder: a systematic review of findings from magnetic resonance studies. Neuropsychopharmacology 43(8):1681–1690
Bergen SE, Petryshen TL (2012) Genome-wide association studies (GWAS) of schizophrenia: does bigger lead to better results? Curr Opin Psychiatry 25(2):76
Ripke S, Neale BM, Corvin A, Walters JT, Farh K-H, Holmans PA, Lee P, Bulik-Sullivan B et al (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511(7510):421
Aliev G, Beeraka NM, Nikolenko VN, Svistunov AA, Rozhnova T, Kostyuk S, Cherkesov I, Gavryushova LV et al (2020) Neurophysiology and psychopathology underlying PTSD and recent insights into the PTSD therapies—a comprehensive review. J Clin Med 9(9):2951
Balu DT (2016) The NMDA receptor and schizophrenia: from pathophysiology to treatment. In: Advances in Pharmacology, vol 76. Elsevier, pp 351–382
Coyle JT, Balu D, Benneyworth M, Basu A, Roseman A (2010) Beyond the dopamine receptor: novel therapeutic targets for treating schizophrenia. Dialogues Clin Neurosci 12(3):359
Schmidt-Kastner R, Guloksuz S, Kietzmann T, Van Os J, Rutten BP (2020) Analysis of GWAS-derived schizophrenia genes for links to ischemia-hypoxia response of the brain. Front Psych 11:393
Bitanihirwe BK, Mauney SA, Woo T-UW (2016) Weaving a net of neurobiological mechanisms in schizophrenia and unraveling the underlying pathophysiology. Biol Psychiat 80(8):589–598
Goff DC, Romero K, Paul J, Perez-Rodriguez MM, Crandall D, Potkin SG (2016) Biomarkers for drug development in early psychosis: current issues and promising directions. Eur Neuropsychopharmacol 26(6):923–937
Noto C, Ota VK, Gadelha A, Noto MN, Barbosa DS, Bonifácio KL, Nunes SO, Cordeiro Q et al (2015) Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res 68:210–216
Davis J, Moylan S, Harvey BH, Maes M, Berk M (2014) Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry 48(6):512–529
Gallego JA, Blanco EA, Husain-Krautter S, Fagen EM, Moreno-Merino P, del Ojo-Jiménez JA, Ahmed A, Rothstein TL et al (2018) Cytokines in cerebrospinal fluid of patients with schizophrenia spectrum disorders: new data and an updated meta-analysis. Schizophr Res 202:64–71
Garver DL, Tamas RL, Holcomb JA (2003) Elevated interleukin-6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology 28(8):1515–1520
O’Rourke R, Kay T, Lyle E, Traxler S, Deveney C, Jobe B, Roberts C Jr, Marks D et al (2006) Alterations in peripheral blood lymphocyte cytokine expression in obesity. Clin Exp Immunol 146(1):39–46
Chase KA, Cone JJ, Rosen C, Sharma RP (2016) The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry 16(1):1–7
Jordan W, Dobrowolny H, Bahn S, Bernstein H-G, Brigadski T, Frodl T, Isermann B, Lessmann V et al (2018) Oxidative stress in drug-naïve first episode patients with schizophrenia and major depression: effects of disease acuity and potential confounders. Eur Arch Psychiatry Clin Neurosci 268(2):129–143
Dwir D, Giangreco B, Xin L, Tenenbaum L, Cabungcal J-H, Steullet P, Goupil A, Cleusix M, Jenni R, Baumann PS (2019) MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: a reverse translation study in schizophrenia patients. Molecular psychiatry 25(11):2889–2904
Sarandol A, Kirli S, Akkaya C, Altin A, Demirci M, Sarandol E (2007) Oxidative–antioxidative systems and their relation with serum S100 B levels in patients with schizophrenia: effects of short term antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry 31(6):1164–1169
Liu M-L, Zheng P, Liu Z, Xu Y, Mu J, Guo J, Huang T, Meng H-Q et al (2014) GC-MS based metabolomics identification of possible novel biomarkers for schizophrenia in peripheral blood mononuclear cells. Mol BioSyst 10(9):2398–2406
Kim S-Y, Cohen BM, Chen X, Lukas SE, Shinn AK, Yuksel AC, Li T, Du F et al (2017) Redox dysregulation in schizophrenia revealed by in vivo NAD+/NADH measurement. Schizophr Bull 43(1):197–204
Copoglu US, Virit O, Kokacya MH, Orkmez M, Bulbul F, Erbagci AB, Semiz M, Alpak G et al (2015) Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res 229(1–2):200–205
Lijuan Huo XL, Fengchun Wu, Chang C, Ning Y, Zhang XY (November 2021) Elevated activity of superoxide dismutase in male late-life schizophrenia and its correlation with clinical symptoms and cognitive deficits. BMC Psychiatry 21:606
Đorđević VV, Lazarević D, Ćosić V, Knežević MZ, Đorđević VB (2017) Age-related changes of superoxide dismutase activity in patients with schizophrenia. Vojnosanit Pregl 74(1):31–37
Radonjić NV, Knežević ID, Vilimanovich U, Kravić-Stevović T, Marina LV, Nikolić T, Todorović V, Bumbaširević V et al (2010) Decreased glutathione levels and altered antioxidant defense in an animal model of schizophrenia: long-term effects of perinatal phencyclidine administration. Neuropharmacology 58(4–5):739–745
Reddy R, Keshavan M, Yao JK (2003) Reduced plasma antioxidants in first-episode patients with schizophrenia. Schizophr Res 62(3):205–212
Ohnuma T, Nishimon S, Takeda M, Sannohe T, Katsuta N, Arai H (2018) Carbonyl stress and microinflammation-related molecules as potential biomarkers in schizophrenia. Front Psych 9:82
Noto C, Maes M, Ota VK, Teixeira AL, Bressan RA, Gadelha A, Brietzke E (2015) High predictive value of immune-inflammatory biomarkers for schizophrenia diagnosis and association with treatment resistance. The World Journal of Biological Psychiatry 16(6):422–429
Noto C, Gadelha A, Belangero SI, Spindola LM, Rocha NP, de Miranda AS, Teixeira AL, Smith MAC et al (2013) Circulating levels of sTNFR1 as a marker of severe clinical course in schizophrenia. J Psychiatr Res 47(4):467–471
Nishimon S, Ohnuma T, Takebayashi Y, Katsuta N, Takeda M, Nakamura T, Sannohe T, Higashiyama R et al (2017) High serum soluble tumor necrosis factor receptor 1 predicts poor treatment response in acute-stage schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 76:145–154
Haack M, Hinze-Selch D, Fenzel T, Kraus T, Kühn M, Schuld A, Pollmächer T (1999) Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res 33(5):407–418
Cuenod M, Steullet P, Cabungcal J-H, Dwir D, Khadimallah I, Klauser P, Conus P, Do KQ (2021) Caught in vicious circles: a perspective on dynamic feed-forward loops driving oxidative stress in schizophrenia. Molecular Psychiatry 1–12
Pérez-Santiago J, Diez-Alarcia R, Callado LF, Zhang JX, Chana G, White CH, Glatt SJ, Tsuang MT et al (2012) A combined analysis of microarray gene expression studies of the human prefrontal cortex identifies genes implicated in schizophrenia. J Psychiatr Res 46(11):1464–1474
Karagiannis A, Gallopin T, Dávid C, Battaglia D, Geoffroy H, Rossier J, Hillman EM, Staiger JF et al (2009) Classification of NPY-expressing neocortical interneurons. J Neurosci 29(11):3642–3659
Kubota Y (2014) Untangling GABAergic wiring in the cortical microcircuit. Curr Opin Neurobiol 26:7–14
Tremblay R, Lee S, Rudy B (2016) GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91(2):260–292
Vruwink M, Schmidt HH, Weinberg RJ, Burette A (2001) Substance P and nitric oxide signaling in cerebral cortex: anatomical evidence for reciprocal signaling between two classes of interneurons. Journal of Comparative Neurology 441(4):288–301
Bowen EF, Burgess JL, Granger R, Kleinman JE, Rhodes CH (2019) DLPFC transcriptome defines two molecular subtypes of schizophrenia. Transl Psychiatry 9(1):1–10
Cardis R, Cabungcal J-H, Dwir D, Do KQ, Steullet P (2018) A lack of GluN2A-containing NMDA receptors confers a vulnerability to redox dysregulation: consequences on parvalbumin interneurons, and their perineuronal nets. Neurobiol Dis 109:64–75
Nakazawa K, Sapkota K (2020) The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol Ther 205:107426
Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, Deppen P, Preisig M et al (2007) Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci 104(42):16621–16626
Baxter PS, Bell KF, Hasel P, Kaindl AM, Fricker M, Thomson D, Cregan SP, Gillingwater TH et al (2015) Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat Commun 6(1):1–13
Mubarak B, Soriano FX, Hardingham GE (2009) Synaptic NMDAR activity suppresses FOXO1 expression via a cis-acting FOXO binding site: FOXO1 is a FOXO target gene. Channels 3(4):233–239
Mumtaz F, Khan MI, Zubair M, Dehpour AR (2018) Neurobiology and consequences of social isolation stress in animal model—a comprehensive review. Biomed Pharmacother 105:1205–1222
Yao JK, Keshavan MS (2011) Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxidants & redox signaling 5(7):2011–2035
Sampaio LRL, Cysne Filho FMS, de Almeida JC, dos Santos DD, Patrocínio CFV, de Sousa CNS, Patrocínio MCA, Macêdo D et al (2018) Advantages of the alpha-lipoic acid association with chlorpromazine in a model of schizophrenia induced by ketamine in rats: behavioral and oxidative stress evidences. Neuroscience 373:72–81
Zuo D-Y, Wu Y-L, Yao W-X, Cao Y, Wu C-F, Tanaka M (2007) Effect of MK-801 and ketamine on hydroxyl radical generation in the posterior cingulate and retrosplenial cortex of free-moving mice, as determined by in vivo microdialysis. Pharmacol Biochem Behav 86(1):1–7
Wesseling H, Want EJ, Guest PC, Rahmoune H, Holmes E, Bahn S (2015) Hippocampal proteomic and metabonomic abnormalities in neurotransmission, oxidative stress, and apoptotic pathways in a chronic phencyclidine rat model. J Proteome Res 14(8):3174–3187
Genius J, Geiger J, Dölzer A-L, Benninghoff J, Giegling I, Hartmann AM, Möller H-J, Rujescu D (2013) Glutamatergic dysbalance and oxidative stress in in vivo and in vitro models of psychosis based on chronic NMDA receptor antagonism. PloS one 8(7):e59395
Gunduz-Bruce H (2009) The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev 60(2):279–286
Frodl T, Amico F (2014) Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Prog Neuropsychopharmacol Biol Psychiatry 48:295–303
Kayser MS, Dalmau J (2016) Anti-NMDA receptor encephalitis, autoimmunity, and psychosis. Focus 14(4):510–515
Nerurkar A, Bitton A, Davis RB, Phillips RS, Yeh G (2013) When physicians counsel about stress: results of a national study. JAMA Intern Med 173(1):76–77
Steullet P, Cabungcal J, Monin A, Dwir D, O’donnell P, Cuenod M, Do K (2016) Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? Schizophr Res 176(1):41–51
Bhandari R, Kaur J, Kaur S, Kuhad A (2021) The Nrf2 pathway in psychiatric disorders: pathophysiological role and potential targeting. Expert Opin Ther Targets 25(2):115–139
Zhang J-c, Yao W, Dong C, Han M, Shirayama Y, Hashimoto K (2018) Keap1–Nrf2 signaling pathway confers resilience versus susceptibility to inescapable electric stress. Eur Arch Psychiatry Clin Neurosci 268(8):865–870
Behrens MM, Ali SS, Dugan LL (2008) Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci 28(51):13957–13966
Sorce S, Schiavone S, Tucci P, Colaianna M, Jaquet V, Cuomo V, Dubois-Dauphin M, Trabace L et al (2010) The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci 30(34):11317–11325
Barron H, Hafizi S, Andreazza AC, Mizrahi R (2017) Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int J Mol Sci 18(3):651
Hou Y, Zhang H, Xie G, Cao X, Zhao Y, Liu Y, Mao Z, Yang J et al (2013) Neuronal injury, but not microglia activation, is associated with ketamine-induced experimental schizophrenic model in mice. Prog Neuropsychopharmacol Biol Psychiatry 45:107–116
Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H, Chen L, Li M et al (2018) Antidepressant effect of repeated ketamine administration on kynurenine pathway metabolites in patients with unipolar and bipolar depression. Brain Behav Immun 74:205–212
Reus GZ, Becker IR, Scaini G, Petronilho F, Oses JP, Kaddurah-Daouk R, Ceretta LB, Zugno AI et al (2018) The inhibition of the kynurenine pathway prevents behavioral disturbances and oxidative stress in the brain of adult rats subjected to an animal model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 81:55–63
Das TK, Javadzadeh A, Dey A, Sabesan P, Théberge J, Radua J, Palaniyappan L (2019) Antioxidant defense in schizophrenia and bipolar disorder: a meta-analysis of MRS studies of anterior cingulate glutathione. Prog Neuropsychopharmacol Biol Psychiatry 91:94–102
Demro C, Rowland L, Wijtenburg SA, Waltz J, Gold J, Kline E, Thompson E, Reeves G et al (2017) Glutamatergic metabolites among adolescents at risk for psychosis. Psychiatry Res 257:179–185
Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E, Takanashi J, Matsuda T, Shimizu E, Ikehira H (2008) Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PloS one 3(4):e1944
Tsai M-C, Liou C-W, Lin T-K, Lin I-M, Huang T-L (2013) Changes in oxidative stress markers in patients with schizophrenia: the effect of antipsychotic drugs. Psychiatry Res 209(3):284–290
Şimşek Ş, Gençoğlan S, Yüksel T, Kaplan İ, Alaca R, Aktaş H (2016) Oxidative stress and DNA damage in untreated first-episode psychosis in adolescents. Neuropsychobiology 73(2):92–97
Miljevic C, Nikolic M, Nikolic-Kokic A, Jones DR, Niketic V, Lecic-Tosevski D, Spasic MB (2010) Lipid status, anti-oxidant enzyme defence and haemoglobin content in the blood of long-term clozapine-treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 34(2):303–307
Steullet P, Lavoie S, Kraftsik R, Guidi R, Gysin R, Cuénod M, Do KQ (2008) A glutathione deficit alters dopamine modulation of L-type calcium channels via D2 and ryanodine receptors in neurons. Free Radical Biol Med 44(6):1042–1054
Stojković T, Radonjić NV, Velimirović M, Jevtić G, Popović V, Doknić M, Petronijević ND (2012) Risperidone reverses phencyclidine induced decrease in glutathione levels and alterations of antioxidant defense in rat brain. Prog Neuropsychopharmacol Biol Psychiatry 39(1):192–199
Breier A, Liffick E, Hummer TA, Vohs JL, Yang Z, Mehdiyoun NF, Visco AC, Metzler E et al (2018) Effects of 12-month, double-blind N-acetyl cysteine on symptoms, cognition and brain morphology in early phase schizophrenia spectrum disorders. Schizophr Res 199:395–402
Bulut M, Savas HA, Altindag A, Virit O, Dalkilic A (2009) Beneficial effects of N-acetylcysteine in treatment resistant schizophrenia. The World Journal of Biological Psychiatry 10(4–2):626–628
Klauser P, Xin L, Fournier M, Griffa A, Cleusix M, Jenni R, Cuenod M, Gruetter R et al (2018) N-acetylcysteine add-on treatment leads to an improvement of fornix white matter integrity in early psychosis: a double-blind randomized placebo-controlled trial. Transl Psychiatry 8(1):1–8
Cabungcal J-H, Steullet P, Kraftsik R, Cuenod M, Do KQ (2013) Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiat 73(6):574–582
Phensy A, Duzdabanian HE, Brewer S, Panjabi A, Driskill C, Berz A, Peng G, Kroener S (2017) Antioxidant treatment with N-acetyl cysteine prevents the development of cognitive and social behavioral deficits that result from perinatal ketamine treatment. Front Behav Neurosci 11:106
Bošković M, Vovk T, Koprivšek J, Plesničar BK, Grabnar I (2016) Vitamin E and essential polyunsaturated fatty acids supplementation in schizophrenia patients treated with haloperidol. Nutr Neurosci 19(4):156–161
Faizi M, Salimi A, Rasoulzadeh M, Naserzadeh P, Pourahmad J (2014) Schizophrenia induces oxidative stress and cytochrome C release in isolated rat brain mitochondria: a possible pathway for induction of apoptosis and neurodegeneration. Iran J Pharm Res 13(Suppl):93
Özyurt H, Özyurt B, Sarsılmaz M, Kuş İ, Songür A, Akyol Ö (2014) Potential role of some oxidant/antioxidant status parameters in prefrontal cortex of rat brain in an experimental psychosis model and the protective effects of melatonin 18(15):2137–2144
Pertierra LR, Hughes KA, Vega GC, Olalla-Tárraga MÁ (2017) High resolution spatial mapping of human footprint across Antarctica and its implications for the strategic conservation of avifauna. PloS One 12(1):e0168280
Vickers NJ (2017) Animal communication: when i’m calling you, will you answer too? Curr Biol 27(14):R713–R715
Kulak A, Cuenod M, Do KQ (2012) Behavioral phenotyping of glutathione-deficient mice: relevance to schizophrenia and bipolar disorder. Behav Brain Res 226(2):563–570
Steullet P, Cabungcal J, Bukhari S, Ardelt M, Pantazopoulos H, Hamati F, Salt T, Cué nod M et al (2018) The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbuminexpressing neuron networks and oxidative stress. Mol Psychiatry 23:2057–2065
Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K (2010) Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci 13(1):76
Jadi MP, Behrens MM, Sejnowski TJ (2016) Abnormal gamma oscillations in N-methyl-D-aspartate receptor hypofunction models of schizophrenia. Biol Psychiat 79(9):716–726
Tatard-Leitman VM, Jutzeler CR, Suh J, Saunders JA, Billingslea EN, Morita S, White R, Featherstone RE et al (2015) Pyramidal cell selective ablation of N-methyl-D-aspartate receptor 1 causes increase in cellular and network excitability. Biol Psychiat 77(6):556–568
Huria T, Beeraka NM, Al-Ghamdi B, Fern R (2015) Premyelinated central axons express neurotoxic NMDA receptors: relevance to early developing white-matter injury. J Cereb Blood Flow Metab 35(4):543–553
Huria T, Beeraka N, Elgradawi M, Elzwei S (2005) Picrotoxin (GABAA receptor antagonist) shows a protective role in brain injury during neonatal development
Lewis DA, Curley AA, Glausier JR, Volk DW (2012) Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35(1):57–67
Anderson KM, Collins MA, Chin R, Ge T, Rosenberg MD, Holmes AJ (2020) Transcriptional and imaging-genetic association of cortical interneurons, brain function, and schizophrenia risk. Nat Commun 11(1):1–15
Behrens MM, Sejnowski TJ (2009) Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology 57(3):193–200
Jiang Z, Cowell RM, Nakazawa K (2013) Convergence of genetic and environmental factors on parvalbumin-positive interneurons in schizophrenia. Front Behav Neurosci 7:116
Steullet P, Cabungcal J, Coyle J, Didriksen M, Gill K, Grace A, Hensch T, LaMantia A et al (2017) Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol Psychiatry 22(7):936–943
Powell SB, Sejnowski TJ, Behrens MM (2012) Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology 62(3):1322–1331
Yolland CO, Hanratty D, Neill E, Rossell SL, Berk M, Dean OM, Castle DJ, Tan EJ et al (2020) Meta-analysis of randomised controlled trials with N-acetylcysteine in the treatment of schizophrenia. Aust N Z J Psychiatry 54(5):453–466
Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F et al (2008) N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiat 64(5):361–368
Farokhnia M, Azarkolah A, Adinehfar F, Khodaie-Ardakani M-R, Yekehtaz H, Tabrizi M, Rezaei F, Salehi B et al (2013) N-acetylcysteine as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol 36(6):185–192
Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI et al (2008) Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology 33(9):2187–2199
Zheng W, Zhang QE, Cai DB, Yang XH, Qiu Y, Ungvari G, Ng C, Berk M et al (2018) N-acetylcysteine for major mental disorders: a systematic review and meta-analysis of randomized controlled trials. Acta Psychiatr Scand 137(5):391–400
Rapado-Castro M, Dodd S, Bush A, Malhi G, Skvarc D, On Z, Berk M, Dean O (2017) Cognitive effects of adjunctive N-acetyl cysteine in psychosis. Psychol Med 47(5):866–876
Sepehrmanesh Z, Heidary M, Akasheh N, Akbari H, Heidary M (2018) Therapeutic effect of adjunctive N-acetyl cysteine (NAC) on symptoms of chronic schizophrenia: a double-blind, randomized clinical trial. Prog Neuropsychopharmacol Biol Psychiatry 82:289–296
Conus P, Seidman LJ, Fournier M, Xin L, Cleusix M, Baumann PS, Ferrari C, Cousins A et al (2018) N-acetylcysteine in a double-blind randomized placebo-controlled trial: toward biomarker-guided treatment in early psychosis. Schizophr Bull 44(2):317–327
McQueen G, Lay A, Lally J, Gabay AS, Collier T, Lythgoe DJ, Barker GJ, Stone JM et al (2020) Effect of single dose N-acetylcysteine administration on resting state functional connectivity in schizophrenia. Psychopharmacology 237(2):443–451
Singh V, Singh SP, Chan K (2010) Review and meta-analysis of usage of ginkgo as an adjunct therapy in chronic schizophrenia. Int J Neuropsychopharm 13(2):257–271
Doruk A, Uzun Ö, Özsahin A (2008) A placebo-controlled study of extract of ginkgo biloba added to clozapine in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol 23(4):223–227
Zhang W-F, Tan Y-L, Zhang X-Y, Chan RC, Wu H-R, Zhou D-F (2010) Extract of Ginkgo biloba treatment for tardive dyskinesia in schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 71 (5):0–0
Rathbone J, Zhang L, Zhang M, Xia J, Liu X, Yang Y (2005) Chinese herbal medicine for schizophrenia. Cochrane Database of Systematic Reviews 4(190):379–384
Amiri A, Noorbala AA, Nejatisafa AA, Ghoreishi A, Derakhshan MK, Khodaie-Ardakani MR, Hajiazim M, Raznahan M et al (2008) Efficacy of selegiline add on therapy to risperidone in the treatment of the negative symptoms of schizophrenia: a double-blind randomized placebo-controlled study. Hum Psychopharmacol Clin Exp 23(2):79–86
Ermakov EA, Dmitrieva EM, Parshukova DA, Kazantseva DV, Vasilieva AR, Smirnova LP (2021) Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxidative Medicine and Cellular Longevity 8881770
Bodkin JA, Siris SG, Bermanzohn PC, Hennen J, Cole JO (2005) Double-blind, placebo-controlled, multicenter trial of selegiline augmentation of antipsychotic medication to treat negative symptoms in outpatients with schizophrenia. Am J Psychiatry 162(2):388–390
Akhondzadeh S, Safarcherati A, Amini H (2005) Beneficial antipsychotic effects of allopurinol as add-on therapy for schizophrenia: a double blind, randomized and placebo controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 29(2):253–259
Brunstein MG, Ghisolfi ES, Ramos FL, Lara DR (2005) A clinical trial of adjuvant allopurinol therapy for moderately refractory schizophrenia. J Clin Psychiatry 66(2):213–219
Dickerson FB, Stallings CR, Origoni AE, Sullens A, Khushalani S, Sandson N, Yolken RH (2009) A double-blind trial of adjunctive allopurinol for schizophrenia. Schizophr Res 109(1–3):66–69
Weiser M, Gershon AA, Rubinstein K, Petcu C, Ladea M, Sima D, Podea D, Keefe RS et al (2012) A randomized controlled trial of allopurinol vs. placebo added on to antipsychotics in patients with schizophrenia or schizoaffective disorder. Schizophr Res 138(1):35–38
Adler LA (1993) Vitamin E in tardive dyskinesia: time course of effect after. Psychopharmacol Bull 29(3):371
Adler LA, Edson R, Lavori P, Peselow E, Duncan E, Rosenthal M, Rotrosen J (1998) Long-term treatment effects of vitamin E for tardive dyskinesia. Biol Psychiat 43(12):868–872
Lohr JB, Caligiuri MP (1996) A double-blind placebo-controlled study of vitamin E treatment of tardive dyskinesia. J Clin Psychiatry 57(4):167–173
Zhang XY, Zhou DF, Cao LY, Xu CQ, Wu GY (2004) The effect of vitamin E treatment on tardive dyskinesia and blood superoxide dismutase: a double-blind placebo-controlled trial. J Clin Psychopharmacol 24(1):83–86
Sajjad SHA (1998) Vitamin E in the treatment of tardive dyskinesia: a preliminary study over 7 months at different doses. International Clinical Psychopharmacology 13(4):147–55
Soares‐Weiser K, Maayan N, Bergman H (2018) Vitamin E for antipsychotic‐induced tardive dyskinesia. Cochrane Database of Systematic Reviews 1(1):CD000209
Dakhale G, Khanzode S, Khanzode S, Saoji A (2005) Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology 182(4):494–498
Michael N, Sourgens H, Arolt V, Erfurth A (2002) Severe tardive dyskinesia in affective disorders: treatment with vitamin E and C. Neuropsychobiology 46(Suppl. 1):28–30
Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP (2003) Supplementation with a combination of ω-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr Res 62(3):195–204
Sivrioglu E, Kirli S, Sipahioglu D, Gursoy B, Sarandöl E (2007) The impact of ω-3 fatty acids, vitamins E and C supplementation on treatment outcome and side effects in schizophrenia patients treated with haloperidol: An open-label pilot study. Prog Neuropsychopharmacol Biol Psychiatry 31(7):1493–1499
Emsley R, Myburgh C, Oosthuizen P, van Rensburg SJ (2002) Randomized, placebo-controlled study of ethyl-eicosapentaenoic acid as supplemental treatment in schizophrenia. Am J Psychiatry 159(9):1596–1598
Emsley R, Niehaus DJ, Koen L, Oosthuizen PP, Turner HJ, Carey P, van Rensburg SJ, Maritz JS et al (2006) The effects of eicosapentaenoic acid in tardive dyskinesia: a randomized, placebo-controlled trial. Schizophr Res 84(1):112–120
Fenton WS, Dickerson F, Boronow J, Hibbeln JR, Knable M (2001) A placebo-controlled trial of omega-3 fatty acid (ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. Am J Psychiatry 158(12):2071–2074
Peet M, Brind J, Ramchand C, Shah S, Vankar G (2001) Two double-blind placebo-controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophr Res 49(3):243–251
Pawełczyk T, Grancow M, Kotlicka-Antczak M, Trafalska E, Gębski P, Szemraj J, Żurner N, Pawełczyk A (2015) Omega-3 fatty acids in first-episode schizophrenia-a randomized controlled study of efficacy and relapse prevention (OFFER): rationale, design, and methods. BMC Psychiatry 15(1):1–13
Sedlak TW, Nucifora LG, Koga M, Shaffer LS, Higgs C, Tanaka T, Wang AM, Coughlin JM et al (2017) Sulforaphane augments glutathione and influences brain metabolites in human subjects: a clinical pilot study. Mol Neuropsychiatry 3(4):214–222
Shiina A, Kanahara N, Sasaki T, Oda Y, Hashimoto T, Hasegawa T, Yoshida T, Iyo M et al (2015) An open study of sulforaphane-rich broccoli sprout extract in patients with schizophrenia. Clin Psychopharm Neurosci 13(1):62
Shirai Y, Fujita Y, Hashimoto R, Ohi K, Yamamori H, Yasuda Y, Ishima T, Suganuma H et al (2015) Dietary intake of sulforaphane-rich broccoli sprout extracts during juvenile and adolescence can prevent phencyclidine-induced cognitive deficits at adulthood. PLoS One 10(6):e0127244
Xu L-L, Wu Y-F, Yan F, Li C-C, Dai Z, You Q-D, Jiang Z-Y, Di B (2019) 5-(3, 4-Difluorophenyl)-3-(6-methylpyridin-3-yl)-1, 2, 4-oxadiazole (DDO-7263), a novel Nrf2 activator targeting brain tissue, protects against MPTP-induced subacute Parkinson’s disease in mice by inhibiting the NLRP3 inflammasome and protects PC12 cells against oxidative stress. Free Radical Biol Med 134:288–303
Miodownik C, Lerner V, Kudkaeva N, Lerner PP, Pashinian A, Bersudsky Y, Eliyahu R, Kreinin A et al (2019) Curcumin as add-on to antipsychotic treatment in patients with chronic schizophrenia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol 42(4):117–122
Zortea K, Franco VC, Francesconi LP, Cereser KM, Lobato MIR, Belmonte-de-Abreu PS (2016) Resveratrol supplementation in schizophrenia patients: a randomized clinical trial evaluating serum glucose and cardiovascular risk factors. Nutrients 8(2):73
Cao Y, Yan Z, Zhou T, Wang G (2017) SIRT1 regulates cognitive performance and ability of learning and memory in diabetic and nondiabetic models. Journal of Diabetes Research 7121827
Magaji MG, Iniaghe LO, Abolarin M, Abdullahi OI, Magaji RA (2017) Neurobehavioural evaluation of resveratrol in murine models of anxiety and schizophrenia. Metab Brain Dis 32(2):437–442
Huang Q, Ye X, Wang L, Pan J (2019) Salvianolic acid B abolished chronic mild stress-induced depression through suppressing oxidative stress and neuro-inflammation via regulating NLRP3 inflammasome activation. J Food Biochem 43(3):e12742
Yu L, An C, Jia L, Li Y, Chen Q, Zhen F, Wang S, Wang M (2016) Combination therapy of salvianolic acid and fluoxetine improves the cognitive function of rats with chronic stress-induced depression. World Neurosurg 86:173–180
Liao D, Chen Y, Guo Y, Wang C, Liu N, Gong Q, Fu Y, Fu Y et al (2020) Salvianolic acid B improves chronic mild stress-induced depressive behaviors in rats: involvement of AMPK/SIRT1 signaling pathway. J Inflamm Res 13:195
Jiang W-L, Cai D-B, Yin F, Zhang L, Zhao X-W, He J, Ng CH, Ungvari GS et al (2020) Adjunctive metformin for antipsychotic-induced dyslipidemia: a meta-analysis of randomized, double-blind, placebo-controlled trials. Transl Psychiatry 10(1):1–9
Pérez-Torres I, Guarner-Lans V, Rubio-Ruiz ME (2017) Reductive stress in inflammation-associated diseases and the pro-oxidant effect of antioxidant agents. Int J Mol Sci 18(10):2098
Large CH (2007) Do NMDA receptor antagonist models of schizophrenia predict the clinical efficacy of antipsychotic drugs? J Psychopharmacol 21(3):283–301
Kriisa K, Haring L, Vasar E, Koido K, Janno S, Vasar V, Zilmer K, Zilmer M (2016) Antipsychotic treatment reduces indices of oxidative stress in first-episode psychosis patients. Oxidative medicine and cellular longevity 9616593
Flatow J, Buckley P, Miller BJ (2013) Meta-analysis of oxidative stress in schizophrenia. Biol Psychiat 74(6):400–409
Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, Arndt T, Bäckers L et al (2019) Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 394(10202):939–951
Chikowe I, Domingo M, Mwakaswaya V, Parveen S, Mafuta C, Kampira E (2019) Adverse drug reactions experienced by out-patients taking chlorpromazine or haloperidol at Zomba Mental Hospital. Malawi BMC Research notes 12(1):1–6
Boyda HN, Ho AA, Tse L, Procyshyn RM, Yuen JW, Kim DD, Honer WG, Barr AM (2020) Differential effects of acute treatment with antipsychotic drugs on peripheral catecholamines. Frontiers in Psychiatry 11:617428
Boll KM, Noto C, Bonifácio KL, Bortolasci CC, Gadelha A, Bressan RA, Barbosa DS, Maes M et al (2017) Oxidative and nitrosative stress biomarkers in chronic schizophrenia. Psychiatry Res 253:43–48
Zhang XY, Zhou DF, Qi LY, Chen S, Cao LY, Xiu MH, Wang F, Wu GY et al (2009) Superoxide dismutase and cytokines in chronic patients with schizophrenia: association with psychopathology and response to antipsychotics. Psychopharmacology 204(1):177–184
Bai Z-L, Li X-S, Chen G-Y, Du Y, Wei Z-X, Chen X, Zheng G-E, Deng W et al (2018) Serum oxidative stress marker levels in unmedicated and medicated patients with schizophrenia. J Mol Neurosci 66(3):428–436
Zhang XY, Zhou DF, Shen YC, Zhang PY, Zhang WF, Liang J, Xiu MH, Kosten TA et al (2012) Effects of risperidone and haloperidol on superoxide dismutase and nitric oxide in schizophrenia. Neuropharmacology 62(5–6):1928–1934
Kulaksizoglu B, Kulaksizoglu S (2018) Thiol/disulfide homeostasis in schizophrenic patients. Neurochem J 12(1):102–106
Topcuoglu C, Bakirhan A, Yilmaz FM, Neselioglu S, Erel O, Sahiner SY (2017) Thiol/disulfide homeostasis in untreated schizophrenia patients. Psychiatry Res 251:212–216
Ünal K, Erzin G, Yüksel RN, Alisik M, Erel Ö (2018) Thiol/disulphide homeostasis in schizophrenia patients with positive symptoms. Nord J Psychiatry 72(4):281–284
An H, Du X, Huang X, Qi L, Jia Q, Yin G, Xiao C, Huang X-F et al (2018) Obesity, altered oxidative stress, and clinical correlates in chronic schizophrenia patients. Transl Psychiatry 8(1):1–7
Orlovska-Waast S, Köhler-Forsberg O, Brix SW, Nordentoft M, Kondziella D, Krogh J, Benros ME (2019) Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry 24(6):869–887
González-Blanco L, García-Portilla MP, García-Álvarez L, de la Fuente-Tomás L, García CI, Sáiz PA, Rodríguez-González S, Coto-Montes A et al (2018) Oxidative stress biomarkers and clinical dimensions in first 10 years of schizophrenia. Rev Psiquiatr Salud Ment (Engl Ed) 11(3):130–140
Smaga I, Niedzielska E, Gawlik M, Moniczewski A, Krzek J, Pera J, Filip M, Przegalin E (2015) Pharmacological reports oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2 Depression, anxiety, schizophrenia and autism. Pharmacol Rep 67:569–580
Ng F, Berk M, Dean O, Bush AI (2008) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 11(6):851–876
Nikitina IL, Beeraka NM, Gaisina GG, Bulygin KV, Galimova EF, Galimov SN, Nikolenko VN, Mikhaleva LM, Somasundaram SG, Kirkland CE (2020) In vivo antidepressant efficacy of 3-substituted Thietane-1, 1-dioxide derivative—a preliminary study for novel anti-depression therapy in neurological disorders
Beeraka NM, Doreswamy SH, Sadhu SP, Srinivasan A, Pragada RR, Madhunapantula SV, Aliev G (2020) The role of exosomes in stemness and neurodegenerative diseases—chemoresistant-cancer therapeutics and phytochemicals. Int J Mol Sci 21(18):6818
Cunha AS, Matheus FC, Moretti M, Sampaio TB, Poli A, Santos DB, Colle D, Cunha MP, Blum-Silva CH, Sandjo LP (2016) Agmatine attenuates reserpine-induced oral dyskinesia in mice: role of oxidative stress, nitric oxide and glutamate NMDA receptors. Behav Brain Res 312:64–76
Funding
This work was supported by GALLY International Research Institute, San Antonio, Texas, USA, and by the Russian Academic Excellence project "5–100″ for the Sechenov University, Moscow, Russia.
Author information
Authors and Affiliations
Contributions
Narasimha M Beeraka (NMB), Marco F. Avila-Rodriguez (MAR), and Gjumrakch Aliev (GA) conceptualized, designed the study, collected, analyzed the data, and performed the formal analysis. All of authors’ discussed the analyses, the results and their interpretation, wrote the original manuscript, revised and improved the original draft. All authors have reviewed and approved the manuscript before submission.
Corresponding author
Ethics declarations
Ethics Approval
No animal or human used in this study. All of the data was collected from open scientific and public sources.
Consent to Participate
Not applicable.
Consent for Publication
None.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beeraka, N.M., Avila-Rodriguez, M.F. & Aliev, G. Recent Reports on Redox Stress-Induced Mitochondrial DNA Variations, Neuroglial Interactions, and NMDA Receptor System in Pathophysiology of Schizophrenia. Mol Neurobiol 59, 2472–2496 (2022). https://doi.org/10.1007/s12035-021-02703-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02703-4