Abstract
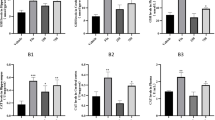
Depression is a disorder with a high incidence that has been increasing worldwide although the pathophysiology remains unclear. Moreover, some studies revealed a higher concentration of glutamate and oxidative stress in the patients’ brain, which causes cell death by excitotoxicity. Morus nigra L. is known as black mulberry and its leaves are popularly used to treat affections related to menopause, obesity and high cholesterol. M. nigra leaves are a rich fount of phenolics which well-known by the antioxidant property. Herein, we examined the phenolic profile and the antidepressant-like effect of the Morus nigra aqueous extract (MN) and its major phenolic constituent, syringic acid (SA). Furthermore, the involvement of antioxidant and neuroprotective activities were further evaluated. Our results show that acute and subchronic MN or SA administration exerted antidepressant-like property in the behavioral testes in mice. The results suggest that the antidepressant-like effect of MN, at least in part, could be due to the SA influence. Moreover, the observed effect involves the nitro-oxidative system modulation in both the serum and brain of mice. Furthermore, MN or SA was able to contain the glutamate-induced cell death in the hippocampal and cortical slices implicating the neuroprotection activity in the antidepressant-like effect.






Similar content being viewed by others
References
Araujo CA, Lucio KP, Silva ME et al (2015) Morus nigra leaf extract improves glycemic response and redox in the liver of diabetic rats. Food Funct 6:3490–3499. doi:10.1039/C5FO00474H
Bai F, Li X, Clay M et al (2001) Intra and interstrain differences in models of “behavioral despair”. Pharmacol Biochem Behav 70:187–192. doi:10.1016/S0091-3057(01)00599-8
Berton O, Nestler EJ (2006) New approaches to antidepressant discovery: beyond monoamines. Neuroscience 7:137–151. doi:10.1038/nrn1846
Cervo L, Rozio M, Ekalle-Soppo CB et al (2002) Role of hyperforin in the antidepressant-like activity of Hypericum perforatum extracts. J Psychopharmacol 164:423–428. doi:10.1007/s00213-002-1229-5
Davison KM, Kaplan BJ (2012) Nutrient intakes are correlated with overall psychiatric functioning in adults with mood disorders. Can J Psychiatr 57:85–92
Diers JA, Ivey KD, El-Alfy A et al (2008) Identification of antidepressant drug leads through the evaluation of marine natural products with neuropsychiatric pharmacophores. Pharmacol Biochem Behav 89:46–53. doi:10.1016/j.pbb.2007.10.021
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Engel DF, Oliveira J, Lopes JB et al (2016) Is there an association between hypercholesterolemia and depression? Behavioral evidence from the LDLr (−/−) mouse experimental model. Behav Brain Res 15:31:318. doi: 10.1016/j.bbr.2016.05.029
Gibson SA, Korade BAZ, Shelton RC (2012) Oxidative stress and glutathione response in tissue cultures from persons with major depression. J Psychiatr Res 46:1326–1332. doi:10.1016/j.jpsychires.2012.06.008
Griess P (1879) Bemerkungen zu der abhandlung der H.H. Weselsky und Benedikt “Ueber einige azoverbindungen.” Ber Dtsch Chem Ges 12:426–428
Gundogdu M, Muradoglu F, Sensoy RIG et al (2011) Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci Hortic 132:37–41. doi:10.1016/j.scienta.2011.09.035
Hashimoto K, Sawa A, Iyo M (2007) Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry 62:1310–1316. doi:10.1016/j.biopsych.2007.03.017
Holzmann I, Silva LM, Silva JAC et al (2015) Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharmacol Biochem Behav 136:55–63. doi:10.1016/j.pbb.2015.07.003
Lee S, Han C, Patkar AA et al (2013) Oxidative/nitrosative stress and antidepressants: targets for novel antidepressants. Prog Neuro-Psychopharmacol Biol Psychiatry 46:224–235. doi:10.1016/j.pnpbp.2012.09.008
Lenzi J, Rodrigues AF, Rós A et al (2015) Ferulic acid chronic treatment exerts antidepressant-like effect:role of antioxidant defense system. Metab Brain Dis 30:1453–1463. doi:10.1007/s11011-015-9725-6
Liao JC, Tsai JC, Chia YL et al (2013) Antidepressant-like activity of turmerone in behavioral despair tests in mice. BMC Complement Altern Med 13:1–8. doi:10.1186/1472-6882-13-299
Lowry OH, Rosebrough NJ, Farr A et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lucca G, Comima CM, Valvassori SS et al (2009) Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int 54:358–362. doi:10.1016/j.neuint.2009.01.001
Maes M (1999) Major depression and activation of the inflammatory response system. Adv Exp Med Biol 461:25–46. doi:10.1007/978-0-585-37970-8_2
Maes M, Galecki P, Chang YS et al (2011) A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuro-Psychopharmacol Biol Psychiatry 35:676–692. doi:10.1016/j.pnpbp.2010.05.004
Malhi TH, Qadir MI, Khan YH et al (2014) Hepatoprotective activity of aqueous methanolic extract of Morus nigra against paracetamol-induced hepatotoxicity in mice. Bangladesh J Pharmacol 9:60–66. doi:10.3329/bjp.v9i1.17337
Mauri MC, Ferrara A, Boscati L et al (1998) Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology 37:124–129. doi:10.1159/000026491
Memon AA, Luthria D, Memon N et al (2010) Phenolic acids profiling and antioxidant potential of mulberry (Morus laevigata W., Morus nigra L., Morus alba L.) leaves and fruits grown in Pakistan. Pol J Food Nutr Sci 60:25–32
Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65:732–741. doi:10.1016/j.biopsych.2008.11.029
Miranda MA, Vieira GD, Alves MS et al (2010) Uso etnomedicinal do chá de Morus nigra L. no tratamento dos sintomas do climatério de mulheres de Muriaé, Minas Gerais, Brasil. HU Revista 36:61–68
Moreira TD (2015) Phytochemical and, hypolipidemic and antioxidant effects study of amoreira preta (Morus nigra L.) aqueous extract on acute hyperlipidemia in rats. Dissertation, Universidade Regional de Blumenau, Brazil
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity. J Immunol Methods 65:55–63
Müller LG, Salles LA, Stein AC et al (2012) Antidepressant-like effect of Valeriana glechomifolia Meyer (Valerianaceae) in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 36:101–109. doi:10.1016/j.pnpbp.2011.08.015
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Oliveira IR, Meersch BV, Dardennes R et al (1990) Self-inhibiting action of nortriptyline’s anti-immobility effect at high plasma and brain levels in mice. J Psychopharmacol 102:553–556
Oliveira ACB, Oliveira AP, Guimarães AL et al (2013) Avaliação toxicológica pré-clínica do chá das folhas de Morus nigra L. (Moraceae). Rev Bras Plantas Med 15:244–249. doi:10.1590/S1516-05722013000200012
Padilha MM, Vilela FC, Silva MJ et al (2009) Antinociceptive effect of the extract of Morus nigra leaves in mice. J Med Food 12:1381–1385. doi:10.1089/jmf.2009.0012
Padilha MM, Vilela FC, Rocha CQ et al (2010) Antiinflammatory properties of Morus nigra leaves. Phytother Res 24:1496–1500. doi:10.1002/ptr.3134
Pandya CD, Howell KR, Pillai A (2013) Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog Neuro-Psychopharmacol Biol Psychiatry 46:214–223. doi:10.1016/j.pnpbp.2012.10.017
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732. doi:10.1038/266730a0
Queiroz GT, Santos TR, Macedo R et al (2012) Efficacy of Morus nigra L. on reproduction in female Wistar rats. Food Chem Toxicol 50:816–822. doi:10.1016/j.fct.2011.12.014
Rekha KG, Selvakumar GP, Sivakamasundari RI (2014) Effects of syringic acid on chronic MPTP/probenecid induced motor dysfunction, dopaminergic markers expression and neuroinflammation in C57BL/6 mice. Biomedicine & Aging Pathology 4:95–104. doi:10.1016/j.biomag.2014.02.004
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Rojas P, Serrano-García N, Medina-Campos ON et al (2011) Antidepressant-like effect of a Ginkgo biloba extract (EGb761) in the mouse forced swimming test: role of oxidative stress. Neurochem Int 59:628–636. doi:10.1016/j.neuint.2011.05.007
Sałat K, Siwek A, Starowicz G et al (2015) Antidepressant-like effects of ketamine, norketamine and dehydronorketamine in forced swim test: role of activity at NMDA receptor. Neuropharmacology 99:301–307. doi:10.1016/j.neuropharm.2015.07.037
Sanacora G, Treccani G, Popoli M (2012) Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77. doi:10.1016/j.neuropharm.2011.07.036
Sánchez-Salcedo EM, Mena P, García-Viguera C et al (2015) (poly) phenolic compounds and antioxidant activity of white (Morus alba) and black (Morus nigra) mulberry leaves: their potential for new products rich in phytochemicals. J Funct Foods 18:1039–1046. doi:10.1016/j.jff.2015.03.053
Savas HA, Gergerlioglu HS, Armutcu F et al (2006) Elevated serum nitric oxide and superoxide dismutase in euthymic bipolar patients: impact of past episodes. World J Biol Psychiatry 7:51–55. doi:10.1080/15622970510029993
Selek S, Savas AH, Gergerlioglu HS et al (2008) The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. J Affect Disord 107:89–94. doi:10.1016/j.jad.2007.08.006
Sharpley AL, McGavin CL, Whale R et al (1998) Antidepressant-like effect of Hypericum perforatum (St John's wort) on the sleep polysomnogram. J Psychopharmacol 139:286–287
Silva FC, Cito MCO, Silva MI et al (2010) Behavioral alterations and pro-oxidant effect of a single ketamine administration to mice. Brain Res Bull 83:9–15. doi:10.1016/j.brainresbull.2010.05.011
Srinivasan S, Muthukumaran J, Muruganathan U et al (2014) Antihyperglycemic effect of syringic acid on attenuating the key enzymes of carbohydrate metabolism in experimental diabetic rats. Biomed Prev Nutr 4:595–602. doi:10.1016/j.bionut.2014.07.010
Steru L, Chermat R, Thierry B et al (1985) The tail suspension test: a new method for screening antidepressants in mice. J Psychopharmacol 85:367–370
Tedders SH, Fokong KD, McKenzie LE et al (2011) Low cholesterol is associated with depression among US household population. J Affect Disord 135:115–121. doi:10.1016/j.jad.2011.06.045
Thierry B, Steru L, Simon P et al (1986) The tail suspension test: ethical considerations. Psychopharmacology 90:284–285
Tyrovolas S, Lionis C, Zeimbekis A et al (2009) Increased body mass and depressive symptomatology are associated with hypercholesterolemia, among elderly individuals; results from the MEDIS study. Lipids Health Dis 8:1–7. doi:10.1186/1476-511X-8-10
Volpato GT, Calderon IM, Sinzato S et al (2011) Effect of Morus nigra aqueous extract treatment on the maternal-fetal outcome, oxidative stress status and lipid profile of streptozotocin-induced diabetic rats. J Ethnopharmacol 138:691–696. doi:10.1016/j.jep.2011.09.044
Wysokinski A, Strzelecki D, Kloszewska I (2015) Levels of triglycerides, cholesterol, LDL, HDL and glucose in patients with schizophrenia, unipolar depression and bipolar disorder. Diabetes Metab Syndr 9:168–176. doi:10.1002/jcp.25488
Xu Y, Ku B-S, Yao HY et al (2005a) The effects of curcumin on depressive-like behaviors in mice. Eur J Pharmacol 518:40–46. doi:10.1016/j.ejphar.2005.06.002
Xu Y, Ku B-S, Yao HY et al (2005b) Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav 82:200–206. doi:10.1016/j.pbb.2005.08.009
Xu Y, Wang Z, You W et al (2010) Antidepressant-like effect of trans-resveratrol: involvement of serotonin and noradrenaline system. Eur Neuropsychopharmacol 20:405–413. doi:10.1016/j.euroneuro.2010.02.013
Zeni AL, Zomkowski AD, Dal-Cim T et al (2011) Antidepressant-like and neuroprotective effects of Aloysia gratissima: investigation of involvement of L-arginine-nitric oxide-cyclic guanosine monophosphate pathway. J Ethnopharmacol 137:864–874. doi:10.1016/j.jep.2011.07.009
Zeni ALB, Dias A, Zomkowski E et al (2012) Ferulic acid exerts antidepressant-like effect in the tail suspension test in mice: evidence for the involvement of the serotonergic system. Eur J Pharmacol 679:68–74. doi:10.1016/j.ejphar.2011.12.041
Zeni ALB, Albuquerque CAC, Gonçalves F et al (2013) Phytochemical profile, toxicity and antioxidant activity of Aloysia gratissima (Verbenaceae). Quim Nova 36:69–73
Zeni ALB, Vandresen-Filho S, Dal-Cim T et al (2014) Aloysia gratissima prevents cellular damage induced by glutamatergic excitotoxicity. J Pharm Pharmacol 66:1294–1302. doi:10.1111/jphp.12250
Zhen L, Zhu J, Zhao X et al (2012) The antidepressant-like effect of fisetin involves the serotonergic and noradrenergic system. Behav Brain Res 228:359–366. doi:10.1016/j.bbr.2011.12.017
Acknowledgements
This work was supported by grants from Universidade Regional de Blumenau (FURB), PIBIC-FURB, PIBIC-CNPq and FAPESC scholarships. The authors thank the English language review by professors Luiz Henrique da Silva and Marta Helena Caetano (FURB). There is a patent request (PI10 2016 029764 8, INPI, Brazil) associated with this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dalmagro, A.P., Camargo, A. & Zeni, A.L.B. Morus nigra and its major phenolic, syringic acid, have antidepressant-like and neuroprotective effects in mice. Metab Brain Dis 32, 1963–1973 (2017). https://doi.org/10.1007/s11011-017-0089-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0089-y