Abstract
The present study aimed to evaluate the antidepressant-like effect of essential oils from Schisandra chinensis (Turcz.) Baill. (SEO) and its possible mechanisms of SEO. The behavioral despair mouse model in vivo and H2O2-induced PC12 cells model in vitro were employed. And the potential effective components were identified by the spectrum-effect relationships analysis. SEO significantly decreased the immobility time in the forced swimming test and tail suspension test, which indicated a promising antidepressant-like effect of SEO in depressed mice. The decreased levels of SOD, GSH, and CAT, and increased levels of MDA were significantly reversed by SEO treatment, which showed good antioxidant activities both in vitro and in vivo. Besides, SEO significantly promoted the nuclear translocation of Nrf2 and the expression of HO-1 in depressed mice and H2O2-induced PC12 cells. The histopathological examination results showed a potential neuronal protective effect of SEO in the hippocampus and cortex. Furthermore, the upregulation of PI3K/AKT/GSK3β signaling was observed after SEO treatment in the H2O2-induced PC12 cells. Additionally, based on the spectrum-effect relationship analysis, 9 peaks were identified as positively correlated with the antioxidant activity of SEO. These results suggested that SEO promoted Nrf2/HO-1 pathway to improve the oxidative stress status and exerted the antidepressant-like effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder (MDD) is one of the most disabling disorders causing a great burden globally on patients and societies. Especially, the COVID-19 pandemic worldwide is concerning, it has affected depressive patients’ physical and mental health fundamentally due to social isolation and lack of care. However, the pathogenesis of MDD remains unclear, oxidative stress, neuroinflammation, gut microbiome disorder, and HPA axis dysfunction can induce the occurrence of depression. The available treatment for depression is still insufficient since 30% of patients are treatment-resistant. Thus, there is an urgent need to find new antidepressants to treat depression.
Traditional Chinese medicine (TCM) has been widely used to treat mood disorders for many years in China. According to TCM theory, herbs systematically alter the physiological functions of the whole body and improve depressive symptoms gradually. Essential oils (EOs) are the main components of TCM with promising pharmacological activities, such as anti-oxidant, anti-bacterial, anti-cancer, and anti-allergic effects. In addition, EOs also have great potential effects on depression, insomnia, and neuronal injury due to the high hydrophobicity which can make EOs are easier to pass through the blood-brain barrier (BBB) and play positive roles in the central nervous system (CNS) disease treatment (Zhang et al. 2021b). However, EOs contain multicomponent which can aim at multiple targets in vivo, so it is difficult to identify the active compounds. Spectrum-effect relationships study has been widely used to screen the effective components in TCM. The fingerprint spectrum is usually obtained by using the GC-MS method. The correlation between the fingerprint spectrum and bioactivity of EOs could locate the possible bioactivity-related chemicals. In this study, the spectrum-effect relationship analysis was applied to identify the components with the anti-oxidant effect of EOs.
Schisandra chinensis (Turcz.) Baill. (SCH) is mainly distributed in East Asia, including China, Russia, and Japan, and has long been used to treat hepatitis, insomnia, and convulsions. Moreover, our lab previously found that lignans of SCH showed good antidepressant-like effects on different depressed animal models. Recent studies reported that EOs of SCH exhibited inhibitory activity on ROS production which could be used as an excellent anti-oxidant (Lee et al. 2021). Therefore, we intend to investigate the antidepressant-like effect of EOs of SCH and explore the detailed anti-oxidant mechanism and identify the components with antidepressant-like effect by the spectrum-effect relationship analysis in the present study.
Materials and methods
Chemicals and reagents
DPPH and ABTS were purchased from Dalian Meilunbio Institute (Dalian, China). ELISA kits used for the determination of MDA, SOD, CAT, and GSH were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other reagents were of analytical grade.
SEO extraction
The fruits of S. Chinensis were purchased from trading companies in China and identified by Professor Ying Jia (Department of Pharmacognosy, Shenyang Pharmaceutical University) according to the guidelines of the Chinese Pharmacopoeia (2020). SEO was obtained using the hydrodistillation system-Clevenger type apparatus. The detailed procedure is as in our previous report (Yan et al. 2021). The SEO was dried over Na2SO4 and stored at 4 °C until further analysis.
Animals and treatment
Adult male C57BL/6 mice (20 ± 2 g) were obtained from the animal center of Shenyang Pharmaceutical University. All mice were allowed to habituate to the novel environment for 1 week prior to the experiment with food and water available ad libitum. All experiments were approved by the animal care committee of Shenyang Pharmaceutical University and performed in compliance with the national institutes of health and institutional guidelines for the humane care of animals. The mice were randomly divided into 4 groups (10 mice/group): Vehicle group, Fluoxetine (Flu) group (15 mg/kg, i.g.), SEO 250 group (250 mg/kg, i.g.) and SEO 750 group (750 mg/kg, i.g.). Flu and SEO were suspended in 0.5% sodium carboxymethylcellulose (CMC-Na), respectively. For the Vehicle group, the animals were given the same amount of 0.5% CMC-Na. The repeated drug treatment was performed once daily and continuously for 9 days.
Forced swimming test (FST)
FST was conducted according to the methods described previously (Armario 2021). In brief, the immobility time of each mouse was detected during the last 4 min of a single 6-min forced swim session.
Tail suspension test (TST)
TST was conducted as described previously with slight modifications (Zhang et al. 2021a). In brief, the immobility time of each mouse was recorded for the last 4 min following 2 min habituation during a 6 min test period.
ELISA assay
After the behavioral tests, the blood was collected and the supernatant was centrifuged at 4000 r/min for 15 min and stored at −80 °C with a rapidly stripped hippocampus and cortex for subsequent assays. The levels of MDA, SOD, CAT, and GSH in serum, liver, brain, and cell supernatant were detected using ELISA kits according to the manufacturer’s protocol.
Western blot
The hippocampus, cortex, and cell supernatant were processed following the western blot procedure (Zhang et al. 2021c). The primary antibodies used in the present study including Nrf2 (Abcam, 1:1000), HO-1 (Abcam, 1:1000), PI3K (CST, 1:1000), p-PI3K (phosphor Y607) (CST, 1:1000), AKT (CST, 1:1000), p-AKT (phosphor Ser 473) (CST, 1:1000), GSK3β (CST, 1:1000), p-GSK3β (phosphor Ser 9) (CST, 1:1000), Lamin B (Proteintech, 1:1000) and β-actin (CST, 1:1000). Anti-rabbit IgG (CST, 1:2000) was used as the secondary antibody. The relative densities were detected using Image-Pro plus 6.0 analysis software.
Histopathological examination
After the behavioral tests, mice brains were removed immediately and fixed in formaldehyde solution, and then stained with hematoxylin and eosin after being cut in the coronal plane. The slices of the hippocampus and cortex were examined under light microscopy.
Drug treatment and cell viability
PC12 cells were cultured in RPMI-1640 with 10% fetal calf serum at 37 °C in an incubator supplemented with 5% CO2. Then, the cells were seeded and pre-incubated with various concentrations of SEO for 24 h, followed by the treatment of 300 μmol/L H2O2 for a further 24 h incubation. Subsequently, 20 μL MTT solution was added, followed by the MTT assay protocol. The experimental groups were as follow: Control, Vehicle (300 μmol/L H2O2), SEO (0.78–25 μg/mL + 300 μmol/L H2O2). SEO and H2O2 were dissolved in DMSO to certain concentrations, and the concentrations were selected by our preliminary experiments, and data was not shown. Fluoxetine (15 μmol/L) served as positive control drug as previously reported (Han and Lee 2009; Park et al. 2021).
Immunofluorescence assay
Transfected PC12 cell slides were deparaffinized and permeabilized with PBS/0.1% Triton X100 as described previously (Liu et al. 2021b). Slides were then incubated with anti-Nrf2 overnight at 4 °C, washed 3 times in PBS, then incubated with secondary antibody at 37 °C for 1 h. Cell nuclei were counterstained with DAPI/Fluorescence quenching agent (Beyotime). Stained cells were observed using a Leica fluorescence microscope.
GC-MS analysis
18 batches of SEO (Table 1) were analyzed by a GC-MS, and the analyses were carried out on devices Agilent 7000C equipped with an HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm). In brief, the injector and detector temperatures were set as 230 °C and 230 °C, respectively. 1.0 μL of the sample was injected using split mode (split ratio 1:50). Helium was used as a carrier gas (1.2 mL/min). The column temperature was programmed as follows: the initial temperature was 60 °C for 2 min, then increased to 110 °C at 5 °C/min and maintained for 10 min, increased to 120 °C at 2 °C/min and maintained for 7 min, and finally increased to 180 °C at 5 °C/min for last 2 min. The total time for analysis was 47 min. The MSD (EI mode) was operated at 70 eV, and the scan range was set to 30–500 amu. Constituents were identified by comparison of their retention indices with those reported in the published literature. Further identification was made by comparison of the mass spectra with those listed in the NIST 2014 MS library. Relative concentrations of components were calculated by the area normalization method.
DPPH assay
DPPH radical scavenging activity of samples was detected as described previously with some modifications (Wang et al. 2021b). Briefly, 2 mL of 0.2 mM DPPH was added to 0.2 mL of various concentrations (2–14 mg/mL) SEO solution. The mixture was shaken vigorously and left to stand for 30 min in the dark, and the absorbance was then measured at 517 nm. The assay was performed in triplicate. Ascorbic acid at the same concentrations was used as a reference. The radical scavenging activity was calculated by the following formula:
Where A0 is the absorbance of the control, A1 is the absorbance in the presence of the extract, and A2 is the absorbance of the sample only. The IC50 value represented the concentration of the compounds that caused 50% inhibition of DPPH radical formation.
ABTS assay
The ability to scavenge the ABTS radical cation (ABTS+) was determined according to the previously reported method (Ong et al. 2021). The ABTS+ solution was prepared by the reaction of 7 mM ABTS (10 mL) and 2.45 mM (10 mL) potassium persulphate after incubation at room temperature in the dark for 16 h. It was then diluted with 80% ethanol to give an absorbance value of 0.700 ± 0.005 at 734 nm. The ABTS+ solution (5 mL) was thoroughly mixed with the test samples at various concentrations (0.1 mL). The reaction mixture was allowed to stand at 30 °C for 30 min, and the absorbance at 734 nm was immediately recorded. Ascorbic acid at the same concentrations was used as a reference. The level of radical scavenging was calculated using the aforementioned equation for DPPH.
Spectrum-effect relationship analysis
To investigate the anti-oxidative stress effects of SEO, the partial least-squares regression (PLS) method was employed. The areas of common peaks of each fingerprint were set as the independent variables X, and the IC50 values of DPPH and ABTS were set as Y. If the regression coefficient of one peak was larger than zero, then it contained antioxidant components as previously reported (Zhang et al. 2018).
Statistical analysis
All data were analyzed with Analysis of Variance (ANOVA) using GraphPad Prism and descriptively expressed as mean ± SEM analyzed. p < 0.05 was considered statistically significant.
Results
Effects of SEO on the immobility time in TST and FST
The data of TST (Table 2) and FST (Table 3) both showed that SEO 250 and SEO 750 could significantly reduce the immobility time compared to the Vehicle group. SEO showed a promising antidepressant-like effect in depressed mice.
Effects of SEO on levels of SOD, MDA, CAT, and GSH in depressed mice
Figure 1 showed the levels of SOD, MDA, CAT, and GSH in the hippocampus, cortex, and serum. SEO 750 could significantly increase the levels of SOD, GSH, and CAT in the hippocampus, cortex, and serum, and reduce the levels of MDA in the hippocampus, cortex, and serum. These results indicated that SEO could effectively alleviate oxidative stress in depressed mice.
Effects of SEO on the expressions of Nrf2 in depressed mice
After FST and TST, expressions of nuclear Nrf2 in the hippocampus and cortex were significantly decreased. SEO treatment could significantly reverse these changes (Fig. 2), which illustrated that SEO might promote Nrf2 translocation to reduce oxidative stress.
The Effect of SEO on nuclear of Nrf2 expression in mice. (A) The expression of nuclear Nrf2 was detected by Western Blot using specific antibodies in mice. (B) graphs represented the arbitary density of blot signal. The values represent the mean ± SEM (n = 3 in each group), *p < 0.05, **p < 0.01, ***p < 0.001 compared with the Vehicle group
Effects of SEO on histopathological changes in hippocampus and cortex
The neuronal integrity and orderliness of the hippocampus and cortex were detected by HE staining. In the Vehicle group, neurons in the hippocampus (DG and CA1 sections) and cortex were sparsely arranged, and the nuclei were deeply stained as well as condensed. SEO treatments significantly reversed neuronal loss and injury, the neurons in DG, CA1, and cortex were arranged neatly in rows, oval or round, rarely depigmented, and the nuclei were clearly visible (Fig. 3).
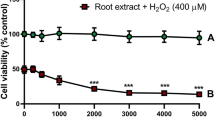
Effects of SEO on cell viability in H2O2-induced PC12 cells
MTT results showed in Fig. 4.Cell viability significantly decreased after H2O2 exposure, and SEO (1.56, 3.125, 6.25, 12.5, and 25 μg/mL) significantly increased the cell viability that indicating that SEO could effectively inhibit the oxidative stress caused by H2O2.
Effects of SEO on levels of SOD, GSH, CAT, and MDA in H2O2-induced PC12 cells
H2O2 induced significant decreases in levels of SOD (Fig. 5A), GSH (Fig. 5B), and CAT (Fig. 5C), and increases in levels of MDA (Fig. 5D). However, SEO (6.25 μg/mL) treatment dramatically increased the levels of SOD, GSH, and CAT, and reduced the levels of MDA.
Effects of SEO on Nrf2/HO-1 signaling in H2O2-induced PC12 cells
Figure 6A showed that SEO significantly promoted the Nrf2 translocation compared with the Vehicle group. Besides, the expression of HO-1 was significantly reduced by H2O2 treatment (Fig. 6B), and SEO could significantly increase the expression of HO-1, which indicated that the effects of anti-oxidative stress of SEO might be via the upregulation of Nrf2/HO-1 signaling.
Effects of SEO on PI3K/AKT/GSK3β signaling in H2O2-induced PC12 cells
As shown in Fig. 7, the ratios of p-PI3K/PI3K, p-AKT/AKT, and p-GSK3β/GSK3β were significantly reduced by H2O2 exposure, and SEO treatments significantly increased these ratios. These results suggested that PI3K/AKT/GSK3β signaling might be involved in the mechanism of anti-oxidative stress effects of SEO in H2O2-induced PC12 cells.
The Effect of administration on PI3K, p-PI3K, AKT, p-AKT, GSK3β and p-GSK3β expression in H2O2-treated PC12. The expression of p-PI3K/PI3K, p-AKT/AKT and p-GSK3β/GSK3β were detected by Western Blot (A). (B), (C) and (D) graphs represented the arbitary density of blot signal. The values represent the mean ± SEM (n = 3 in each group), *p < 0.05, **p < 0.01, ***p < 0.001 compared with the Vehicle group; ###p < 0.001 compared with the Control group
In vitro antioxidant activities
The DPPH radical scavenging activities of different batches of SEO are presented in Table 4. The IC50 value of the DPPH radical inhibitory concentrations of the 18 batches of SEO ranged from 2.99–8.24 mg/mL. S5 showed the weakest DPPH radical inhibitory capacity, and S6 showed the strongest capacity. The results of ABTS radical scavenging activities of different batches of SEO are presented in Table 5. The IC50 value of the ATBS radical inhibitory concentrations of the 18 batches of SEO ranged from 0.5–2.96 mg/mL. S9 showed the weakest capacity and S18 showed the strongest capacity.
GC-MS fingerprint
Total ion chromatograms were acquired for 18 batches of SEO (Fig. 8A). 19 common peaks were chosen by comparing GC-MS retention time in 18 chromatograms and are denoted in the reference fingerprint (Fig. 8B). The reference fingerprint was computed and determined by the professional software Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine composed by the Chinese Pharmacopeia Committee (version 2009). The identification of 19 common peaks was based on matching the recorded mass spectra with the standard mass spectra in the NIST 2014 MS library and comparing their RIs with those from the literature. The identified 19 common peaks are listed in Table 6, and the relative percentage content of these peaks is listed in Table 7.
Spectrum-effect relationship
Figure 9 showed the regression coefficients calculated by PLS method between fingerprint and DPPH IC50 values (Fig. 9A), ATBS IC50 values (Fig. 9B). The regression coefficients of peaks 1, 2, 3, 4, 6, 7, 8, 9, 10, 12 and 13 were larger than zero, which means these common peaks were positively correlated with DPPH radical scavenging activity. The regression coefficients of peaks 1, 2, 3, 4, 6, 7, 8, 10 and 13 were larger than zero, which means these common peaks were positively correlated with ATBS radical scavenging activity. Combine the results of these two regressions, peaks 1 (Benzene, 1-methoxy-4-methyl-2-(1-methylethyl)-), 2 (Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S-endo)-), 3 (1,2,4-Metheno-1H-indene, octahydro-1,7a-dimethyl-5-(1-methylethyl)-, [1S-(1.alpha.,2.alpha.,3a.beta.,4.alpha.,5.alpha.,7a.beta.,8S*)]-), 4 (Ylangene), 6 (Spiro[5.5]undec-2-ene, 3,7,7-trimethyl-11-methylene-), 7 (α-Muurolene), 8 ((1R,3aS,4aS,8aS)-1,4,4,6-Tetramethyl-1,2,3,3a,4,4a,7,8-octahydrocyclopenta[1,4]cyclobuta[1,2]benzene), 10 (β-Bisabolene) and 13 ((1R,3aS,4aS,8aS)-1,4,4,6-Tetramethyl-1,2,3,3a,4,4a,7,8-octahydrocyclopenta[1,4]cyclobuta[1,2]benzene) may greatly contribute to the antioxidant activities of SEO.
Discussion
Based on the results of FST and TST, we found SEO can significantly improve the depression-like behavior in depressed mice, which indicates a promising antidepressant-like effect of SEO. Oxidative stress is caused by an imbalance between ROS production and antioxidant defenses, and these are responsible for the altered brain function associated with depression (Bhatt et al. 2020). In line with the published data, the levels of SOD, GSH, and CAT in the hippocampus, cortex, and plasma were significantly increased, and the levels of MDA in the hippocampus, cortex, and plasma were significantly decreased, which suggests an oxidative stress status in the depressed mice’s brain. Besides, the histopathological examination of the hippocampus and cortex showed the brain structure has been altered. However, SEO treatment restored neuron loss and injury and attenuated the levels of oxidative stress significantly. In addition, we can draw the same conclusion in the H2O2-induced PC12 cells after SEO treatment by cell viability evaluation and SOD, GSH, CAT, and MDA levels detection. Therefore, these results illustrated that alleviation of oxidative stress might involve the mechanism of the antidepressant-like effect of SEO both in vitro and in vivo.
Nrf2 is known as a master regulator of redox homeostasis (Bouvier et al. 2017). During oxidative stress, Nrf2 is over-activated in the cytoplasm and translocases to the nucleus, which then leads to many antioxidant enzymes activation, such as heme-oxygenase1 (HO-1). HO-1 plays an important role in the maintenance of cell homeostasis and cell damage (Huang et al. 2021), and mediates antioxidants as a critical Nrf2-dependent transcription factor. Our data first showed that H2O2 did not increase the nuclear translocation of Nrf2, and the expressions of its downstream antioxidant enzyme HO-1 which consistent with the previous reports (Lu et al. 2022). Then, we found SEO promoted the translocations of Nrf2 to the nucleus both in the hippocampus and cortex in depressed mice, and the same results were observed in H2O2-induced PC12 cells as well by immunofluorescence analysis. Furthermore, SEO increased the expression of HO-1 in H2O2-induced PC12 cells, which indicates SEO attenuates the oxidative stress via the Nrf2/HO-1 signaling pathway regulation. PI3K/AKT/GSK3β signaling could alleviate oxidative stress by modulating the Nrf2/HO-1 pathway (Meng et al. 2021; Shin et al. 2019). PI3K/AKT is involved in the regulation of cell and physiological regeneration and pathological states (Wang et al. 2021a). GSK3β contributes to the export and degradation of Nrf2 (Liao et al. 2020). PI3K activates AKT through phosphorylation and consequently inhibits GSK3β by phosphorylation as well (Zhang et al. 2020). Consistent with published data, we found SEO could significantly increase the ratios of p-PI3K/PI3K, p-AKT/AKT, and p-GSK3β/ GSK3β in H2O2-induced PC12 cells. These results suggest that PI3K/AKT/GSK3β signaling promoted Nrf2/HO-1 pathway would be important in the antioxidant effect of SEO.
Based on the above results of in vivo mechanism study, we try to find out the components with good antioxidant activities in SEO. The spectrum-effect relationship analysis is to combine the chemical compositions of the fingerprint of TCM with the results of the pharmacological efficacy. The chemical fingerprint of 18 batches of SEO was established by the GC-MS method, 19 common peaks were identified and the relative percentage content was calculated. As shown in Tables 4 and 5, DPPH radical scavenging and ABTS values can reflect the antioxidant activity. The antioxidant activity increased with decreases of IC50 of DPPH radical scavenging and ABTS values. Combining the results of the regression coefficients plot of IC50 values of DPPH and ABTS, 9 peaks (peaks 1, 2, 3, 4, 6, 7, 8, 10, and 13) were identified and these peaks were positively correlated with the antioxidant effect of SEO. Previous studies reported that Ylangene (peak 4) (Liu et al. 2021a), α-Muurolene (peak 7) (Mennai et al. 2021), and β-Bisabolene (peak 10) (Obasi and Ogugua 2021) were showed promising antioxidant activity. The spectrum-effect relationship analysis results suggested that these components might be positively correlated with the antidepressant-like effect of SEO. A further study of the identified components will be performed in our future investigation, including their antioxidant effects and antidepressant-like effects.
Conclusion
In the present study, we found SEO showed a promising antidepressant-like effect in mice, and the mechanisms of this effect might be contributed to the antioxidant activities of SEO according to the results of in vitro and in vivo studies. The mechanism study indicated SEO could promote Nrf2/HO-1 pathway to improve the oxidative stress status and exert the antidepressant-like effects, and the PI3K/AKT/GSK3β signaling pathway might be involved as well. Based on the spectrum-effect relationship analysis, we found 9 peaks positively correlated with the antioxidant activity of SEO, and that is positively correlated with the antidepressant-like effects of SEO as well.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to confidential but are available from the corresponding author on reasonable request.
References
Armario A (2021) The forced swim test: historical, conceptual and methodological considerations and its relationship with individual behavioral traits. Neurosci Biobehav Rev 128:74–86
Bhatt S, Nagappa AN, Patil CR (2020) Role of oxidative stress in depression. Drug Discov Today 25(7):1270–1276
Bouvier E, Brouillard F, Molet J, Claverie D, Cabungcal JH, Cresto N, Doligez N, Rivat C, Do KQ, Bernard C, Benoliel JJ, Becker C (2017) Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol Psychiatry 22(12):1701–1713
Han Y, Lee C (2009) Antidepressants reveal differential effect against 1-methyl-4-phenylpyridinium toxicity in differentiated PC12 cells. Eur J Pharmacol 604:36–44
Huang Y, Yang Y, Xu Y, Ma Q, Guo F, Zhao Y, Tao Y, Li M, Guo J (2021) Nrf2/HO-1 Axis regulates the angiogenesis of gastric Cancer via targeting VEGF. Cancer Manag Res 13:3155–3169
Lee J-H, Lee Y-Y, Lee J, Jang Y-J, Jang H-W (2021) Chemical composition, antioxidant, and anti-inflammatory activity of essential oil from Omija (Schisandra chinensis (Turcz.) Baill.) produced by supercritical fluid extraction using CO2. Foods 10:1619. https://doi.org/10.3390/foods10071619
Liao S, Wu J, Liu R, Wang S, Luo J, Yang Y, Qin Y, Li T, Zheng X, Song J, Zhao X, Xiao C, Zhang Y, Bian L, Jia P, Bai Y, Zheng X (2020) A novel compound DBZ ameliorates neuroinflammation in LPS-stimulated microglia and ischemic stroke rats: role of Akt(Ser473)/GSK3β(Ser9)-mediated Nrf2 activation. Redox Biol 36:101644
Liu C, Zhang S, Zhang J, Liang Q, Li D (2021) Chemical composition and antioxidant activity of essential oil from berries of Schisandra chinensis (Turcz.) Baill. Nat Prod Res 26(23):2199–2203
Liu L, Zheng B, Wang Z (2021b) Protective effects of the knockdown of lncRNA AK139328 against oxygen glucose deprivation/reoxygenation-induced injury in PC12 cells. Mol Med Rep 24(3):621. https://doi.org/10.3892/mmr.2021.12260
Lu C, Day C, Kuo C, Wang T, Ho T, Lai P, Chen R, Yao C, Viswanadha V, Kuo W, Huang C (2022) Calycosin alleviates H O -induced astrocyte injury by restricting oxidative stress through the Akt/Nrf2/HO-1 signaling pathway. Environ Toxicol 37(4):858–867
Meng M, Zhang L, Ai D, Wu H, Peng W (2021) β-Asarone ameliorates β-amyloid-induced neurotoxicity in PC12 cells by activating P13K/Akt/Nrf2 signaling pathway. Front Pharmacol 12:659955
Mennai I, Lamera E, Slougui N, Benaicha B, Gasmi S, Samai Z, Rahmounia N, Bensouici C, Pinto DCGA (2021) Chemical composition and antioxidant, antiparasitic, cytotoxicity and antimicrobial potential of the Algerian Limonium oleifolium mill. Essential oil and organic extracts. Chem Biodiversity 18(9):e2100278. https://doi.org/10.1002/cbdv.202100278
Obasi D, Ogugua V (2021) GC-MS analysis, pH and antioxidant effect of Ruzu herbal bitters on alloxan-induced diabetic rats. Biochem Biophys Rep 27:101057
Ong ES, Oh CLY, Tan JCW, Foo SY, Leo CH (2021) Pressurized hot water extraction of okra seeds reveals antioxidant, antidiabetic and vasoprotective activities. Plants (Basel, Switzerland) 10(8):1645. https://doi.org/10.3390/plants10081645
Park S, Lee Y, Yang H, Song G (2021) Fluoxetine potentiates phagocytosis and autophagy in microglia. Front Pharmacol 12:770610
Shin JH, Kim KM, Jeong JU, Shin JM, Kang JH, Bang K, Kim J-H (2019) Nrf2-Heme Oxygenase-1 attenuates high-glucose-induced epithelial-to-mesenchymal transition of renal tubule cells by inhibiting ROS-mediated PI3K/Akt/GSK-3β signaling. J Diabetes Res 2019:2510105–2510105
Wang D, Liu J, Jiang H (2021a) Triclosan regulates the Nrf2/HO-1 pathway through the PI3K/Akt/JNK signaling cascade to induce oxidative damage in neurons. Environ Toxicol 36(9):1953–1964. https://doi.org/10.1002/tox.23315
Wang Y, Chen Y, Jia Y, Xue Z, Chen Z, Zhang M, Panichayupakaranant P, Yang S, Chen H (2021b) Chrysophyllum cainito. L alleviates diabetic and complications by playing antioxidant, antiglycation, hypoglycemic roles and the chemical profile analysis. J Ethnopharmacol 281:114569. https://doi.org/10.1016/j.jep.2021.114569
Yan T, Wang N, Liu B, Wu B, Xiao F, He B, Jia Y (2021) Schisandra chinensis ameliorates depressive-like behaviors by regulating microbiota-gut-brain axis via its anti-inflammation activity. Phytother Res: PTR 35(1):289–296
Zhang X, Chen J, Yang J, Shi Y (2018) UPLC-MS/MS analysis for antioxidant components of Lycii Fructus based on spectrum-effect relationship. Talanta 180:389–395
Zhang J, Ding C, Zhang S, Xu Y (2020) Neuroprotective effects of astaxanthin against oxygen and glucose deprivation damage via the PI3K/Akt/GSK3β/Nrf2 signalling pathway in vitro. J Cell Mol Med 24(16):8977–8985
Zhang L, Tang M, Xie X, Zhao Q, Hu N, He H, Liu G, Huang S, Peng C, Xiao Y, You Z (2021a) Ginsenoside Rb1 induces a pro-neurogenic microglial phenotype via PPARγ activation in male mice exposed to chronic mild stress. J Neuroinflammation 18(1):171
Zhang Y, Long Y, Yu S, Li D, Yang M, Guan Y, Zhang D, Wan J, Liu S, Shi A, Li N, Peng W (2021b) Natural volatile oils derived from herbal medicines: a promising therapy way for treating depressive disorder. Pharmacol Res 164:105376
Zhang Z, Yang Y, Liu X, Qin Z, Li S, Bai L, Li J (2021c) The protective effect of aspirin eugenol Ester on oxidative stress to PC12 cells stimulated with HO through regulating PI3K/Akt signal pathway. Oxidative Med Cell Longev 2021:5527475
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 82173961). Key Laboratory of polysaccharide bioactivity evaluation of TCM of Liaoning Province. Liaoning Distinguished Professor Project for Ying Jia (2017). The Doctoral Scientific Research Foundation of Liaoning Province (2019-BS-233). High-level innovation and entrepreneurship team of Liaoning Province (XLYC2008029). Development Project of Qinghai Provincial Key Laboratory (2022-ZJ-Y18).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Liang Tan, Yunfang Yang, Jing Peng and Yue Zhang. The first draft of the manuscript was written by Liang Tan and Tingxu Yan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All experiments were approved by the animal care committee of Shenyang Pharmaceutical University and performed in compliance with the national institutes of health and institutional guidelines for the humane care of animals.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tan, L., Yang, Y., Peng, J. et al. Schisandra chinensis (Turcz.) Baill. essential oil exhibits antidepressant-like effects and against brain oxidative stress through Nrf2/HO-1 pathway activation. Metab Brain Dis 37, 2261–2275 (2022). https://doi.org/10.1007/s11011-022-01019-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01019-z