Abstract
Cardiovascular diseases represent the major cause of morbidity mainly due to chronic heart failure. Epicardial (EAT) and perivascular adipose tissues (PVAT) are considered major contributors to the pathogenesis of cardiometabolic pathologies. Monoamine oxidases (MAOs) are mitochondrial enzymes recognized as sources of reactive oxygen species (ROS) in cardiometabolic pathologies. Methylene blue (MB) is one of the oldest protective agents, yet no data are available about its effects on adipose tissue. The present pilot study was aimed at assessing the effects of MB: (i) on MAO expression and (ii) oxidative stress in EAT and PVAT harvested from patients with heart failure subjected to cardiac surgery (n = 25). Adipose tissue samples were incubated with MB (0.1 µM/24 h) and used for the assessment of MAO gene and protein expression (qPCS and immune fluorescence) and ROS production (confocal microscopy and spectrophotometry). The human cardiovascular adipose tissues contain both MAO isoforms, predominantly MAO-A. Incubation with MB reduced MAOs expression and oxidative stress; co-incubation with serotonin, the MAO-A substrate, further augmented ROS generation, an effect partially reversed by MB. In conclusion, MAO-A is the major isoform expressed in EAT and PVAT and contribute to local oxidative stress; both effects can be mitigated by methylene blue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary heart disease (CHD) is the leading cause of mortality due to myocardial infarction and morbidity due to heart failure [1]; with the aging of the population and increased number of hospitalizations, the latter is currently a major challenge for the healthcare systems worldwide [2].
Epicardial adipose tissue (EAT), the fat depot on the myocardial surface being in direct contact with the coronary arteries, has been widely recognized for more than one decade as a biologically active organ whose volume has emerged as quantifiable risk factor for CHD, particularly in the setting of obesity [3]. Indeed, the expansion of EAT under obesogenic conditions is promoting the development of atherosclerosis (primary in coronary arteries surrounded by it) via the activation of local inflammation and apoptosis and also acting as metabolic transducer within the paracrine crosstalk with the subjacent myocardium (excellently reviewed in refs. [4, 5]). More recently, EAT dysfunction has emerged as a central pathomechanism and therapeutic target for both heart failure with preserved ejection fraction (HFpEF) and atrial fibrillation in patients with cardiometabolic diseases and chronic systemic inflammation [6].
Perivascular adipose tissue (PVAT), the fat depot surrounding arteries with crucial roles in regulation and maintenance of vascular tone and endothelial function, expresses an inflammatory phenotype in obesity and is responsible, together with the EAT, for the emergence of the so-called “outside-to-inside” model of inflammation in CHD [7, 8].
Increased oxidative stress is another pathomechanism responsible for the dysfunction of the epicardial and perivascular adipose pools. One potential source of reactive oxygen species (ROS) is monoamine oxidases (MAO) with two isoforms, MAO-A and MAO-B at the outer mitochondrial membrane. We have firstly reported the presence of both MAOs in the PVAT surrounding the mammary arteries isolated from patients with CHD undergoing revascularization procedures [9]. Whether the enzyme is also expressed in the EAT and PVAT surrounding the large arteries it is not known.
Methylene blue (MB, 3,7-bis (dimethylamino)-phenazathionium chloride), a more than 100 years-old synthetic compound, was approved by FDA for the treatment of methemoglobinemia, cyanide poisoning and malaria and has recaptured the researchers attention for its neuroprotective properties in neurodegenerative disorders, ischemic and traumatic brain injury via anti-apoptotic, anti-inflammatory, and anti-oxidant effects besides the increased energetic metabolism, since it acts as an alternative electron carrier in the case of the dysfunctional electron transport system (ETS). Also, it acts as a potent antidepressant due to its preferential accumulation in the brain where it readily penetrates neuronal mitochondria, inhibits monoamine oxidase (MAO) and activates signaling pathways involved in mitochondrial biogenesis and autophagy [10, 11]. More recently, MB has been reported to protect the rat kidney mitochondria from cisplatin-induced cytotoxicity by increasing the expression of genes involved in the mtDNA repair pathway [12]. We have previously demonstrated that MB improved mitochondrial respiration in rat heart mitochondria isolated from diabetic and non-diabetic animals [13] and alleviated endothelial dysfunction together with a reduction in oxidative stress in aortas harvested from diabetic rats [14].
We hypothesized that MAOs are expressed in human EAT and PVAT, contribute to the oxidative stress, and can be modulated by MB.
Materials and methods
This study conforms with the ethical principles for medical research involving human subjects outlined in the Declaration of Helsinki. The Commission for Research Ethics of “Victor Babeș” University for Medicine and Pharmacy of Timişoara and the Commision for Ethics in Research and Development of the Institute for Cardiovascular Diseases of Timișoara (no. 371/20.01.2021) approved the study protocol. Written informed consent was obtained from all patients prior to surgery.
Adipose samples were harvested from 25 patients with indication for elective heart surgery for cardiac pathologies (valvular disorders and coronary heart disease) and heart failure (HF) with either mildly reduced or preserved left ventricle ejection fraction (LVEF = 47.7% ± 6.23). Patients’ characteristics and preoperative medication were collected from medical records and are presented in Table 1.
Preparation of samples
EAT samples were harvested from the anterior wall of the right ventricle, while PVAT samples from peri-aortic and peri-pulmonary artery adipose tissue. EAT and PVAT samples were placed in an ice-cold buffer containing: 10 mM Ca-EGTA (ethylene glycol tetraacetic acid), 0.1 μM free calcium, 20 mM imidazole, 20 mM taurine, 50 mM K-MES (2-(N-morpholino)ethanesulfonic acid), 0.5 mM DTT (dithiothreitol), 6.56 mM MgCl2, 5.77 mM ATP (adenosine-5'-triphosphate), 15 mM phosphocreatine and immediately transferred to the laboratory, as previously described [15]. Adipose tissue samples were incubated for 24 h at 37 °C with or without 0.1 µM MB, then snap-frozen for further experimental procedures.
Assessment of MAO A and B expression
Both gene and protein expressions of MAO isoforms were determined according to previously described techniques [16]. In order to assess the gene expression of both MAO isoforms in adipose tissues total RNA was isolated (with the Aurum Total RNA Mini Kit, Biorad) and used for reverse transcription (with the iScript Advanced cDNA Synthesis Kit, Biorad). MAO-gene expression was evaluated by quantitative real time polymerase chain reaction (qRT-PCR), in the presence vs. the absence of MB (0.1 µM, 24 h incubation period). The sequence information used from the NCBI database to design the primers against MAOs was as follows: (5'- > 3')—human MAO-A fw AGG ACT ATC TGC TGC CAA AC; human MAO-A rev AAG CTC CAC CAA CAT CTA CG; human MAO-B fw GAA GAG TGG GAC AAC ATG AC; human MAO-B rev CTC CAC ACT GCT TCA CAT AC). The housekeeping gene and its primers were as follows: GADPH (fw): 5' CTC ATG ACC ACA GTC CAT GC -3'and GADPH (rv): 5'- TTC AGC TCT GGG ATG ACC TT -3', respectively.
Protein expression of MAOs isoforms was quantified in frozen sections of EAT and PVAT using both MAO-A (Abcam, ab126751) and MAO-B (Abcam, ab175136) primary antibodies and a secondary goat anti-rabbit antibody Alexa Fluor labeled (Invitrogen, A32731). Nuclei were stained with DAPI (Santa Cruz, SC3598). The slides were analyzed in confocal microscopy (Olympus Fluoview FV1000 confocal microscope) by means of the Image J software.
Evaluation of oxidative stress
ROS production in the adipose tissue samples was determined in the presence vs. the absence of MB (0.1 µM, 24 h incubation period) using 2 previously described techniques, immune fluorescence (IF) [17] and spectrophotometry [18], respectively.
Assessment of oxidative stress by means of IF used the dihydroethidium probe (DHE, Sigma-Aldrich-Merck). The superoxide indicator DHE, exhibits blue-fluorescence in the cytosol until oxidized, where it intercalates within the cells’ DNA, staining the nuclei a bright fluorescent red. Briefly, the adipose tissue fragments embedded and frozen in the optimal cutting temperature compound (OCT) were cut in 8 µm cryosections (Slee, MTC, Mainz) and placed on glass slides. After 3 washes with PBS, cryosections were incubated with DHE at room temperature. The slides were mounted with Vectashield (Vector Laboratories) and assessed in confocal microscope (Olympus Fluoview FV1000). Images were obtained using laser excitation at 488 nm 488 nm and emission at 610 nm and analyzed with the above mentioned software (Image J).
Hydrogen peroxide production was assessed in EAT and VAT samples by means of the Ferrous iron xylenol orange OXidation (FOX) assay (PeroxiDetect Kit, Sigma Aldrich Merck). The principle of the assay is that in the presence of peroxides, ferrous iron (Fe2+) is oxidized to the ferric (Fe3+) iron; the latter ion will then form a colored adduct with xylenol orange (3,3′-bis-N,N-bis(carboxymethyl)aminomethyl-o-cresolsulfonephthalein, sodium salt) that is measured at 560 nm. The H2O2 production is then calculated using a standard curve and results are expressed in nmol H2O2/mg tissue/h.
Chemicals
All reagents were purchased from Sigma Aldrich Merck unless otherwise stated.
Data analysis
Data analysis was performed by GraphPad Prism version 9.0 for Windows (GraphPad Software, USA). Data were expressed as mean ± S.E.M. Student t test, and for multiple comparisons, one-way ANOVA followed by Bonferroni post hoc analysis were used, differences between groups being considered significant at p < 0.05.
Results
MAO-A is the major isoform in human eat and pvat and both isoforms expression was reduced by acute incubation with methylene blue
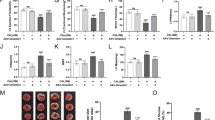
Our data showed that both MAO isoforms are present in human EAT and PVAT, with a higher expression in the former as compared to the latter (by analyzing the IF intensity). However, the protein expression of MAO-A isoform was significantly higher.
as compared to the one of MAO-B (Fig. 1).
The gene expression of MAOs in EAT and PVAT was further assessed by RT-PCR, in the presence vs. the absence of MB. Acute incubation of the samples with MB (0.1 µM, 24 h) significantly decreased the expression of both MAO isoforms in human adipose tissue (Fig. 2).
Acute incubation with mb mitigated oxidative stress in human EAT and PVAT
Since the increased oxidative stress is a classic feature of all dysfunctional adipose tissue, ROS production in EAT and PVAT was assessed using the DHE probe and measured as a fluorescent red staining in confocal microscopy (Fig. 3).
As showed in Fig. 3, a higher intensity of the DHE stain (arbitrary units) was present in EAT as compared to PVAT, a result that recapitulates the observation on MAOs expression in the two types of adipose tissues presented in Fig. 1.
Ex vivo incubation with methylene blue (0.1 µM, 24 h) elicited a significant and comparable ROS reduction in both EAT and PVAT, confirming its antioxidant property in human cardiovascular adipose tissue (Fig. 3).
Since DHE is classically regarded as a superoxide probe, we further determined the H2O2 production in EAT and PVAT by means of FOX assay, in the presence vs. the absence of methylene blue. As shown in Fig. 4, H2O2 production was significantly reduced in the presence of MB in both types of adipose tissues, apparently in a higher degree in EAT as compared to PVAT. Since MAO is a constant source of H2O2 and its expression was mitigated by MB particularly in EAT observation we might speculate on the role of MAOs as important source of oxidative stress in diseased human adipose tissue.
Finally, in order to confirm that MAO-A, the major isoform in human adipose tissue, can be targeted with MB, we recapitulated the experiments in the presence vs the absence of serotonin (10 µM), the MAO-A substrate. Incubation with SR more than doubled the H2O2 production in both types of adipose tissue, an effect that was partially reversed by MB (Fig. 5). Since the H2O2 values in the presence of SR and MB did not reach the ones obtained with MB alone, we might speculate that other ROS sources contribute to the hydrogen peroxide generation and might be induced by SR (or even MB might be responsible for the generation of H2O2 in small amounts).
Discussion
In the present study we recruited cardiac patients with HFpEF and HFmrEF referred for open-heart surgery, since it has been reported in the literature that EAT accumulation was associated with adverse prognosis in these categories of patients [19].
Over the past decade, both epicardial and perivascular adipose pools have been increasingly acknowledged as modulators of progression of cardiometabolic diseases (associated with chronic inflammation and insulin resistance) and pharmacological therapeutic targets [7, 20]. Both tissues are major sources of pro-inflammatory cytokines and growth factors that exert deleterious effects on both heart and vessels by paracrine signaling via direct diffusion. In particular, EAT isolated from obese, but also from overweight patients was found to have higher expression and levels of pro-inflammatory cytokines (IL-1, IL-6, TNF-α, and IFN-γ) than the paired subcutaneous fat [21]. Of note, in the studied group, BMI was 27.7 ± 4.9 (see Table 1) and only 3 patients presented a BMI over 30 (data not shown). We acknowledge as a limitation of our study the lack of measurement of serum inflammatory cytokines and markers (e.g., CRP) and of cardiac injury markers reported to be increased in the settings of cardiopulmonary bypass [22]. Also, this pilot study included more males (20) as compared to females (5); whether sex differences occur in response to MB is worth further investigation.
Moreover, EAT accumulation and its inflammation promote an arrhythmogenic substrate via fibrotic remodeling [23]. Left atrial myocardial fibrosis was positively correlated with the level of proinflammatory and profibrotic cytokines/chemokines, IL‐6, MCP‐1, and TNF‐α, in EAT [24] and myocardial fibrosis is a known substrate for atrial fibrillation. In the present study, 2 patients had atrial fibrillation and one atrial flutter (see Table 1) albeit a causal relation cannot be affirmed.
This pilot study was purported to assess both MAO expression as novel source of oxidative stress in EAT and PVAT samples, as well as the possibility to counteract it by acute exposure to MB. The first major finding is that MAO-A is the predominant isoform expressed in human cardiovascular adipose tissue that can be targeted (at least ex vivo) with submicromolar concentrations of MB. Furthermore, ROS generation assessed in confocal microscopy and spectrophotometry was reduced when the EAT and PVAT samples were acutely incubated with MB, as proof of the antioxidant effect of the redox dye in human adipose tissue. Last but not least, in the presence of serotonin, the MAO-A substrate, ROS production was further increased in both samples, an effect partially reversed by MB.
Besides inflammation, oxidative stress is also increased in the EAT but the sources of ROS are far from being fully elucidated. Salgado-Somoza et al. reported in their pioneering study that EAT produces a higher level of ROS than subcutaneous adipose tissue in patients with CHD. They found, among others, mRNA differences for catalase, glutathione S-transferase P, and protein disulfide isomerase [25]. Since EAT expands from epicardium into the myocardium, following the adventitia of the coronary arteries without any separation from myocardium, it provides harmful signaling via paracrine or vasocrine secretion and the oxidative stress in EAT may induce an increased oxidative stress in both coronary or myocardial tissue [26]. One year later, in EAT harvested from patients with severe stable CHD, Sacks et al. showed an increase mRNAs for 7 molecules involved in oxidative stress and/or oxygen species regulation along with 17 inflammatory adipokines or proteins involved in inflammation. One of the largest increases were reported for NADPH components gp91phox and p47 phox [27].
We have previously reported an increased oxidative stress in visceral adipose tissue (VAT) harvested from obese patients subjected to elective abdominal surgery and that MAO-A was the major isoform overexpressed. The fact that this finding was a particularity of obesity was further proven by the fact that ex vivo inhibition of MAO-A with clorgyline significantly reduced oxidative stress in VAT samples isolated from obese patients and had no effect in those harvested from the non-obese group [28].
The finding that MAO-A is also the predominant isoform in human EAT and PVAT is in line with the pioneering study published by Pizzinat et al. [29] in the late 90 s. Indeed, these authors reported that both MAO-A and MAO-B were expressed in human abdominal adipose tissue with MAO-A representing 70–80% of the total enzyme activity; also, the concomitant expression of noradrenaline transporter in human white adipocytes supports their role in the clearance of peripheral catecholamines. Our results confirm the fact that MAO-A is the predominant isoform in the diseased EAT (and PVAT) and its expression was mitigated by MB. We acknowledge as another limitation of the study the lack of use of MAO inhibitors in this study, in addition to MB, to (indirectly) assess the contribution of the latter to MAO inhibition.
PVAT is the fat depot surrounding most blood vessels, which in health presents anti-inflammatory and anti-contractile properties. At variance, in cardiometabolic pathologies associated with low-grade inflammation, PVAT-derived adipocytes generate various ROS, including superoxide anion and hydrogen peroxide that might signal to the vascular wall, underlying vascular injury. Classic sources of ROS in vascular beds include NADPH oxidase, uncoupled eNOS and dysfunctional ETS at the inner mitochondrial membrane [30, 31]. Moreover, superoxide is able to generate peroxynitrite (ONOO-) in the presence of NO and H2O2 can be converted into the highly reactive hydroxyl radical (–•OH) with further oxidation of lipids and DNA, thus leading to cell damage [30]. Interestingly, in healthy mouse mesenteric resistance arteries, it has been reported that PVAT acts as a reservoir for norepinephrine, preventing it from reaching the vessel and causing contraction [32].
We reported here that MAO-A is an important source of H2O2 in PVAT. We have also demonstrated that acute incubation of mesenteric arteries samples harvested form patients undergoing elective abdominal surgery with IL-6 increased MAO-A gene expression, as evidence of the fact that inflammation also potentiate the oxidative stress in the vascular wall [33]. Whether this observation can be recapitulated at the level of EAT and PVAT remains to be determined.
The first study reporting that MB is a potent reversible inhibitor of MAO-A was published almost two decades ago by Ramsay et al. These authors reported that MB, at concentrations reported to occur after intravenous administration, completely inhibited MAO-A (and partially MAO-B), due to its action as an oxidizing substrate and a one-electron reductant [34]. This MAO inhibitor effect, also common for other MB analogues [35], has been reported to mediate, at least partially, its antidepressant effects. Of note, the central inhibition of MAO-A by MB has also been linked to serotonin toxicity which may arise only when MB was used in combination with serotonergic drugs [36].
Methylene blue (MB) is known as a mild redox agent, which has been used as an electron carrier to prevent free radicals production and enhance cellular metabolic activity because it will not excessively accumulate in mitochondria and will not compromise the oxidation state of the physiological redox centers [37]. MB can reroute electrons in the ETS directly from NADH to cytochrome c, increasing the activity of complex IV activity and promoting ATP generation, while mitigating oxidative stress and delaying cellular aging by reversing neuroinflammation [38, 39].
The group of Adam–Vizi performed an elegant study aimed at elucidating the favorable energetic effects of MB in isolated guinea pig brain mitochondria treated with inhibitors of complex I or complex III of ETS. When the flow of electrons was compromised, MB transferred electrons to cytochrome c, increased the rate of ATP production, restored mitochondrial membrane potential, and improved the rate of calcium uptake. In rat heart mitochondria isolated from healthy and 2 months (streptozotocin-induced) diabetic rats, we have also demonstrated that addition of MB (0.1 μmol·L−1) elicited an increase in oxygen consumption of mitochondria energized with complex I and II substrates. In our hands, MB elicited a significant increase in H2O2 release in the presence of complex I substrates (glutamate and malate), but had an opposite effect in mitochondria energized with complex II substrate (succinate) [13].
More recently, the group of Mariana Rosca showed, in isolated diabetic cardiac mitochondria harvested from mice treated orally with MB that the redox agent facilitated NADH oxidation, increased NAD+, the activity of deacetylase sirtuin 3, and reduces protein lysine acetylation. Thus, by providing an alternative route for mitochondrial electron transport, MB alleviated the metabolic inflexibility in the diabetic heart [40].
MB has been extensively studied for its neuroprotective effects in animal models and patients with neurodegenerative diseases, in particular with Alzheimer disease, by targeting several molecular pathways that ultimately protect the brain mitochondria (comprehensively reviewed in ref. [41]). As an antidepressant, MB has been reported to act via various mechanisms. Accordingly, it restores mitochondrial function by acting as an alternative electron acceptor/donor, enhancing mitochondrial respiration, improving energy production and inhibiting the formation of superoxide. Also, MB has been also acknowledged as a non-selective inhibitor of NOS and modulator of the nitric oxide cyclic guanosine monophosphate (NO-cGMP) cascade, which enhances its antidepressant response, since dysfunction of the NO-cGMP cascade is involved in the neurobiology of mood, anxiety, and psychosis [42].
Recently, Pluta et al. [43] reported the successful reversal of vasoplegic shock by MB and ascribed the effect to the selective inhibition of iNOS (the inducible form of nitric oxide synthase), which prevented vasodilation in response to the pro-inflammatory cytokines.
We showed here, for the first time, that MAO is expressed in the human EAT and cardiac PVAT and MB, in submicromolar concentrations, can mitigate the oxidative stress in these two types of adipose tissue involved in the pathophysiology of cardio-metabolic diseases. Whether MB might reduce oxidative stress when administered as adjunctive pharmacological therapy during revascularization procedures, and improve the outcome of CHD patients with/without DM worth further investigation.
Pharmacological targeting of the epicardial and perivascular adipose tissues signaling pathways will remain a potential disease modifying approach in cardiometabolic syndromes. Whether MB will find a place in this scenario remains to be confirmed by future clinical studies.
Conclusion
MAO-A expression and ROS generation are increased in the epicardial and perivascular adipose tissues harvested from overweight and obese patients with heart failure with preserved and mildly reduced ejection fraction. Methylene blue is able to reduce both MAO expression and the oxidative stress. Further studies are required to elucidate the signal transduction of these observations.
Data availability
No datasets were generated or analysed during the current study.
References
Mensah GA, Fuster V, Murray CJL, Roth GA (2023) Global burden of cardiovascular diseases and risks, 1990–2022. J Am Coll Cardiol 82:2350–2473. https://doi.org/10.1016/j.jacc.2023.11.007
Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS (2023) Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res 118:3272–3287. https://doi.org/10.1093/cvr/cvac013
Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DT (2014) Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther 4:416–429. https://doi.org/10.3978/j.issn.2223-3652.2014.11.05
Madonna R, Massaro M, Scoditti E, Pescetelli I, De Caterina R (2019) The epicardial adipose tissue and the coronary arteries: dangerous liaisons. Cardiovasc Res 115:1013–1025. https://doi.org/10.1093/cvr/cvz062
Packer M (2018) Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 71:2360–2372. https://doi.org/10.1016/j.jacc.2018.03.509
Packer M (2019) Drugs that ameliorate epicardial adipose tissue inflammation may have discordant effects in heart failure with a preserved ejection fraction as compared with a reduced ejection fraction. J Card Fail 25:986–1003. https://doi.org/10.1016/j.cardfail.2019.09.002
Fitzgibbons TP, Czech MP (2014) Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc 3:e000582. https://doi.org/10.1161/jaha.113.000582
Omar A, Chatterjee TK, Tang Y, Hui DY, Weintraub NL (2014) Proinflammatory phenotype of perivascular adipocytes. Arterioscler Thromb Vasc Biol 34:1631–1636. https://doi.org/10.1161/atvbaha.114.303030
Lighezan R, Sturza A, Duicu OM, Ceausu RA, Vaduva A, Gaspar M, Feier H, Vaida M, Ivan V, Lighezan D, Muntean DM, Mornos C (2016) Monoamine oxidase inhibition improves vascular function in mammary arteries from nondiabetic and diabetic patients with coronary heart disease. Can J Physiol Pharmacol 94:1040–1047. https://doi.org/10.1139/cjpp-2015-0580
Yang L, Youngblood H, Wu C, Zhang Q (2020) Mitochondria as a target for neuroprotection: role of methylene blue and photobiomodulation. Transl Neurodegener 9:19. https://doi.org/10.1186/s40035-020-00197-z
Gureev AP, Sadovnikova IS, Popov VN (2022) Molecular mechanisms of the neuroprotective effect of methylene blue. Biochemistry (Mosc) 87:940–956. https://doi.org/10.1134/s0006297922090073
Samoylova NA, Gureev AP, Popov VN (2023) Methylene Blue Induces Antioxidant Defense and Reparation of Mitochondrial DNA in a Nrf2-Dependent Manner during Cisplatin-Induced Renal Toxicity. Int J Mol Sci. https://doi.org/10.3390/ijms24076118
Duicu OM, Privistirescu A, Wolf A, Petruş A, Dănilă MD, Raţiu CD, Muntean DM, Sturza A (2017) Methylene blue improves mitochondrial respiration and decreases oxidative stress in a substrate-dependent manner in diabetic rat hearts. Can J Physiol Pharmacol 95:1376–1382. https://doi.org/10.1139/cjpp-2017-0074
Privistirescu AI, Sima A, Duicu OM, Timar R, Roșca MG, Sturza A, Muntean DM (2018) Methylene blue alleviates endothelial dysfunction and reduces oxidative stress in aortas from diabetic rats. Can J Physiol Pharmacol 96:1012–1016. https://doi.org/10.1139/cjpp-2018-0119
Duicu OM, Lighezan R, Sturza A, Balica R, Vaduva A, Feier H, Gaspar M, Ionac A, Noveanu L, Borza C, Muntean DM, Mornos C (2016) Assessment of mitochondrial dysfunction and monoamine oxidase contribution to oxidative stress in human diabetic hearts. Oxid Med Cell Longev 2016:8470394. https://doi.org/10.1155/2016/8470394
Lascu A, Ionică LN, Buriman DG, Merce AP, Deaconu L, Borza C, Crețu OM, Sturza A, Muntean DM, Feier HB (2023) Metformin and empagliflozin modulate monoamine oxidase-related oxidative stress and improve vascular function in human mammary arteries. Mol Cell Biochem 478:1939–1947. https://doi.org/10.1007/s11010-022-04633-8
Ionica M, Aburel OM, Vaduva A, Petrus A, Rațiu S, Olariu S, Sturza A, Muntean DM (2020) Vitamin D alleviates oxidative stress in adipose tissue and mesenteric vessels from obese patients with subclinical inflammation. Can J Physiol Pharmacol 98:85–92. https://doi.org/10.1139/cjpp-2019-0340
Ionică LN, Gaiță L, Bînă AM, Soșdean R, Lighezan R, Sima A, Malița D, Crețu OM, Burlacu O, Muntean DM, Sturza A (2021) Metformin alleviates monoamine oxidase-related vascular oxidative stress and endothelial dysfunction in rats with diet-induced obesity. Mol Cell Biochem 476:4019–4029. https://doi.org/10.1007/s11010-021-04194-2
van Woerden G, van Veldhuisen DJ, Manintveld OC, van Empel VPM, Willems TP, de Boer RA, Rienstra M, Westenbrink BD, Gorter TM (2022) Epicardial adipose tissue and outcome in heart failure with mid-range and preserved ejection fraction. Circ Heart Fail 15:e009238. https://doi.org/10.1161/circheartfailure.121.009238
Rafeh R, Viveiros A, Oudit GY, El-Yazbi AF (2020) Targeting perivascular and epicardial adipose tissue inflammation: therapeutic opportunities for cardiovascular disease. Clin Sci (Lond) 134:827–851. https://doi.org/10.1042/cs20190227
Vyas V, Blythe H, Wood EG, Sandhar B, Sarker SJ, Balmforth D, Ambekar SG, Yap J, Edmondson SJ, Di Salvo C, Wong K, Roberts N, Uppal R, Adams B, Shipolini A, Oo AY, Lawrence D, Kolvekar S, Lall KS, Finlay MC, Longhi MP (2021) Obesity and diabetes are major risk factors for epicardial adipose tissue inflammation. JCI Insight. https://doi.org/10.1172/jci.insight.145495
Zakkar M, Ascione R, James AF, Angelini GD, Suleiman MS (2015) Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol Ther 154:13–20. https://doi.org/10.1016/j.pharmthera.2015.06.009
Patel KHK, Hwang T, Se Liebers C, Ng FS (2022) Epicardial adipose tissue as a mediator of cardiac arrhythmias. Am J Physiol Heart Circ Physiol 322:H129-h144. https://doi.org/10.1152/ajpheart.00565.2021
Takahashi N, Abe I, Kira S, Ishii Y (2023) Role of epicardial adipose tissue in human atrial fibrillation. J Arrhythm 39:93–110. https://doi.org/10.1002/joa3.12825
Salgado-Somoza A, Teijeira-Fernández E, Fernández AL, González-Juanatey JR, Eiras S (2010) Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol 299:H202–H209. https://doi.org/10.1152/ajpheart.00120.2010
Iacobellis G (2022) Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol 19:593–606. https://doi.org/10.1038/s41569-022-00679-9
Sacks HS, Fain JN, Cheema P, Bahouth SW, Garrett E, Wolf RY, Wolford D, Samaha J (2011) Depot-specific overexpression of proinflammatory, redox, endothelial cell, and angiogenic genes in epicardial fat adjacent to severe stable coronary atherosclerosis. Metab Syndr Relat Disord 9:433–439. https://doi.org/10.1089/met.2011.0024
Sturza A, Muntean DM, Crețu OM (2021) Monoamine Oxidase, Obesity and Related Comorbidities: Discovering Bonds. In: Tappia PS, Ramjiawan B, Dhalla NS (eds) Cellular and Biochemical Mechanisms of Obesity. Springer International Publishing, Cham, pp 199–213
Pizzinat N, Marti L, Remaury A, Leger F, Langin D, Lafontan M, Carpéné C, Parini A (1999) High expression of monoamine oxidases in human white adipose tissue: evidence for their involvement in noradrenaline clearance. Biochem Pharmacol 58:1735–1742. https://doi.org/10.1016/s0006-2952(99)00270-1
Victorio JA, Davel AP (2020) Perivascular adipose tissue oxidative stress on the pathophysiology of cardiometabolic diseases. Curr Hypertens Rev 16:192–200. https://doi.org/10.2174/1573402115666190410153634
Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, Mikhed Y, Münzel T, Daiber A, Förstermann U, Li H (2016) Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice. Arterioscler Thromb Vasc Biol 36:78–85. https://doi.org/10.1161/atvbaha.115.306263
Saxton SN, Ryding KE, Aldous RG, Withers SB, Ohanian J, Heagerty AM (2018) Role of sympathetic nerves and adipocyte catecholamine uptake in the vasorelaxant function of perivascular adipose tissue. Arterioscler Thromb Vasc Biol 38:880–891. https://doi.org/10.1161/atvbaha.118.310777
Sturza A, Popoiu CM, Ionică M, Duicu OM, Olariu S, Muntean DM, Boia ES (2019) Monoamine oxidase-related vascular oxidative stress in diseases associated with inflammatory burden. Oxid Med Cell Longev 2019:8954201. https://doi.org/10.1155/2019/8954201
Ramsay RR, Dunford C, Gillman PK (2007) Methylene blue and serotonin toxicity: inhibition of monoamine oxidase A (MAO A) confirms a theoretical prediction. Br J Pharmacol 152:946–951. https://doi.org/10.1038/sj.bjp.0707430
de Beer F, Petzer JP, Petzer A (2020) Monoamine oxidase inhibition by selected dye compounds. Chem Biol Drug Des 95:355–367. https://doi.org/10.1111/cbdd.13654
Delport A, Harvey BH, Petzer A, Petzer JP (2017) The monoamine oxidase inhibition properties of selected structural analogues of methylene blue. Toxicol Appl Pharmacol 325:1–8. https://doi.org/10.1016/j.taap.2017.03.026
Atamna H, Mackey J, Dhahbi JM (2012) Mitochondrial pharmacology: electron transport chain bypass as strategies to treat mitochondrial dysfunction. BioFactors 38:158–166. https://doi.org/10.1002/biof.197
Tucker D, Lu Y, Zhang Q (2018) From mitochondrial function to neuroprotection-an emerging role for methylene blue. Mol Neurobiol 55:5137–5153. https://doi.org/10.1007/s12035-017-0712-2
Zhou L, Flores J, Noël A, Beauchet O, Sjöström PJ, LeBlanc AC (2019) Methylene blue inhibits Caspase-6 activity, and reverses Caspase-6-induced cognitive impairment and neuroinflammation in aged mice. Acta Neuropathol Commun 7:210. https://doi.org/10.1186/s40478-019-0856-6
Berthiaume JM, Hsiung CH, Austin AB, McBrayer SP, Depuydt MM, Chandler MP, Miyagi M, Rosca MG (2017) Methylene blue decreases mitochondrial lysine acetylation in the diabetic heart. Mol Cell Biochem 432:7–24. https://doi.org/10.1007/s11010-017-2993-1
Hashmi MU, Ahmed R, Mahmoud S, Ahmed K, Bushra NM, Ahmed A, Elwadie B, Madni A, Saad AB, Abdelrahman N (2023) Exploring methylene blue and its derivatives in alzheimer’s treatment: a comprehensive review of randomized control trials. Cureus 15:e46732. https://doi.org/10.7759/cureus.46732
Delport A, Harvey BH, Petzer A, Petzer JP (2017) Methylene blue and its analogues as antidepressant compounds. Metab Brain Dis 32:1357–1382. https://doi.org/10.1007/s11011-017-0081-6
Pluta MP, Putowski Z, Czempik PF, Krzych ŁJ (2023) Successful use of methylene blue in catecholamine-resistant septic shock: a case report and short literature review. Int J Mol Sci. https://doi.org/10.3390/ijms241310772
Acknowledgements
We thank Andreea Anechitei for the expert technical support. The experiments were performed in the Centre for Translational Research and Systems Medicine, advanced research centre of “Victor Babeș” University of Medicine and Pharmacy of Timișoara, Romania.
Funding
This research was funded by the” Victor Babeș” University of Medicine and Pharmacy grant MITO-MB-CURAT:2POSTDOC/1387/03.02.2020.
Author information
Authors and Affiliations
Contributions
Conceptualization, O.M.A. and A.S.; Methodology, A.S.; Validation, O.M.A., A.S. and C.M.; Formal analysis, O.M.A., A.S.; Investigation, L.B., A.P.M., D.G.B., A.M.B.; Writing-original draft preparation, O.M.A.; Writing-review and editing, O.M.A., A.S., D.M.M.; Visualization, Supervision, O.M.A, C.B., C.M., A.S., D.M.M; Project administration, O.M.A.; Funding acquisition, O.M.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Commission for Research Ethics of “Victor Babeș” University of Medicine and Pharmacy of Timișoara (no. 35/21.09.2020) and the Commision for Ethics in Research and Development of the Institute for Cardiovascular Diseases of Timișoara (no. 371/20.01.2021).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aburel, OM., Brăescu, L., Buriman, D.G. et al. Methylene blue reduces monoamine oxidase expression and oxidative stress in human cardiovascular adipose tissue. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-05092-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-05092-z