Abstract
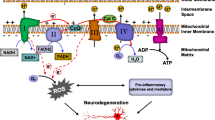
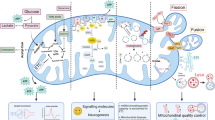
Methylene blue (MB) is a well-established drug with a long history of use, owing to its diverse range of use and its minimal side effect profile. MB has been used classically for the treatment of malaria, methemoglobinemia, and carbon monoxide poisoning, as well as a histological dye. Its role in the mitochondria, however, has elicited much of its renewed interest in recent years. MB can reroute electrons in the mitochondrial electron transfer chain directly from NADH to cytochrome c, increasing the activity of complex IV and effectively promoting mitochondrial activity while mitigating oxidative stress. In addition to its beneficial effect on mitochondrial protection, MB is also known to have robust effects in mitigating neuroinflammation. Mitochondrial dysfunction has been identified as a seemingly unifying pathological phenomenon across a wide range of neurodegenerative disorders, which thus positions methylene blue as a promising therapeutic. In both in vitro and in vivo studies, MB has shown impressive efficacy in mitigating neurodegeneration and the accompanying behavioral phenotypes in animal models for such conditions as stroke, global cerebral ischemia, Alzheimer’s disease, Parkinson’s disease, and traumatic brain injury. This review summarizes recent work establishing MB as a promising candidate for neuroprotection, with particular emphasis on the contribution of mitochondrial function to neural health. Furthermore, this review will briefly examine the link between MB, neurogenesis, and improved cognition in respect to age-related cognitive decline.



Similar content being viewed by others
References
Schirmer RH, Adler H, Pickhardt M, Mandelkow E (2011) Lest we forget you—methylene blue. Neurobiol Aging 32(12):2325 e2327–2316. doi:10.1016/j.neurobiolaging.2010.12.012
Ginimuge PR, Jyothi SD (2010) Methylene blue: revisited. J Anaesthesiol Clin Pharmacol 26(4):517–520
Stawicki SP, Sims C, Sarani B, Grossman MD, Gracias VH (2008) Methylene blue and vasoplegia: who, when, and how? Mini-Rev Med Chem 8(5):472–490
Akbar M, Essa MM, Daradkeh G, Abdelmegeed MA, Choi Y, Mahmood L, Song BJ (2016) Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Res 1637:34–55. doi:10.1016/j.brainres.2016.02.016
Yang SH, Li W, Sumien N, Forster M, Simpkins JW, Liu R (2015) Alternative mitochondrial electron transfer for the treatment of neurodegenerative diseases and cancers: methylene blue connects the dots. Prog Neurobiol. doi:10.1016/j.pneurobio.2015.10.005
P. Guttman PE (1891) On the action of methylene blue on malaria. In: Himmelwelt F (ed) The collected papers of Paul Ehrlich: chemotherapy, vol III. Elsevier, pp 15–20
Schaefer B (2015) Natural products in the chemical industry. Springer, Illustrated edn
DE Hughes EL (1901) The use of methylene blue as a seditive. In: Roland G. Curtin DEH (ed) Philadelphia hospital reports, vol 4. Detre & Blackburn, pp 272–282
Gillman PK (2011) CNS toxicity involving methylene blue: the exemplar for understanding and predicting drug interactions that precipitate serotonin toxicity. J Psychopharmacol 25(3):429–436. doi:10.1177/0269881109359098
Eroglu L, Caglayan B (1997) Anxiolytic and antidepressant properties of methylene blue in animal models. Pharmacol Res 36(5):381–385. doi:10.1006/phrs.1997.0245
Alda M, McKinnon M, Blagdon R, Garnham J, MacLellan S, O'Donovan C, Hajek T, Nair C et al (2017) Methylene blue treatment for residual symptoms of bipolar disorder: randomised crossover study. Br J Psychiatry J Ment Sci 210(1):54–60. doi:10.1192/bjp.bp.115.173930
Peter C, Hongwan D, Kupfer A, Lauterburg BH (2000) Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol 56(3):247–250
DiSanto AR, Wagner JG (1972) Pharmacokinetics of highly ionized drugs. 3. Methylene blue—blood levels in the dog and tissue levels in the rat following intravenous administration. J Pharm Sci 61(7):1090–1094
Gauthier S, Feldman HH, Schneider LS, Wilcock GK, Frisoni GB, Hardlund JH, Moebius HJ, Bentham P et al (2016) Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer's disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 388(10062):2873–2884. doi:10.1016/S0140-6736(16)31275-2
Baddeley TC, McCaffrey J, Storey JM, Cheung JK, Melis V, Horsley D, Harrington CR, Wischik CM (2015) Complex disposition of methylthioninium redox forms determines efficacy in tau aggregation inhibitor therapy for Alzheimer's disease. J Pharmacol Exp Ther 352(1):110–118. doi:10.1124/jpet.114.219352
Coleman MD, Coleman NA (1996) Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf 14(6):394–405
Bradberry SM (2003) Occupational methaemoglobinaemia. Mechanisms of production, features, diagnosis and management including the use of methylene blue. Toxicol Rev 22(1):13–27
Cawein M, Behlen CH 2nd, Lappat EJ, Cohn JE (1964) Hereditary diaphorase deficiency and methemoglobinemia. Arch Intern Med 113:578–585
Oz M, Lorke DE, Hasan M, Petroianu GA (2011) Cellular and molecular actions of methylene blue in the nervous system. Med Res Rev 31(1):93–117. doi:10.1002/med.20177
Alici-Evcimen Y, Breitbart WS (2007) Ifosfamide neuropsychiatric toxicity in patients with cancer. Psycho-Oncology 16(10):956–960. doi:10.1002/pon.1161
Shanmugam G (2005) Vasoplegic syndrome—the role of methylene blue. Eur J Cardiothorac Surg 28(5):705–710. doi:10.1016/j.ejcts.2005.07.011
(2015) WHO model list of essential medicines. http://www.who.int/medicines/publications/essentialmedicines/en/
Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R et al (2011) Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem 286(18):16504–16515. doi:10.1074/jbc.M110.208447
Opii WO, Nukala VN, Sultana R, Pandya JD, Day KM, Merchant ML, Klein JB, Sullivan PG et al (2007) Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J Neurotrauma 24(5):772–789. doi:10.1089/neu.2006.0229
Wong-Riley MT (2012) Bigenomic regulation of cytochrome c oxidase in neurons and the tight coupling between neuronal activity and energy metabolism. Adv Exp Med Biol 748:283–304. doi:10.1007/978-1-4614-3573-0_12
Callaway NL, Riha PD, Bruchey AK, Munshi Z, Gonzalez-Lima F (2004) Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol Biochem Behav 77(1):175–181
Gureev AP, Syromyatnikov MY, Gorbacheva TM, Starkov AA, Popov VN (2016) Methylene blue improves sensorimotor phenotype and decreases anxiety in parallel with activating brain mitochondria biogenesis in mid-age mice. Neurosci Res 113:19–27. doi:10.1016/j.neures.2016.07.006
Wu HM, Lee CG, Hwang SJ, Kim SG (2014) Mitigation of carbon tetrachloride-induced hepatic injury by methylene blue, a repurposed drug, is mediated by dual inhibition of GSK3beta downstream of PKA. Br J Pharmacol 171(11):2790–2802. doi:10.1111/bph.12637
Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, Aplin AE, Tai YT et al (2005) Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem 280(17):16916–16924. doi:10.1074/jbc.M410690200
Stack C, Jainuddin S, Elipenahli C, Gerges M, Starkova N, Starkov AA, Jove M, Portero-Otin M et al (2014) Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity. Hum Mol Genet 23(14):3716–3732. doi:10.1093/hmg/ddu080
Mergenthaler P, Lindauer U, Dienel GA, Meisel A (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci 36(10):587–597. doi:10.1016/j.tins.2013.07.001
Howarth C, Gleeson P, Attwell D (2012) Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab 32(7):1222–1232. doi:10.1038/jcbfm.2012.35
Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W (2008) Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A 105(17):6409–6414. doi:10.1073/pnas.0710766105
Wallace DC (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39:359–407. doi:10.1146/annurev.genet.39.110304.095751
Roth AD, Nunez MT (2016) Oligodendrocytes: functioning in a delicate balance between high metabolic requirements and oxidative damage. Adv Exp Med Biol 949:167–181. doi:10.1007/978-3-319-40764-7_8
Song BJ, Akbar M, Abdelmegeed MA, Byun K, Lee B, Yoon SK, Hardwick JP (2014) Mitochondrial dysfunction and tissue injury by alcohol, high fat, nonalcoholic substances and pathological conditions through post-translational protein modifications. Redox Biol 3:109–123. doi:10.1016/j.redox.2014.10.004
Calabrese V, Lodi R, Tonon C, D'Agata V, Sapienza M, Scapagnini G, Mangiameli A, Pennisi G et al (2005) Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia. J Neurol Sci 233(1–2):145–162. doi:10.1016/j.jns.2005.03.012
Johri A, Beal MF (2012) Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther 342(3):619–630. doi:10.1124/jpet.112.192138
Sanderson TH, Raghunayakula S, Kumar R (2015) Release of mitochondrial Opa1 following oxidative stress in HT22 cells. Mol Cell Neurosci 64:116–122. doi:10.1016/j.mcn.2014.12.007
Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P (2011) Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev 67(1–2):103–118. doi:10.1016/j.brainresrev.2010.11.004
Chen H, McCaffery JM, Chan DC (2007) Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130(3):548–562. doi:10.1016/j.cell.2007.06.026
Giannoccaro MP, La Morgia C, Rizzo G, Carelli V (2017) Mitochondrial DNA and primary mitochondrial dysfunction in Parkinson's disease. Mov Disord 32(3):346–363. doi:10.1002/mds.26966
Lu Q, Tucker D, Dong Y, Zhao N, Zhang Q (2016) Neuroprotective and functional improvement effects of methylene blue in global cerebral ischemia. Mol Neurobiol 53(8):5344–5355. doi:10.1007/s12035-015-9455-0
Ryou MG, Choudhury GR, Li W, Winters A, Yuan F, Liu R, Yang SH (2015) Methylene blue-induced neuronal protective mechanism against hypoxia-reoxygenation stress. Neuroscience 301:193–203. doi:10.1016/j.neuroscience.2015.05.064
Wiklund L, Basu S, Miclescu A, Wiklund P, Ronquist G, Sharma HS (2007) Neuro- and cardioprotective effects of blockade of nitric oxide action by administration of methylene blue. Ann N Y Acad Sci 1122:231–244. doi:10.1196/annals.1403.016
Pakavathkumar P, Sharma G, Kaushal V, Foveau B, LeBlanc AC (2015) Methylene blue inhibits caspases by oxidation of the catalytic cysteine. Sci Rep 5:13730. doi:10.1038/srep13730
Weingartner R, Oliveira E, Oliveira ES, Sant'Anna UL, Oliveira RP, Azambuja LA, Friedman G (1999) Blockade of the action of nitric oxide in human septic shock increases systemic vascular resistance and has detrimental effects on pulmonary function after a short infusion of methylene blue. Braz J Med Biol Res 32(12):1505–1513
Li L, Yang R, Li P, Lu H, Hao J, Tucker D, Zhang Q (2017) Combination treatment with methylene blue and hypothermia in global cerebral ischemia. Mol Neurobiol. doi:10.1007/s12035-017-0470-1
Wiklund L, Zoerner F, Semenas E, Miclescu A, Basu S, Sharma HS (2013) Improved neuroprotective effect of methylene blue with hypothermia after porcine cardiac arrest. Acta Anaesthesiol Scand 57(8):1073–1082. doi:10.1111/aas.12106
Jiang Z, Watts LT, Huang S, Shen Q, Rodriguez P, Chen C, Zhou C, Duong TQ (2015) The effects of methylene blue on autophagy and apoptosis in MRI-defined normal tissue, ischemic penumbra and ischemic core. PLoS One 10(6):e0131929. doi:10.1371/journal.pone.0131929
Di Y, He YL, Zhao T, Huang X, Wu KW, Liu SH, Zhao YQ, Fan M et al (2015) Methylene blue reduces acute cerebral ischemic injury via the induction of mitophagy. Mol Med 21:420–429. doi:10.2119/molmed.2015.00038
Rodriguez P, Jiang Z, Huang S, Shen Q, Duong TQ (2014) Methylene blue treatment delays progression of perfusion-diffusion mismatch to infarct in permanent ischemic stroke. Brain Res 1588:144–149. doi:10.1016/j.brainres.2014.09.007
Shen Q, Du F, Huang S, Rodriguez P, Watts LT, Duong TQ (2013) Neuroprotective efficacy of methylene blue in ischemic stroke: an MRI study. PLoS One 8(11):e79833. doi:10.1371/journal.pone.0079833
Ahmed ME, Tucker D, Dong Y, Lu Y, Zhao N, Wang R, Zhang Q (2016) Methylene blue promotes cortical neurogenesis and ameliorates behavioral deficit after photothrombotic stroke in rats. Neuroscience 336:39–48. doi:10.1016/j.neuroscience.2016.08.036
Xu H, Li J, Wang Z, Feng M, Shen Y, Cao S, Li T, Peng Y et al (2017) Methylene blue attenuates neuroinflammation after subarachnoid hemorrhage in rats through the Akt/GSK-3beta/MEF2D signaling pathway. Brain Behav Immun. doi:10.1016/j.bbi.2017.04.020
Rodriguez P, Zhao J, Milman B, Tiwari YV, Duong TQ (2016) Methylene blue and normobaric hyperoxia combination therapy in experimental ischemic stroke. Brain Behav 6(7):e00478. doi:10.1002/brb3.478
Li L, Qin L, Lu HL, Li PJ, Song YJ, Yang RL (2017) Methylene blue improves streptozotocin-induced memory deficit by restoring mitochondrial function in rats. Brain Res 1657:208–214. doi:10.1016/j.brainres.2016.12.024
Melis V, Magbagbeolu M, Rickard JE, Horsley D, Davidson K, Harrington KA, Goatman K, Goatman EA et al (2015) Effects of oxidized and reduced forms of methylthioninium in two transgenic mouse tauopathy models. Behav Pharmacol 26(4):353–368. doi:10.1097/FBP.0000000000000133
Wischik CM, Staff RT, Wischik DJ, Bentham P, Murray AD, Storey JM, Kook KA, Harrington CR (2015) Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer's disease. J Alzheimers Dis 44(2):705–720. doi:10.3233/JAD-142874
Mori T, Koyama N, Segawa T, Maeda M, Maruyama N, Kinoshita N, Hou H, Tan J et al (2014) Methylene blue modulates beta-secretase, reverses cerebral amyloidosis, and improves cognition in transgenic mice. J Biol Chem 289(44):30303–30317. doi:10.1074/jbc.M114.568212
Paban V, Manrique C, Filali M, Maunoir-Regimbal S, Fauvelle F, Alescio-Lautier B (2014) Therapeutic and preventive effects of methylene blue on Alzheimer's disease pathology in a transgenic mouse model. Neuropharmacology 76(Pt A):68–79. doi:10.1016/j.neuropharm.2013.06.033
Hosokawa M, Arai T, Masuda-Suzukake M, Nonaka T, Yamashita M, Akiyama H, Hasegawa M (2012) Methylene blue reduced abnormal tau accumulation in P301L tau transgenic mice. PLoS One 7(12):e52389. doi:10.1371/journal.pone.0052389
Necula M, Breydo L, Milton S, Kayed R, van der Veer WE, Tone P, Glabe CG (2007) Methylene blue inhibits amyloid Abeta oligomerization by promoting fibrillization. Biochemistry 46(30):8850–8860. doi:10.1021/bi700411k
Medina DX, Caccamo A, Oddo S (2011) Methylene blue reduces abeta levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol 21(2):140–149. doi:10.1111/j.1750-3639.2010.00430.x
Atamna H, Kumar R (2010) Protective role of methylene blue in Alzheimer's disease via mitochondria and cytochrome c oxidase. J Alzheimers Dis 20(Suppl 2):S439–S452. doi:10.3233/JAD-2010-100414
Congdon EE, Wu JW, Myeku N, Figueroa YH, Herman M, Marinec PS, Gestwicki JE, Dickey CA et al (2012) Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy 8(4):609–622. doi:10.4161/auto.19048
Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR (1996) Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci U S A 93(20):11213–11218
Hattori M, Sugino E, Minoura K, In Y, Sumida M, Taniguchi T, Tomoo K, Ishida T (2008) Different inhibitory response of cyanidin and methylene blue for filament formation of tau microtubule-binding domain. Biochem Biophys Res Commun 374(1):158–163. doi:10.1016/j.bbrc.2008.07.001
Crowe A, James MJ, Lee VM, Smith AB 3rd, Trojanowski JQ, Ballatore C, Brunden KR (2013) Aminothienopyridazines and methylene blue affect Tau fibrillization via cysteine oxidation. J Biol Chem 288(16):11024–11037. doi:10.1074/jbc.M112.436006
Zhao M, Liang F, Xu H, Yan W, Zhang J (2016) Methylene blue exerts a neuroprotective effect against traumatic brain injury by promoting autophagy and inhibiting microglial activation. Mol Med Rep 13(1):13–20. doi:10.3892/mmr.2015.4551
Talley Watts L, Long JA, Boggs RC, Manga H, Huang S, Shen Q, Duong TQ (2016) Delayed methylene blue improves lesion volume, multi-parametric quantitative magnetic resonance imaging measurements, and behavioral outcome after traumatic brain injury. J Neurotrauma 33(2):194–202. doi:10.1089/neu.2015.3904
Talley Watts L, Long JA, Chemello J, Van Koughnet S, Fernandez A, Huang S, Shen Q, Duong TQ (2014) Methylene blue is neuroprotective against mild traumatic brain injury. J Neurotrauma 31(11):1063–1071. doi:10.1089/neu.2013.3193
Fenn AM, Skendelas JP, Moussa DN, Muccigrosso MM, Popovich PG, Lifshitz J, Eiferman DS, Godbout JP (2015) Methylene blue attenuates traumatic brain injury-associated neuroinflammation and acute depressive-like behavior in mice. J Neurotrauma 32(2):127–138. doi:10.1089/neu.2014.3514
Mangus DB, Huang L, Applegate PM, Gatling JW, Zhang J, Applegate RL 2nd (2014) A systematic review of neuroprotective strategies after cardiac arrest: from bench to bedside (part I—protection via specific pathways). Med Gas Res 4:9. doi:10.1186/2045-9912-4-9
Schneider A, Bottiger BW, Popp E (2009) Cerebral resuscitation after cardiocirculatory arrest. Anesth Analg 108(3):971–979. doi:10.1213/ane.0b013e318193ca99
Kim YM, Yim HW, Jeong SH, Klem ML, Callaway CW (2012) Does therapeutic hypothermia benefit adult cardiac arrest patients presenting with non-shockable initial rhythms?: a systematic review and meta-analysis of randomized and non-randomized studies. Resuscitation 83(2):188–196. doi:10.1016/j.resuscitation.2011.07.031
Nolan JP, Neumar RW, Adrie C, Aibiki M, Berg RA, Bbttiger BW, Callaway C, Clark RS et al (2010) Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a scientific statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke (Part II). Int Emerg Nurs 18(1):8–28. doi:10.1016/j.ienj.2009.07.001
Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WG, Billi J, Bottiger BW, Okada K et al (2003) Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation 108(1):118–121. doi:10.1161/01.CIR.0000079019.02601.90
Scirica BM (2013) Therapeutic hypothermia after cardiac arrest. Circulation 127(2):244–250. doi:10.1161/CIRCULATIONAHA.111.076851
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S et al (2016) Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 133(4):e38–360. doi:10.1161/CIR.0000000000000350
Victor Li XB, Paul Szelemej and Jiming Kong. Delayed neuronal death in ischemic stroke: molecular pathways. In: Balestrino DM (ed) Advances in the preclinical study of ischemic stroke. InTech,
Kim J, Park JE, Nahrendorf M, Kim DE (2016) Direct thrombus imaging in stroke. J Stroke 18(3):286–296. doi:10.5853/jos.2016.00906
Kirmani JF, Alkawi A, Panezai S, Gizzi M (2012) Advances in thrombolytics for treatment of acute ischemic stroke. Neurology 79(13 Suppl 1):S119–S125. doi:10.1212/WNL.0b013e3182695882
The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333(24):1581–1587. doi:10.1056/NEJM199512143332401
Ferrer I (2006) Apoptosis: future targets for neuroprotective strategies. Cerebrovasc Dis 21(Suppl 2):9–20. doi:10.1159/000091699
Siesjo BK (1981) Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab 1(2):155–185. doi:10.1038/jcbfm.1981.18
Kaur C, Ling EA (2008) Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res 5(1):71–81
Giulian D, Li J, Leara B, Keenen C (1994) Phagocytic microglia release cytokines and cytotoxins that regulate the survival of astrocytes and neurons in culture. Neurochem Int 25(3):227–233
Bothe HW, Bosma HJ, Hofer H, Hossmann KA, Angermeier WF (1986) Selective vulnerability of hippocampus and disturbances of memory storage after mild unilateral ischemia of gerbil brain. Stroke 17(6):1160–1163
Sharma HS, Miclescu A, Wiklund L (2011) Cardiac arrest-induced regional blood-brain barrier breakdown, edema formation and brain pathology: a light and electron microscopic study on a new model for neurodegeneration and neuroprotection in porcine brain. J Neural Transm 118(1):87–114. doi:10.1007/s00702-010-0486-4
Martijn C, Wiklund L (2010) Effect of methylene blue on the genomic response to reperfusion injury induced by cardiac arrest and cardiopulmonary resuscitation in porcine brain. BMC Med Genet 3:27. doi:10.1186/1755-8794-3-27
Xie L, Li W, Winters A, Yuan F, Jin K, Yang S (2013) Methylene blue induces macroautophagy through 5′ adenosine monophosphate-activated protein kinase pathway to protect neurons from serum deprivation. Front Cell Neurosci 7:56. doi:10.3389/fncel.2013.00056
Fang Q, Yan X, Li S, Sun Y, Xu L, Shi Z, Wu M, Lu Y et al (2016) Methylene blue ameliorates ischemia/reperfusion-induced cerebral edema: an MRI and transmission electron microscope study. Acta Neurochir Suppl 121:227–236. doi:10.1007/978-3-319-18497-5_41
Nixon RA (2007) Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci 120(Pt 23):4081–4091. doi:10.1242/jcs.019265
Arrazola MS, Silva-Alvarez C, Inestrosa NC (2015) How the Wnt signaling pathway protects from neurodegeneration: the mitochondrial scenario. Front Cell Neurosci 9:166. doi:10.3389/fncel.2015.00166
Irwin JA, Wong HE, Kwon I (2013) Different fates of Alzheimer’s disease amyloid-beta fibrils remodeled by biocompatible small molecules. Biomacromolecules 14(1):264–274. doi:10.1021/bm3016994
Supnet C, Bezprozvanny I (2010) Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer's disease. J Alzheimers Dis 20(Suppl 2):S487–S498. doi:10.3233/JAD-2010-100306
Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD (2009) Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 106(34):14670–14675. doi:10.1073/pnas.0903563106
Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y et al (2001) Mitochondrial abnormalities in Alzheimer's disease. J Neurosci 21(9):3017–3023
Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH (2006) Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 15(9):1437–1449. doi:10.1093/hmg/ddl066
Mosconi L (2005) Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging 32(4):486–510. doi:10.1007/s00259-005-1762-7
Devi L, Anandatheerthavarada HK (2010) Mitochondrial trafficking of APP and alpha synuclein: relevance to mitochondrial dysfunction in Alzheimer's and Parkinson's diseases. Biochim Biophys Acta 1802(1):11–19. doi:10.1016/j.bbadis.2009.07.007
Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X et al (2004) ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science 304(5669):448–452. doi:10.1126/science.1091230
Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH (2011) Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer's disease. Hum Mol Genet 20(23):4515–4529. doi:10.1093/hmg/ddr381
Pratico D (2008) Oxidative stress hypothesis in Alzheimer's disease: a reappraisal. Trends Pharmacol Sci 29(12):609–615. doi:10.1016/j.tips.2008.09.001
Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA (2009) S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324(5923):102–105. doi:10.1126/science.1171091
Atamna H (2009) Amino acids variations in amyloid-beta peptides, mitochondrial dysfunction, and new therapies for Alzheimer's disease. J Bioenerg Biomembr 41(5):457–464. doi:10.1007/s10863-009-9246-2
Violet M, Chauderlier A, Delattre L, Tardivel M, Chouala MS, Sultan A, Marciniak E, Humez S et al (2015) Prefibrillar Tau oligomers alter the nucleic acid protective function of Tau in hippocampal neurons in vivo. Neurobiol Dis 82:540–551. doi:10.1016/j.nbd.2015.09.003
Zakaria A, Hamdi N, Abdel-Kader RM (2016) Methylene blue improves brain mitochondrial ABAD functions and decreases Abeta in a neuroinflammatory Alzheimer's disease mouse model. Mol Neurobiol 53(2):1220–1228. doi:10.1007/s12035-014-9088-8
Lim YA, Grimm A, Giese M, Mensah-Nyagan AG, Villafranca JE, Ittner LM, Eckert A, Gotz J (2011) Inhibition of the mitochondrial enzyme ABAD restores the amyloid-beta-mediated deregulation of estradiol. PLoS One 6(12):e28887. doi:10.1371/journal.pone.0028887
Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM et al (2005) ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB J 19(6):597–598. doi:10.1096/fj.04-2582fje
Yarza R, Vela S, Solas M, Ramirez MJ (2015) C-Jun N-terminal kinase (JNK) signaling as a therapeutic target for Alzheimer's disease. Front Pharmacol 6:321. doi:10.3389/fphar.2015.00321
Piedrahita D, Hernandez I, Lopez-Tobon A, Fedorov D, Obara B, Manjunath BS, Boudreau RL, Davidson B et al (2010) Silencing of CDK5 reduces neurofibrillary tangles in transgenic Alzheimer's mice. J Neurosci 30(42):13966–13976. doi:10.1523/JNEUROSCI.3637-10.2010
Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer's disease. J Neurochem 104(6):1433–1439. doi:10.1111/j.1471-4159.2007.05194.x
Mietelska-Porowska A, Wasik U, Goras M, Filipek A, Niewiadomska G (2014) Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci 15(3):4671–4713. doi:10.3390/ijms15034671
Rodriguez-Martin T, Cuchillo-Ibanez I, Noble W, Nyenya F, Anderton BH, Hanger DP (2013) Tau phosphorylation affects its axonal transport and degradation. Neurobiol Aging 34(9):2146–2157. doi:10.1016/j.neurobiolaging.2013.03.015
Sulistio YA, Heese K (2016) The ubiquitin-proteasome system and molecular chaperone deregulation in Alzheimer's disease. Mol Neurobiol 53(2):905–931. doi:10.1007/s12035-014-9063-4
Taniguchi S, Suzuki N, Masuda M, Hisanaga S, Iwatsubo T, Goedert M, Hasegawa M (2005) Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J Biol Chem 280(9):7614–7623. doi:10.1074/jbc.M408714200
Lira-De Leon KI, Garcia-Gutierrez P, Serratos IN, Palomera-Cardenas M, Figueroa-Corona Mdel P, Campos-Pena V, Meraz-Rios MA (2013) Molecular mechanism of tau aggregation induced by anionic and cationic dyes. J Alzheimers Dis 35(2):319–334. doi:10.3233/JAD-121765
Hochgrafe K, Sydow A, Matenia D, Cadinu D, Konen S, Petrova O, Pickhardt M, Goll P et al (2015) Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human Tau. Acta Neuropathol Commun 3:25. doi:10.1186/s40478-015-0204-4
van Bebber F, Paquet D, Hruscha A, Schmid B, Haass C (2010) Methylene blue fails to inhibit Tau and polyglutamine protein dependent toxicity in zebrafish. Neurobiol Dis 39(3):265–271. doi:10.1016/j.nbd.2010.03.023
Fatouros C, Pir GJ, Biernat J, Koushika SP, Mandelkow E, Mandelkow EM, Schmidt E, Baumeister R (2012) Inhibition of tau aggregation in a novel Caenorhabditis elegans model of tauopathy mitigates proteotoxicity. Hum Mol Genet 21(16):3587–3603. doi:10.1093/hmg/dds190
Driver JA, Logroscino G, Gaziano JM, Kurth T (2009) Incidence and remaining lifetime risk of Parkinson disease in advanced age. Neurology 72(5):432–438. doi:10.1212/01.wnl.0000341769.50075.bb
Baumann CR (2012) Epidemiology, diagnosis and differential diagnosis in Parkinson's disease tremor. Parkinsonism Relat Disord 18(Suppl 1):S90–S92. doi:10.1016/S1353-8020(11)70029-3
Martinez-Ramirez D, Almeida L, Giugni JC, Ahmed B, Higuchi MA, Little CS, Chapman JP, Mignacca C et al (2015) Rate of aspiration pneumonia in hospitalized Parkinson's disease patients: a cross-sectional study. BMC Neurol 15:104. doi:10.1186/s12883-015-0362-9
Tjaden K (2008) Speech and swallowing in Parkinson's disease. Top Geriatr Rehabil 24(2):115–126. doi:10.1097/01.TGR.0000318899.87690.44
Iwasaki S, Narabayashi Y, Hamaguchi K, Iwasaki A, Takakusagi M (1990) Cause of death among patients with Parkinson's disease: a rare mortality due to cerebral haemorrhage. J Neurol 237(2):77–79
Lewitt PA (2008) Levodopa for the treatment of Parkinson's disease. N Engl J Med 359(23):2468–2476. doi:10.1056/NEJMct0800326
Arduino DM, Esteves AR, Cortes L, Silva DF, Patel B, Grazina M, Swerdlow RH, Oliveira CR et al (2012) Mitochondrial metabolism in Parkinson's disease impairs quality control autophagy by hampering microtubule-dependent traffic. Hum Mol Genet 21(21):4680–4702. doi:10.1093/hmg/dds309
Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK (2008) Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283(14):9089–9100. doi:10.1074/jbc.M710012200
Elkon H, Don J, Melamed E, Ziv I, Shirvan A, Offen D (2002) Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome C oxidase. J Mol Neurosci 18(3):229–238. doi:10.1385/JMN:18:3:229
Winklhofer KF, Haass C (2010) Mitochondrial dysfunction in Parkinson's disease. Biochim Biophys Acta 1802(1):29–44. doi:10.1016/j.bbadis.2009.08.013
Lee KK, Boelsterli UA (2014) Bypassing the compromised mitochondrial electron transport with methylene blue alleviates efavirenz/isoniazid-induced oxidant stress and mitochondria-mediated cell death in mouse hepatocytes. Redox Biol 2:599–609. doi:10.1016/j.redox.2014.03.003
Todorovic M, Wood SA, Mellick GD (2016) Nrf2: a modulator of Parkinson's disease? J Neural Transm 123(6):611–619. doi:10.1007/s00702-016-1563-0
Tretter L, Horvath G, Holgyesi A, Essek F, Adam-Vizi V (2014) Enhanced hydrogen peroxide generation accompanies the beneficial bioenergetic effects of methylene blue in isolated brain mitochondria. Free Radic Biol Med 77:317–330. doi:10.1016/j.freeradbiomed.2014.09.024
Delport A, Harvey BH, Petzer A, Petzer JP (2014) Azure B and a synthetic structural analogue of methylene blue, ethylthioninium chloride, present with antidepressant-like properties. Life Sci 117(2):56–66. doi:10.1016/j.lfs.2014.10.005
Yonutas HM, Vekaria HJ, Sullivan PG (2016) Mitochondrial specific therapeutic targets following brain injury. Brain Res 1640(Pt A):77–93. doi:10.1016/j.brainres.2016.02.007
Haddad SH, Arabi YM (2012) Critical care management of severe traumatic brain injury in adults. Scand J Trauma Resusc Emerg Med 20:12. doi:10.1186/1757-7241-20-12
Chesnut RM (1995) Secondary brain insults after head injury: clinical perspectives. New Horiz 3(3):366–375
Kimbler DE, Murphy M, Dhandapani KM (2011) Concussion and the adolescent athlete. J Neurosci Nurs 43(6):286–290. doi:10.1097/JNN.0b013e31823858a6
Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC, Manley GT, Gruen RL (2012) Early management of severe traumatic brain injury. Lancet 380(9847):1088–1098. doi:10.1016/S0140-6736(12)60864-2
Werner C, Engelhard K (2007) Pathophysiology of traumatic brain injury. Br J Anaesth 99(1):4–9. doi:10.1093/bja/aem131
Hiebert JB, Shen Q, Thimmesch AR, Pierce JD (2015) Traumatic brain injury and mitochondrial dysfunction. Am J Med Sci 350(2):132–138. doi:10.1097/MAJ.0000000000000506
Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV (2015) Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis Treat 11:97–106. doi:10.2147/NDT.S65815
Maxwell WL (2015) Development of concepts in the pathology of traumatic axonal and traumatic brain injury. In: Kobeissy FH (ed) Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects. Frontiers in Neuroengineering. Boca Raton (FL)
Abdul-Muneer PM, Chandra N, Haorah J (2015) Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol 51(3):966–979. doi:10.1007/s12035-014-8752-3
Raghupathi R (2004) Cell death mechanisms following traumatic brain injury. Brain Pathol 14(2):215–222
Ahmed AI, Bullock MR, Dietrich WD (2016) Hypothermia in traumatic brain injury. Neurosurg Clin N Am 27(4):489–497. doi:10.1016/j.nec.2016.05.004
Bennett MH, Trytko B, Jonker B (2012) Hyperbaric oxygen therapy for the adjunctive treatment of traumatic brain injury. Cochrane Database Syst Rev 12:CD004609. doi:10.1002/14651858.CD004609.pub3
Mendes Arent A, de Souza LF, Walz R, Dafre AL (2014) Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. Biomed Res Int 2014:723060. doi:10.1155/2014/723060
Suliman NA, Mat Taib CN, Mohd Moklas MA, Adenan MI, Hidayat Baharuldin MT, Basir R (2016) Establishing natural nootropics: recent molecular enhancement influenced by natural nootropic. Evid Based Complement Alternat Med 2016:4391375. doi:10.1155/2016/4391375
Camfield DA, Stough C, Farrimond J, Scholey AB (2014) Acute effects of tea constituents L-theanine, caffeine, and epigallocatechin gallate on cognitive function and mood: a systematic review and meta-analysis. Nutr Rev 72(8):507–522. doi:10.1111/nure.12120
Varga MD (2012) Adderall abuse on college campuses: a comprehensive literature review. J Evid Based Soc Work 9(3):293–313. doi:10.1080/15433714.2010.525402
Gonzalez-Lima F, Barksdale BR, Rojas JC (2014) Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochem Pharmacol 88(4):584–593. doi:10.1016/j.bcp.2013.11.010
Rodriguez P, Zhou W, Barrett DW, Altmeyer W, Gutierrez JE, Li J, Lancaster JL, Gonzalez-Lima F et al (2016) Multimodal randomized functional MR imaging of the effects of methylene blue in the human brain. Radiology 281(2):516–526. doi:10.1148/radiol.2016152893
Barrett DW, Gonzalez-Lima F (2013) Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 230:13–23. doi:10.1016/j.neuroscience.2012.11.016
Grimm A, Eckert A (2017) Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. doi:10.1111/jnc.14037
Grimm A, Friedland K, Eckert A (2016) Mitochondrial dysfunction: the missing link between aging and sporadic Alzheimer's disease. Biogerontology 17(2):281–296. doi:10.1007/s10522-015-9618-4
Reddy PH, Reddy TP (2011) Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr Alzheimer Res 8(4):393–409
Voloboueva LA, Giffard RG (2011) Inflammation, mitochondria, and the inhibition of adult neurogenesis. J Neurosci Res 89(12):1989–1996. doi:10.1002/jnr.22768
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132(4):645–660. doi:10.1016/j.cell.2008.01.033
Kernie SG, Parent JM (2010) Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol Dis 37(2):267–274. doi:10.1016/j.nbd.2009.11.002
Zhang RL, Zhang ZG, Zhang L, Chopp M (2001) Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience 105(1):33–41
Liu J, Solway K, Messing RO, Sharp FR (1998) Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci 18(19):7768–7778
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8(9):963–970. doi:10.1038/nm747
Bettio LEB, Rajendran L, Gil-Mohapel J (2017) The effects of aging in the hippocampus and cognitive decline. Neurosci Biobehav Rev 79:66–86. doi:10.1016/j.neubiorev.2017.04.030
Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y (2003) Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol 106(6):518–526. doi:10.1007/s00401-003-0766-2
Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR (2003) Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res 63(14):4021–4027
Hallenbeck JM (2002) The many faces of tumor necrosis factor in stroke. Nat Med 8(12):1363–1368. doi:10.1038/nm1202-1363
Davies CA, Loddick SA, Toulmond S, Stroemer RP, Hunt J, Rothwell NJ (1999) The progression and topographic distribution of interleukin-1beta expression after permanent middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 19(1):87–98. doi:10.1097/00004647-199901000-00010
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer’s disease brains. Neurology 38(8):1285–1291
Monje ML, Toda H, Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302(5651):1760–1765. doi:10.1126/science.1088417
Torroglosa A, Murillo-Carretero M, Romero-Grimaldi C, Matarredona ER, Campos-Caro A, Estrada C (2007) Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells 25(1):88–97. doi:10.1634/stemcells.2006-0131
Fike JR, Rola R, Limoli CL (2007) Radiation response of neural precursor cells. Neurosurg Clin N Am 18(1):115–127, x. doi:10.1016/j.nec.2006.10.010
Cordeau-Lossouarn L, Vayssiere JL, Larcher JC, Gros F, Croizat B (1991) Mitochondrial maturation during neuronal differentiation in vivo and in vitro. Biol Cell 71(1–2):57–65
Kirby DM, Rennie KJ, Smulders-Srinivasan TK, Acin-Perez R, Whittington M, Enriquez JA, Trevelyan AJ, Turnbull DM et al (2009) Transmitochondrial embryonic stem cells containing pathogenic mtDNA mutations are compromised in neuronal differentiation. Cell Prolif 42(4):413–424. doi:10.1111/j.1365-2184.2009.00612.x
Papa S, Petruzzella V, Scacco S, Vergari R, Panelli D, Tamborra R, Corsi P, Picciariello M et al (2004) Respiratory complex I in brain development and genetic disease. Neurochem Res 29(3):547–560
Wong A, Cavelier L, Collins-Schramm HE, Seldin MF, McGrogan M, Savontaus ML, Cortopassi GA (2002) Differentiation-specific effects of LHON mutations introduced into neuronal NT2 cells. Hum Mol Genet 11(4):431–438
Xie L, Choudhury GR, Wang J, Park Y, Liu R, Yuan F, Zhang CL, Yorio T et al (2014) Methylene blue promotes quiescence of rat neural progenitor cells. Front Cell Neurosci 8:315. doi:10.3389/fncel.2014.00315
van der Ven AT, Pape JC, Hermann D, Schloesser R, Genius J, Fischer N, Mossner R, Scherbaum N et al (2017) Methylene blue (tetramethylthionine chloride) influences the mobility of adult neural stem cells: a potentially novel therapeutic mechanism of a therapeutic approach in the treatment of Alzheimer’s disease. J Alzheimers Dis 57(2):531–540. doi:10.3233/JAD-160755
Crooks J (1982) Haemolytic jaundice in a neonate after intra-amniotic injection of methylene blue. Arch Dis Child 57(11):872–873
Porat R, Gilbert S, Magilner D (1996) Methylene blue-induced phototoxicity: an unrecognized complication. Pediatrics 97(5):717–721
Spahr RC, Salsburey DJ, Krissberg A, Prin W (1980) Intraamniotic injection of methylene blue leading to methemoglobinemia in one of twins. Int J Gynaecol Obstet 17(5):477–478
Wolvetang T, Janse R, Ter Horst M (2016) Serotonin syndrome after methylene blue administration during cardiac surgery: a case report and review. J Cardiothorac Vasc Anesth 30(4):1042–1045. doi:10.1053/j.jvca.2015.11.019
Hencken L, To L, Ly N, Morgan JA (2016) Serotonin syndrome following methylene blue administration for vasoplegic syndrome. J Card Surg 31(4):208–210. doi:10.1111/jocs.12705
Smith CJ, Wang D, Sgambelluri A, Kramer RS, Gagnon DJ (2015) Serotonin syndrome following methylene blue administration during cardiothoracic surgery. J Pharm Pract 28(2):207–211. doi:10.1177/0897190014568389
Acknowledgements
This work was supported by Research Grant NS086929 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, USA, and an American Heart Association Grant-in-Aid 15GRNT25240004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors confirm that their contributions to this article are free from conflict of interest.
Rights and permissions
About this article
Cite this article
Tucker, D., Lu, Y. & Zhang, Q. From Mitochondrial Function to Neuroprotection—an Emerging Role for Methylene Blue. Mol Neurobiol 55, 5137–5153 (2018). https://doi.org/10.1007/s12035-017-0712-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0712-2