Abstract
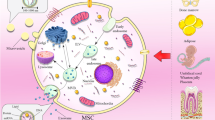
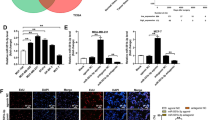
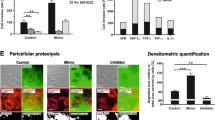
Mesenchymal stem cells (MSCs) may play a pivotal role in shaping the tumor microenvironment (TME), influencing tumor growth. Nonetheless, conflicting evidence exists regarding the distinct impacts of MSCs on tumor progression, with some studies suggesting promotion while others indicate suppression of tumor cell growth. Considering that oxidative stress is implicated in the dynamic interaction between components of the TME and tumor cells, we investigated the contribution of exosomes released by hydrogen peroxide (H2O2)-treated MSCs to murine mammary tumor growth and progression. Additionally, we aimed to identify the underlying mechanism through which MSC-derived exosomes affect breast tumor growth and angiogenesis. Our findings demonstrated that exosomes released by H2O2-treated, stress-induced MSCs (St-MSC Exo) promoted breast cancer cell progression by inducing the expression of vascular endothelial growth factor (VEGF) and markers associated with epithelial-to-mesenchymal transition. Further clarification revealed that the promoting effect of St-MSC Exo on VEGF expression may, in part, depend on activating STAT3 signaling in BC cells. In contrast, exosomes derived from untreated MSCs retarded JAK1/STAT3 phosphorylation and reduced VEGF expression. Additionally, our observations revealed that the activation of the transcription factor NF-κB in BC cells, stimulated with St-MSC Exo, occurs concurrently with an increase in intracellular ROS production. Moreover, we observed that the increase in VEGF secretion into the conditioned media of 4T1 BC, mediated by St-MSC Exo, positively influenced endothelial cell proliferation, migration, and vascular behavior in vitro. In turn, our in vivo studies confirmed that St-MSC Exo, but not exosomes derived from untreated MSCs, exhibited a significant promoting effect on breast tumorigenicity. Collectively, our findings provide new insights into how MSCs may contribute to modulating the TME. We propose a novel mechanism through which exosomes derived from oxidative stress-induced MSCs may contribute to tumor progression and angiogenesis.







Similar content being viewed by others
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Meirson T, Gil-Henn H, Samson AO (2020) Invasion and metastasis: the elusive hallmark of cancer. Oncogene 39:2024–2026
Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z, Li X, Cao K, Deng H, He Y (2020) The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res 39:1–19
Weis SM, Cheresh DA (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17:1359–1370
Mali SB (2015) Review of STAT3 (Signal Transducers and Activators of Transcription) in head and neck cancer. Oral Oncol 51:565–569
Gritsina G, Xiao F, O’Brien SW, Gabbasov R, Maglaty MA, Xu R-H, Thapa RJ, Zhou Y, Nicolas E, Litwin S (2015) Targeted Blockade of JAK/STAT3 Signaling Inhibits Ovarian Carcinoma GrowthTargeting JAK/STAT3 Inhibits Ovarian Cancer. Mol Cancer Ther 14:1035–1047
Ku JM, Kim SR, Hong SH, Choi H-S, Seo HS, Shin YC, Ko S-G (2015) Cucurbitacin D induces cell cycle arrest and apoptosis by inhibiting STAT3 and NF-κB signaling in doxorubicin-resistant human breast carcinoma (MCF7/ADR) cells. Mol Cell Biochem 409:33–43
Masoumi-Dehghi S, Babashah S, Sadeghizadeh M (2020) microRNA-141-3p-containing small extracellular vesicles derived from epithelial ovarian cancer cells promote endothelial cell angiogenesis through activating the JAK/STAT3 and NF-κB signaling pathways. J cell commun signal 14:233–244
Zhou C, Ma J, Su M, Shao D, Zhao J, Zhao T, Song Z, Meng Y, Jiao P (2018) Down-regulation of STAT3 induces the apoptosis and G1 cell cycle arrest in esophageal carcinoma ECA109 cells. Cancer Cell Int 18:53. https://doi.org/10.1186/s12935-018-0549-4
Wu P, Wu D, Zhao L, Huang L, Shen G, Huang J, Chai Y (2016) Prognostic role of STAT3 in solid tumors: a systematic review and meta-analysis. Oncotarget 7:19863
Don-Doncow N, Marginean F, Coleman I, Nelson PS, Ehrnström R, Krzyzanowska A, Morrissey C, Hellsten R, Bjartell A (2017) Expression of STAT3 in prostate cancer metastases. Eur Urol 71:313–316
Liu X, Xiao Q, Bai X, Yu Z, Sun M, Zhao H, Mi X, Wang E, Yao W, Jin F (2014) Activation of STAT3 is involved in malignancy mediated by CXCL12-CXCR4 signaling in human breast cancer. Oncol Rep 32:2760–2768
Katsha A, Arras J, Soutto M, Belkhiri A, El-Rifai W (2014) AURKA regulates JAK2–STAT3 activity in human gastric and esophageal cancers. Mol Oncol 8:1419–1428
Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nat Rev Immunol 8:726–736
Karp JM, Teo GSL (2009) Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4:206–216
Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC (2004) Gastric cancer originating from bone marrow-derived cells. Science 306:1568–1571
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563
Pan W, Chen H, Wang A, Wang F, Zhang X (2023) Challenges and strategies: Scalable and efficient production of mesenchymal stem cells-derived exosomes for cell-free therapy. Life Sci 319:121524. https://doi.org/10.1016/j.lfs.2023.121524
Katsha AM, Ohkouchi S, Xin H, Kanehira M, Sun R, Nukiwa T, Saijo Y (2011) Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther 19:196–203
Dwivedi M, Ghosh D, Saha A, Hasan S, Jindal D, Yadav H, Yadava A, Dwivedi M (2023) Biochemistry of exosomes and their theranostic potential in human diseases. Life Sci 315:121369. https://doi.org/10.1016/j.lfs.2023.121369
Zhang F, Guo J, Zhang Z, Qian Y, Wang G, Duan M, Zhao H, Yang Z, Jiang X (2022) Mesenchymal stem cell-derived exosome: A tumor regulator and carrier for targeted tumor therapy. Cancer Lett 526:29–40
Al-Awsi GRL, Alsaikhan F, Margiana R, Ahmad I, Patra I, Najm MA, Yasin G, Rasulova I, Hammid AT, Kzar HH (2023) Shining the light on mesenchymal stem cell-derived exosomes in breast cancer. Stem Cell Res Ther 14:21
Aslan C, Maralbashi S, Salari F, Kahroba H, Sigaroodi F, Kazemi T, Kharaziha P (2019) Tumor-derived exosomes: Implication in angiogenesis and antiangiogenesis cancer therapy. J Cell Physiol 234:16885–16903
Zhao R, Chen X, Song H, Bie Q, Zhang B (2020) Dual Role of MSC-Derived Exosomes in Tumor Development. Stem Cells Int 2020:8844730. https://doi.org/10.1155/2020/8844730
Jezierska-Drutel A, Rosenzweig SA, Neumann CA (2013) Role of oxidative stress and the microenvironment in breast cancer development and progression. Adv Cancer Res 119:107–125
Guo L, Chen Y, Feng X, Sun D, Sun J, Mou S, Zhao K, An R (2022) Oxidative stress-induced endothelial cells-derived exosomes accelerate skin flap survival through Lnc NEAT1-mediated promotion of endothelial progenitor cell function. Stem Cell Res Ther 13:325. https://doi.org/10.1186/s13287-022-03013-9
Liu Y, Li Q, Hosen MR, Zietzer A, Flender A, Levermann P, Schmitz T, Frühwald D, Goody P, Nickenig G (2019) Atherosclerotic conditions promote the packaging of functional microRNA-92a-3p into endothelial microvesicles. Circ Res 124:575–587
Pu X, Ma S, Gao Y, Xu T, Chang P, Dong L (2020) Mesenchymal stem cell-derived exosomes: biological function and their therapeutic potential in radiation damage. Cells 10:42
Yan C, Xv Y, Lin Z, Endo Y, Xue H, Hu Y, Hu L, Chen L, Cao F, Zhou W (2022) Human umbilical cord mesenchymal stem cell-derived exosomes accelerate diabetic wound healing via ameliorating oxidative stress and promoting angiogenesis. Front Bioeng Biotechnol 10:829868
Anjos‐Afonso F and Bonnet D (2008) Isolation, culture, and differentiation potential of mouse marrow stromal cells. Curr Protocols Stem Cell Biol 7:2B-3
Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T (2005) A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb 12:138–142
Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N, Javan M (2017) MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol 40:457–470
Pakravan K, Mossahebi-Mohammadi M, Ghazimoradi MH, Cho WC, Sadeghizadeh M, Babashah S (2022) Monocytes educated by cancer-associated fibroblasts secrete exosomal miR-181a to activate AKT signaling in breast cancer cells. J Transl Med 20:1–21
Livak KJ and Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Nosaeid MH, Mahdian R, Jamali S, Maryami F, Babashah S, Maryami F, Karimipoor M, Zeinali S (2009) Validation and comparison of two quantitative real-time PCR assays for direct detection of DMD/BMD carriers. Clin Biochem 42:1291–1299
Faustino-Rocha A, Oliveira PA, Pinho-Oliveira J, Teixeira-Guedes C, Soares-Maia R, da Costa RG, Colaço B, Pires MJ, Colaço J, Ferreira R, Ginja M (2013) Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab Anim (NY) 42:217–224. https://doi.org/10.1038/laban.254
Feldman AT and Wolfe D (2014) Tissue processing and hematoxylin and eosin staining. Histopathology 1180:31–43
Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J (2007) In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood, The Journal of the American Society of Hematology 109:4761–4768
Liu T, Niu Y, Feng Y, Niu R, Yu Y, Lv A, Yang Y (2008) Methylation of CpG islands of p16INK4a and cyclinD1 overexpression associated with progression of intraductal proliferative lesions of the breast. Hum Pathol 39:1637–1646
Liao DJ, Thakur A, Wu J, Biliran H, Sarkar FH (2007) Perspectives on c-Myc, Cyclin D1, and their interaction in cancer formation, progression, and response to chemotherapy. Crit Rev Oncog 13:93–158. https://doi.org/10.1615/critrevoncog.v13.i2.10
Serrano-Gomez SJ, Maziveyi M, Alahari SK (2016) Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer 15:1–14
Manore SG, Doheny DL, Wong GL, Lo HW (2022) IL-6/JAK/STAT3 Signaling in Breast Cancer Metastasis: Biology and Treatment. Front Oncol 12:866014. https://doi.org/10.3389/fonc.2022.866014
Qin J-J, Yan L, Zhang J, Zhang W-D (2019) STAT3 as a potential therapeutic target in triple negative breast cancer: a systematic review. J Exp Clin Cancer Res 38:1–16
Ren X, Duan L, He Q, Zhang Z, Zhou Y, Wu D, Pan J, Pei D, Ding K (2010) Identification of niclosamide as a new small-molecule inhibitor of the STAT3 signaling pathway. ACS Med Chem Lett 1:454–459
Hoesel B, Schmid JA (2013) The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 12:1–15
Fan Y, Mao R, Yang J (2013) NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 4:176–185
Chen AC, Arany PR, Huang Y-Y, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS (2011) Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 6:e22453
Lugano R, Ramachandran M, Dimberg A (2020) Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 77:1745–1770
Secord A and Siamakpour-Reihani S (2017) Angiogenesis. Translational Advances in Gynecologic Cancers, Elsevier, pp. 79–109
Xuan X, Tian C, Zhao M, Sun Y, Huang C (2021) Mesenchymal stem cells in cancer progression and anticancer therapeutic resistance. Cancer Cell Int 21:1–16
Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F III (2011) Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem cells 29:11–19
Motavaf M, Pakravan K, Babashah S, Malekvandfard F, Masoumi M, Sadeghizadeh M (2016) Therapeutic application of mesenchymal stem cell-derived exosomes: A promising cell-free therapeutic strategy in regenerative medicine. Cell Mol Biol (Noisy-le-grand) 62:74–79
Del Fattore A, Luciano R, Saracino R, Battafarano G, Rizzo C, Pascucci L, Alessandri G, Pessina A, Perrotta A, Fierabracci A (2015) Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin Biol Ther 15:495–504
Lee J-K, Park S-R, Jung B-K, Jeon Y-K, Lee Y-S, Kim M-K, Kim Y-G, Jang J-Y, Kim C-W (2013) Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 8:e84256
Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, Xu X, Wang M, Qian H, Xu W (2012) Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 315:28–37. https://doi.org/10.1016/j.canlet.2011.10.002
Qi J, Zhou Y, Jiao Z, Wang X, Zhao Y, Li Y, Chen H, Yang L, Zhu H, Li Y (2017) Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth through hedgehog signaling pathway. Cell Physiol Biochem 42:2242–2254
Huang Y-J, Nan G-X (2019) Oxidative stress-induced angiogenesis. J Clin Neurosci 63:13–16
Auyeung K, K and K Ko J, (2017) Angiogenesis and oxidative stress in metastatic tumor progression: pathogenesis and novel therapeutic approach of colon cancer. Curr Pharm Des 23:3952–3961
Mdkhana B, Goel S, Saleh M, Siddiqui R, Khan N and Elmoselhi A (2022) Role of oxidative stress in angiogenesis and the therapeutic potential of antioxidants in breast cancer. Eur Rev Med Pharmacol Sci 26:4677
D’Aiuto N, Hochmann J, Millán M, Di Paolo A, Bologna-Molina R, Sotelo Silveira J, Arocena M (2022) Hypoxia, acidification and oxidative stress in cells cultured at large distances from an oxygen source. Sci Rep 12:21699
Brown NS, Jones A, Fujiyama C, Harris AL, Bicknell R (2000) Thymidine phosphorylase induces carcinoma cell oxidative stress and promotes secretion of angiogenic factors. Can Res 60:6298–6302
Brown NS, Bicknell R (2001) Hypoxia and oxidative stress in breast cancer Oxidative stress-its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res 3:1–5
Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH (2014) Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood, The Journal of the American Society of Hematology 124:3748–3757
To KK, Cho WC (2022) Exosome secretion from hypoxic cancer cells reshapes the tumor microenvironment and mediates drug resistance. Cancer Drug Resistance 5:577
Atienzar-Aroca S, Flores-Bellver M, Serrano-Heras G, Martinez-Gil N, Barcia JM, Aparicio S, Perez-Cremades D, Garcia-Verdugo JM, Diaz-Llopis M, Romero FJ (2016) Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J Cell Mol Med 20:1457–1466
Shojaei S, Hashemi SM, Ghanbarian H, Salehi M, Mohammadi-Yeganeh S (2019) Effect of mesenchymal stem cells-derived exosomes on tumor microenvironment: tumor progression versus tumor suppression. J Cell Physiol 234:3394–3409
Gao F-y, Li X-t, Xu K, R-t W, Guan X-x (2023) c-MYC mediates the crosstalk between breast cancer cells and tumor microenvironment. Cell Communication and Signaling 21:28
Jin W (2020) Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial–mesenchymal transition. Cells 9:217
Gao P, Niu N, Wei T, Tozawa H, Chen X, Zhang C, Zhang J, Wada Y, Kapron CM, Liu J (2017) The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget 8:69139
Wang S-W, Sun Y-M (2014) The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer. Int J Oncol 44:1032–1040
He N, Kong Y, Lei X, Liu Y, Wang J, Xu C, Wang Y, Du L, Ji K, Wang Q (2018) MSCs inhibit tumor progression and enhance radiosensitivity of breast cancer cells by down-regulating Stat3 signaling pathway. Cell Death Dis 9:1026
Lee H, Herrmann A, Deng J-H, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H (2009) Persistently activated Stat3 maintains constitutive NF-κB activity in tumors. Cancer Cell 15:283–293
Patel M, Horgan PG, McMillan DC, Edwards J (2018) NF-κB pathways in the development and progression of colorectal cancer. Transl Res 197:43–56
Weinberg F, Ramnath N, Nagrath D (2019) Reactive oxygen species in the tumor microenvironment: an overview. Cancers 11:1191
Acknowledgements
The authors are grateful to the members of the Department of Molecular Genetics at Tarbiat Modares University for their excellent support. This work was supported financially by Tarbiat Modares University.
Funding
This work was supported financially by Tarbiat Modares University.
Author information
Authors and Affiliations
Contributions
S.B. conceived and designed the study. M.A., K.P., and M.G. performed all the experiments. Z.M.H and M.G. contributed to the provision of study materials. M.A. drafted the manuscript, and B.B., R.M., and S.B. contributed to revising it. S.B. supervised the study and provided administrative support. All the authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflicts of interest.
Ethics approval and consent to participate
All experimental procedures were performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Tarbiat Modares University (IR.MODARES.REC.1400.164).
Consent for publication
All authors have read the final manuscript and agreed for the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almouh, M., Pakravan, K., Ghazimoradi, M.H. et al. Exosomes released by oxidative stress-induced mesenchymal stem cells promote murine mammary tumor progression through activating the STAT3 signaling pathway. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04934-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04934-0