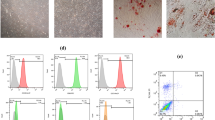
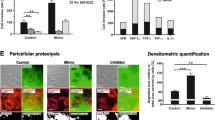
Abstract
Mesenchymal stromal cells (MSCs) play an important role in the development of human cancer. Meanwhile, exosomes released by MSCs can mediate cell–cell communication by delivering microRNAs (miRNAs/miRs). Hence, this study aimed to explore the role of bone marrow mesenchymal stromal cell (BMSC)-derived exosomal miR-551b-3p in breast cancer. In this study, we found that upregulation of miR-551b-5p suppressed the proliferation and migration and induced the apoptosis of breast cancer cells via downregulating tripartite motif-containing protein 31 (TRIM31). In addition, miR-551b-5p could be transferred from BMSCs to breast cancer cells via exosomes; BMSC-derived exosomal miR-551b-3p suppressed the proliferation and migration and promoted the apoptosis and oxidative stress of MDA-MB-231 cells via inhibiting TRIM31. Furthermore, a xenograft mouse model was used to explore the role of BMSC-derived exosomal miR-551b-3p in vivo. We found that BMSC-derived exosomal miR-551b-3p inhibited tumor growth in a mouse xenograft model of breast cancer in vivo. Collectively, these findings indicated that BMSC-derived exosomal miR-551b-3p could suppress the development of breast cancer via downregulating TRIM31. Thus, miR-551b-3p could serve as a potential target for the treatment of breast cancer.








Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Li X, Zeng Z, Wang J, Wu Y, Chen W, Zheng L, et al. MicroRNA-9 and breast cancer. Biomed Pharmacothe = Biomed Pharmacother. 2020;122:109687. https://doi.org/10.1016/j.biopha.2019.109687.
Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global Cancer in women: burden and trends. Cancer Epidemiol, Biomark Prev. 2017;26(4):444–57. https://doi.org/10.1158/1055-9965.Epi-16-0858.
Boukai A, Gonçalves AC, Padoan M, Andrade P, Carvalho N, Lemos F, et al. Outcome of patients with breast cancer treated in a private health care institution in Brazil. J Global Oncol. 2018;4:1–10. https://doi.org/10.1200/jgo.17.00143.
Au FW, Ghai S, Lu FI, Moshonov H, Crystal P. Histological grade and immunohistochemical biomarkers of breast cancer: correlation to ultrasound features. J Ultrasound Med. 2017;36(9):1883–94. https://doi.org/10.1002/jum.14247.
Amin A, Shriver CD, Henry LR, Lenington S, Peoples GE, Stojadinovic A. Breast cancer screening compliance among young women in a free access healthcare system. J Surg Oncol. 2008;97(1):20–4. https://doi.org/10.1002/jso.20895.
Wang R, Zhu Y, Liu X, Liao X, He J, Niu L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer. 2019;19(1):1091. https://doi.org/10.1186/s12885-019-6311-z.
He N, Kong Y, Lei X, Liu Y, Wang J, Xu C, et al. MSCs inhibit tumor progression and enhance radiosensitivity of breast cancer cells by down-regulating Stat3 signaling pathway. Cell Death Dis. 2018;9(10):1026. https://doi.org/10.1038/s41419-018-0949-3.
Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, et al. Mesenchymal stem versus stromal cells: international society for cell & gene therapy (ISCT®) mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy. 2019;21(10):1019–24. https://doi.org/10.1016/j.jcyt.2019.08.002.
Chen K, Liu Q, Tsang LL, Ye Q, Chan HC, Sun Y, et al. Human MSCs promotes colorectal cancer epithelial-mesenchymal transition and progression via CCL5/β-catenin/Slug pathway. Cell Death Dis. 2017;8(5): e2819. https://doi.org/10.1038/cddis.2017.138.
Chu DT, Phuong TNT, Tien NLB, Tran DK, Thanh VV, Quang TL, et al. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21030708.
Zhang X, Sai B, Wang F, Wang L, Wang Y, Zheng L, et al. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol Cancer. 2019;18(1):40. https://doi.org/10.1186/s12943-019-0959-5.
Zhao F, Yu YQ. The prognostic roles of mRNAs of the exosomes derived from bone marrow stromal cells in common malignancies: a bioinformatic study. Onco Targets Ther. 2018;11:7979–86. https://doi.org/10.2147/ott.S172414.
Wang Y, Shao S, Luo M, Huang S, Feng L, Yuan N, et al. Effects of rat bone marrow-derived mesenchymal stem cells on breast cancer cells with differing hormone receptor status. Oncol Lett. 2017;14(6):7269–75. https://doi.org/10.3892/ol.2017.7130.
Lu Y, Zhou Y, Zhang R, Wen L, Wu K, Li Y, et al. Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front Neurosci. 2019;13:209. https://doi.org/10.3389/fnins.2019.00209.
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. https://doi.org/10.1146/annurev-biochem-013118-111902.
Yang F, Ning Z, Ma L, Liu W, Shao C, Shu Y, et al. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol Cancer. 2017;16(1):148. https://doi.org/10.1186/s12943-017-0718-4.
Liang Y, Zhang D, Li L, Xin T, Zhao Y, Ma R, et al. Exosomal microRNA-144 from bone marrow-derived mesenchymal stem cells inhibits the progression of non-small cell lung cancer by targeting CCNE1 and CCNE2. Stem Cell Res Ther. 2020;11(1):87. https://doi.org/10.1186/s13287-020-1580-7.
Naseri Z, Oskuee RK, Jaafari MR, Forouzandeh MM. Exosome-mediated delivery of functionally active miRNA-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int J Nanomed. 2018;13:7727–47. https://doi.org/10.2147/ijn.S182384.
Loh HY, Norman BP, Lai KS, Rahman N, Alitheen NBM, Osman MA. The regulatory role of micrornas in breast cancer. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20194940.
Bertoli G, Cava C, Castiglioni I. MicroRNAs: new biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015;5(10):1122–43. https://doi.org/10.7150/thno.11543.
Song G, Zhang H, Chen C, Gong L, Chen B, Zhao S, et al. miR-551b regulates epithelial-mesenchymal transition and metastasis of gastric cancer by inhibiting ERBB4 expression. Oncotarget. 2017;8(28):45725–35. https://doi.org/10.18632/oncotarget.17392.
Chang W, Wang Y, Li W, Shi L, Geng Z. MicroRNA-551b-3p inhibits tumour growth of human cholangiocarcinoma by targeting Cyclin D1. J Cell Mol Med. 2019;23(8):4945–54. https://doi.org/10.1111/jcmm.14312.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif). 2001;25(4):402–8. https://doi.org/10.1006/meth.2001.1262.
Naseri Z, Oskuee RK, Forouzandeh-Moghadam M, Jaafari MR. Delivery of LNA-antimiR-142-3p by mesenchymal stem cells-derived exosomes to breast cancer stem cells reduces tumorigenicity. Stem Cell Rev Rep. 2020;16(3):541–56. https://doi.org/10.1007/s12015-019-09944-w.
Xu H, Zhao G, Zhang Y, Jiang H, Wang W, Zhao D, et al. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/β-catenin signaling pathway by targeting EZH2. Stem Cell Res Ther. 2019;10(1):381. https://doi.org/10.1186/s13287-019-1446-z.
Ahmad A, Zhang W, Wu M, Tan S, Zhu T. Tumor-suppressive miRNA-135a inhibits breast cancer cell proliferation by targeting ELK1 and ELK3 oncogenes. Genes Gen. 2018;40(3):243–51. https://doi.org/10.1007/s13258-017-0624-6.
Qi L, Sun B, Yang B, Lu S. LINC00665 stimulates breast cancer progression via regulating miR-551b-5p. Cancer Manag Res. 2021;13:1113–21. https://doi.org/10.2147/cmar.S275096.
Zhou Y, Liu X, Lan J, Wan Y, Zhu X. Circular RNA circRPPH1 promotes triple-negative breast cancer progression via the miR-556-5p/YAP1 axis. Am J Transl Res. 2020;12(10):6220–34.
Oltra SS, Peña-Chilet M, Vidal-Tomas V, Flower K, Martinez MT, Alonso E, et al. Methylation deregulation of miRNA promoters identifies miR124–2 as a survival biomarker in breast cancer in very young women. Sci Rep. 2018;8(1):14373. https://doi.org/10.1038/s41598-018-32393-3.
Li H, Zhang Y, Hai J, Wang J, Zhao B, Du L, et al. Knockdown of TRIM31 suppresses proliferation and invasion of gallbladder cancer cells by down-regulating MMP2/9 through the PI3K/Akt signaling pathway. Biomed Pharmacother = Biomed Pharmacother. 2018;103:1272–8. https://doi.org/10.1016/j.biopha.2018.04.120.
Zhang H, Deng Y, Liang L, Shen L, Zhu J, Wang Y, et al. Knockdown of TRIM31 enhances colorectal cancer radiosensitivity by inducing DNA damage and activating apoptosis. Onco Targets Ther. 2019;12:8179–88. https://doi.org/10.2147/ott.S215769.
Shi G, Lv C, Yang Z, Qin T, Sun L, Pan P, et al. TRIM31 promotes proliferation, invasion and migration of glioma cells through Akt signaling pathway. Neoplasma. 2019;66(5):727–35. https://doi.org/10.4149/neo_2019_190106N21.
Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5(1):145. https://doi.org/10.1038/s41392-020-00261-0.
Wörmann B. Breast cancer: basics, screening, diagnostics and treatment. Med Monatsschr Pharm. 2017;40(2):55–64.
Bliss SA, Sinha G, Sandiford OA, Williams LM, Engelberth DJ, Guiro K, et al. Mesenchymal stem cell-derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Can Res. 2016;76(19):5832–44. https://doi.org/10.1158/0008-5472.Can-16-1092.
Sun HY, Qu ZC, Liu DM, Zhang W, Liu G, Zhu L. Decreased expression of miR-551b predicts poor prognosis and promotes tumorigenesis by targeting PTP4A3 in human colorectal cancer. Eur Rev Med Pharmacol Sci. 2019;23(13):5741–51. https://doi.org/10.26355/eurrev_201907_18311.
Lv T, Jiang L, Kong L, Yang J. MicroRNA-29c-3p acts as a tumor suppressor gene and inhibits tumor progression in hepatocellular carcinoma by targeting TRIM31. Oncol Rep. 2020;43(3):953–64. https://doi.org/10.3892/or.2020.7469.
Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2017;312(1):L110–21. https://doi.org/10.1152/ajplung.00423.2016.
Yan W, Wu X, Zhou W, Fong MY, Cao M, Liu J, et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol. 2018;20(5):597–609. https://doi.org/10.1038/s41556-018-0083-6.
Petrov VN, Isaeva EV, Ulyanenko SE, Beketov EE, Yatsenko EM, Sayapina EV, et al. In vivo effects of human bone marrow mesenchymal stromal cells on the development of experimental B16 melanoma in mice. Bull Exp Biol Med. 2020;168(4):561–5. https://doi.org/10.1007/s10517-020-04753-5.
Lin Y, Lu Y, Li X. Biological characteristics of exosomes and genetically engineered exosomes for the targeted delivery of therapeutic agents. J Drug Target. 2020;28(2):129–41. https://doi.org/10.1080/1061186x.2019.1641508.
Kim H, Kim EH, Kwak G, Chi SG, Kim SH, Yang Y. Exosomes: cell-derived nanoplatforms for the delivery of cancer therapeutics. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms22010014.
Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnology. 2020;18(1):10. https://doi.org/10.1186/s12951-019-0563-2.
Lou G, Chen L, Xia C, Wang W, Qi J, Li A, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res: CR. 2020;39(1):4. https://doi.org/10.1186/s13046-019-1512-5.
He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang J, et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9(26):8206–20. https://doi.org/10.7150/thno.37455.
Qin F, Tang H, Zhang Y, Zhang Z, Huang P, Zhu J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J Cell Physiol. 2020;235(5):4734–45. https://doi.org/10.1002/jcp.29351.
Raimondi L, De Luca A, Gallo A, Costa V, Russelli G, Cuscino N, et al. Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs. Carcinogenesis. 2020;41(5):666–77. https://doi.org/10.1093/carcin/bgz130.
Funding
The present study was supported by the Shanghai Pujiang Talent Program (Grant no. 18PJD005).
Author information
Authors and Affiliations
Contributions
ZY, BX and SW made major contributions to the conception and design of this study, and drafted the manuscript. WY, RL, SG, ZX, WJ, XS, XG and HZ were responsible for data acquisition, analysis and interpretation, and manuscript revision. HW made substantial contributions to the conception and design of the study and revised the manuscript. All authors agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Both clinical and animal experiments have been approved by the Ethics Committee of the Zhongshan Hospital, Fudan University (No. Y2014-135). Written informed consent was obtained from all participants.
Consent for publication
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13577_2022_753_MOESM1_ESM.tif
Supplementary file1 Figure. S1 Identification of DEMs in high-grade and low-grade breast cancer tissues. (A, B) Heat maps of DEMs in GSE131599 and TCGA. (C, D) Volcano plot of DEMs in GSE131599 and TCGA. DEMs, differentially expressed miRNAs; TCGA, The Cancer Genome Atlas (TIF 8346 KB)
13577_2022_753_MOESM2_ESM.tif
Supplementary file2 Figure. S2 miR-551b-3p inhibits the proliferation and migration of SK-BR-3 cells. (A) Reverse transcription-quantitative PCR analysis of miR-135a-5p, miR-551b-3p and miR-556-3p expression levels in MCF-10A and MDA-MB-231 cells. (B) SK-BR-3 cells were transfected with miR-551b-3p agomir or miR-551b-3p antagomir. Cell proliferation was detected via EdU staining. (C) Cell migration was measured by using a Transwell migration assay. **P<0.01. miR, microRNA (TIF 7370 KB)
13577_2022_753_MOESM3_ESM.tif
Supplementary file3 Figure. S3 miR-551b-3p inhibits the viability of breast cancer cells via negatively regulating TRIM31. MDA-MB-231 cells were transfected with miR-551b-3p agomir or/and TRIM31 siRNA2. Cell viability was detected via a Cell Counting Kit-8 assay. **P<0.01 (TIF 2196 KB)
13577_2022_753_MOESM4_ESM.tif
Supplementary file4 Figure. S4 Effects of BMSC-Exo on the proliferation and migration of breast cancer cells. (A) RT-qPCR analysis of miR-551b-3p expression levels in BMSCs, MCF-10A and MDA-MB-231 cells. (B) MDA-MB-231 cells were co-cultured with BMSCs. Meanwhile, MDA-MB-231 cells were treated with BMSC-Exo. RT-qPCR analysis of miR-551b-3p and TRIM31 expression levels in MDA-MB-231 cells. (C) Cell proliferation was detected via EdU staining. (D) Cell migration was measured by using a Transwell migration assay. **P<0.01. BMSCs, bone marrow mesenchymal stromal cells; BMSC-Exo, BMSC-derived exosomes; RT-qPCR, reverse transcription-quantitative PCR; miR, microRNA; TRIM31, tripartite motif-containing protein 31 (TIF 13371 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Z., Xu, B., Wu, S. et al. Exosomal microRNA-551b-3p from bone marrow-derived mesenchymal stromal cells inhibits breast cancer progression via regulating TRIM31/Akt signaling. Human Cell 35, 1797–1812 (2022). https://doi.org/10.1007/s13577-022-00753-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00753-x