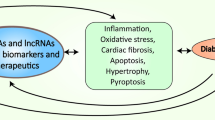
Abstract
Diabetic cardiomyopathy (DCM) represents a distinct myocardial disorder elicited by diabetes mellitus, characterized by aberrations in myocardial function and structural integrity. This pathological condition predominantly manifests in individuals with diabetes who do not have concurrent coronary artery disease or hypertension. An escalating body of scientific evidence substantiates the pivotal role of programmed cell death (PCD)—encompassing apoptosis, autophagy, pyroptosis, ferroptosis, and necroptosis—in the pathogenic progression of DCM, thereby emerging as a prospective therapeutic target. Additionally, numerous non-coding RNAs (ncRNAs) have been empirically verified to modulate the biological processes underlying programmed cell death, consequently influencing the evolution of DCM. This review systematically encapsulates prevalent types of PCD manifest in DCM as well as nascent discoveries regarding the regulatory influence of ncRNAs on programmed cell death in the pathogenesis of DCM, with the aim of furnishing novel insights for the furtherance of research in PCD-associated disorders relevant to DCM.








Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
Gao J, Liu J, Meng Z et al (2021) Ultrasound-assisted C3F8-filled PLGA nanobubbles for enhanced FGF21 delivery and improved prophylactic treatment of diabetic cardiomyopathy. Acta Biomater 130:395–408
Ouyang C, You J, Xie Z (2014) The interplay between autophagy and apoptosis in the diabetic heart. J Mol Cell Cardiol 71:71–80
Elshenawy DSA, Ramadan NM, Abdo VB, Ahour ARH (2022) Sacubitril/valsartan combination enhanced cardiac glycophagy and prevented the progression of murine diabetic cardiomyopathy [published correction appears in Biomed Pharmacother. Dec 156:113867]. Biomed Pharmacother 2022(153):113382
Kabbage M, Kessens R, Bartholomay LC, Williams B (2017) The life and death of a plant cell. Annu Rev Plant Biol 68:375–404
Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN (2019) Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev 99(4):1765–1817
Mishra AP, Salehi B, Sharifi-Rad M et al (2018) Programmed cell death, from a cancer perspective: an overview. Mol Diagn Ther 22(3):281–295
Zhang L, Ai C, Bai M, Niu J, Zhang Z (2022) NLRP3 inflammasome/pyroptosis: a key driving force in diabetic cardiomyopathy. Int J Mol Sci 23(18):10632
Peng F, Liao M, Qin R et al (2022) Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther 7(1):286
Liu X, Yang W, Guan Z et al (2018) There are only four basic modes of cell death, although there are many ad-hoc variants adapted to different situations. Cell Biosci 8:6
Moujalled D, Strasser A, Liddell JR (2021) Molecular mechanisms of cell death in neurological diseases. Cell Death Differ 28(7):2029–2044
Wang E, Zhou S, Zeng D, Wang R (2023) Molecular regulation and therapeutic implications of cell death in pulmonary hypertension. Cell Death Discov 9(1):239
Jiang M, Qi L, Li L, Wu Y, Song D, Li Y (2021) Caspase-8: a key protein of cross-talk signal way in “PANoptosis” in cancer. Int J Cancer 149(7):1408–1420
Kaloni D, Diepstraten ST, Strasser A, Kelly GL (2023) BCL-2 protein family: attractive targets for cancer therapy. Apoptosis 28(1–2):20–38
Zhou X, Zeng Y, Zheng R et al (2023) Natural products modulate cell apoptosis: a promising way for treating endometrial cancer. Front Pharmacol 14:1209412
Carberry S, D’Orsi B, Monsefi N et al (2018) The BAX/BAK-like protein BOK is a prognostic marker in colorectal cancer. Cell Death Dis 9(2):125
Quarato G, Mari L, Barrows NJ et al (2023) Mitophagy restricts BAX/BAK-independent, Parkin-mediated apoptosis. Sci Adv 9(21):eadg8156
Belali OM, Ahmed MM, Mohany M et al (2022) LCZ696 protects against diabetic cardiomyopathy-induced myocardial inflammation, ER stress, and apoptosis through inhibiting AGEs/NF-κB and PERK/CHOP signaling pathways. Int J Mol Sci 23(3):1288
Lin N, Lin H, Yang Q et al (2021) SGLT1 inhibition attenuates apoptosis in diabetic cardiomyopathy via the JNK and p38 pathway. Front Pharmacol 11:598353
Ren BC, Zhang YF, Liu SS et al (2020) Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J Cell Mol Med 24(21):12355–12367
Joubert M, Manrique A, Cariou B, Prieur X (2019) Diabetes-related cardiomyopathy: the sweet story of glucose overload from epidemiology to cellular pathways. Diabetes Metab 45(3):238–247
Shi H, Zhou P, Ni YQ et al (2021) In vivo and in vitro studies of Danzhi Jiangtang capsules against diabetic cardiomyopathy via TLR4/MyD88/NF-κB signaling pathway. Saudi Pharm J 29(12):1432–1440
Wang S, Duan J, Liao J et al (2022) LncRNA H19 inhibits ER stress induced apoptosis and improves diabetic cardiomyopathy by regulating PI3K/AKT/mTOR axis. Aging (Albany NY) 14(16):6809–6828
Ni J, Li Y, Xu Y, Guo R (2021) Salidroside protects against cardiomyocyte apoptosis and ventricular remodeling by AKT/HO-1 signaling pathways in a diabetic cardiomyopathy mouse model. Phytomedicine 82:153406
Taheriazam A, Bayanzadeh SD, Heydari Farahani M et al (2023) Non-coding RNA-based therapeutics in cancer therapy: an emphasis on Wnt/β-catenin control. Eur J Pharmacol 951:175781
Chen R, Chen H, Yang Z et al (2023) Danlou tablet inhibits high-glucose-induced cardiomyocyte apoptosis via the miR-34a-SIRT1 axis. Heliyon 9(3):e14479
Park IH, Song YS, Joo HW et al (2020) Role of MicroRNA-34a in anti-apoptotic effects of granulocyte-colony stimulating factor in diabetic cardiomyopathy. Diabetes Metab J 44(1):173–185
Xia X, Liang Y, Zheng W, Lin D, Sun S (2020) miR-410-5p promotes the development of diabetic cardiomyopathy by suppressing PIM1-induced anti-apoptosis. Mol Cell Probes 52:101558
Qiao Y, Zhao Y, Liu Y et al (2016) miR-483-3p regulates hyperglycaemia-induced cardiomyocyte apoptosis in transgenic mice. Biochem Biophys Res Commun 477(4):541–547
Wang X, Zhang Z, Wang M (2022) MiR-29a regulates cardiomyocyte apoptosis by targeting Bak1 in diabetic cardiomyopathy. J Biochem 171(6):663–671
Liu X, Guo B, Zhang W, Ma B, Li Y (2021) MiR-20a-5p overexpression prevented diabetic cardiomyopathy via inhibition of cardiomyocyte apoptosis, hypertrophy, fibrosis and JNK/NF-κB signalling pathway. J Biochem 170(3):349–362
Xu D, Zhang X, Chen X, Yang S, Chen H (2020) Inhibition of miR-223 attenuates the NLRP3 inflammasome activation, fibrosis, and apoptosis in diabetic cardiomyopathy. Life Sci 256:117980
Liu Y, Zheng W, Pan Y, Hu J (2019) Low expression of miR-186-5p regulates cell apoptosis by targeting toll-like receptor 3 in high glucose-induced cardiomyocytes. J Cell Biochem 120(6):9532–9538
Huang W, Li H, Yu Q, Xiao W, Wang DO (2022) LncRNA-mediated DNA methylation: an emerging mechanism in cancer and beyond [published correction appears in J Exp Clin Cancer Res. 2022 Aug 27;41(1):262]. J Exp Clin Cancer Res. 41(1):100
Toden S, Zumwalt TJ, Goel A (2021) Non-coding RNAs and potential therapeutic targeting in cancer. Biochim Biophys Acta Rev Cancer 1875(1):188491
Wang T, Li N, Yuan L et al (2023) MALAT1/miR-185-5p mediated high glucose-induced oxidative stress, mitochondrial injury and cardiomyocyte apoptosis via the RhoA/ROCK pathway. J Cell Mol Med 27(17):2495–2506
Cheng Y, Li J, Wang C et al (2020) Inhibition of long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 attenuates high glucose-induced cardiomyocyte apoptosis via regulation of miR-181a-5p. Exp Anim 69(1):34–44
Tang H, Zhong H, Liu W et al (2022) Melatonin alleviates hyperglycemia-induced cardiomyocyte apoptosis via regulation of long non-coding RNA H19/miR-29c/MAPK axis in diabetic cardiomyopathy. Pharmaceuticals (Basel) 15(7):821
Zhu C, Zhang H, Wei D, Sun Z (2021) Silencing lncRNA GAS5 alleviates apoptosis and fibrosis in diabetic cardiomyopathy by targeting miR-26a/b-5p. Acta Diabetol 58(11):1491–1501
Zhao SF, Ye YX, Xu JD et al (2021) Long non-coding RNA KCNQ1OT1 increases the expression of PDCD4 by targeting miR-181a-5p, contributing to cardiomyocyte apoptosis in diabetic cardiomyopathy. Acta Diabetol 58(9):1251–1267
Wang C, Liu G, Yang H et al (2021) MALAT1-mediated recruitment of the histone methyltransferase EZH2 to the microRNA-22 promoter leads to cardiomyocyte apoptosis in diabetic cardiomyopathy. Sci Total Environ 766:142191
Chen Y, Zhang Z, Zhu D, Zhao W, Li F (2019) Long non-coding RNA MEG3 serves as a ceRNA for microRNA-145 to induce apoptosis of AC16 cardiomyocytes under high glucose condition. Biosci Rep 39(6):BSR20190444
Zhou X, Zhang W, Jin M et al (2017) lncRNA MIAT functions as a competing endogenous RNA to upregulate DAPK2 by sponging miR-22-3p in diabetic cardiomyopathy. Cell Death Dis 8(7):e2929
Abdelmohsen K, Panda AC, Munk R et al (2017) Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol 14(3):361–369
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J (2019) The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet 20(11):675–691
Shao Y, Li M, Yu Q et al (2022) CircRNA CDR1as promotes cardiomyocyte apoptosis through activating hippo signaling pathway in diabetic cardiomyopathy. Eur J Pharmacol 922:174915
Jiang J, Gao G, Pan Q et al (2022) Circular RNA circHIPK3 is downregulated in diabetic cardiomyopathy and overexpression of circHIPK3 suppresses PTEN to protect cardiomyocytes from high glucose-induced cell apoptosis. Bioengineered 13(3):6272–6279
Peng Q, Liu H, Luo Z et al (2022) Effect of autophagy on ferroptosis in foam cells via Nrf2. Mol Cell Biochem 477(5):1597–1606
Lin L, Zhang MX, Zhang L et al (2022) Autophagy, pyroptosis, and ferroptosis: new regulatory mechanisms for atherosclerosis. Front Cell Dev Biol 9:809955
Khan F, Khan H, Khan A et al (2022) Autophagy in adipogenesis: molecular mechanisms and regulation by bioactive compounds. Biomed Pharmacother 155:113715
Madrigal-Matute J, Cuervo AM, Sluimer JC (2022) Chaperone-mediated autophagy protects against atherosclerosis. Autophagy 18(10):2505–2507
Ravikumar B, Sarkar S, Davies JE et al (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90(4):1383–1435
Al-Bari MAA (2020) A current view of molecular dissection in autophagy machinery. J Physiol Biochem 76(3):357–372
Su Y, Fan X, Li S et al (2022) Scutellarin improves Type 2 diabetic cardiomyopathy by regulating cardiomyocyte autophagy and apoptosis. Dis Mark 2022:3058354
Escobar KA, Cole NH, Mermier CM et al (2019) Autophagy and aging: maintaining the proteome through exercise and caloric restriction. Aging Cell 18(1):e12876
Kim J, Kundu M, Viollet B et al (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13(2):132–141
Wang H, Wang L, Hu F et al (2022) Neuregulin-4 attenuates diabetic cardiomyopathy by regulating autophagy via the AMPK/mTOR signalling pathway. Cardiovasc Diabetol 21(1):205
Lu Q-B, Ding Y, Liu Y et al (2022) Metrnl ameliorates diabetic cardiomyopathy via inactivation of cGAS/STING signaling dependent on LKB1/AMPK/ULK1-mediated autophagy. J Adv Res 51:161–179
Zhao J, Wu Q, Yang T et al (2022) Gaseous signal molecule SO2 regulates autophagy through PI3K/AKT pathway inhibits cardiomyocyte apoptosis and improves myocardial fibrosis in rats with type II diabetes. Korean J Physiol Pharmacol 26(6):541–556
Ning SW, Zhang SQ, Guo ZK (2023) MicroRNA-494 regulates high glucose-induced cardiomyocyte apoptosis and autophagy by PI3K/AKT/mTOR signalling pathway. ESC Heart Fail 10:1401–1411
Xing R, Liu D, Cheng X, Tian X, Yan C, Han Y (2019) MiR-207 inhibits autophagy and promotes apoptosis of cardiomyocytes by directly targeting LAMP2 in type 2 diabetic cardiomyopathy. Biochem Biophys Res Commun 520(1):27–34
Ni T, Lin N, Lu W et al (2020) Dihydromyricetin prevents diabetic cardiomyopathy via miR-34a suppression by activating autophagy. Cardiovasc Drugs Ther 34(3):291–301
Nandi SS, Duryee MJ, Shahshahan HR et al (2015) Induction of autophagy markers is associated with attenuation of miR-133a in diabetic heart failure patients undergoing mechanical unloading. Am J Transl Res 7(4):683–696
Ucar A, Gupta SK, Fiedler J et al (2012) The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat Commun 3:1078
Li X, Meng C, Han F et al (2021) Vildagliptin attenuates myocardial dysfunction and restores autophagy via miR-21/SPRY1/ERK in diabetic mice heart. Front Pharmacol 12:634365
Chen C, Yang S, Li H et al (2017) Mir30c is involved in diabetic cardiomyopathy through regulation of cardiac autophagy via BECN1. Mol Ther Nucleic Acids 7:127–139
Chen D, Zhang M (2021) GAS5 regulates diabetic cardiomyopathy via miR-221-3p/p27 axis-associated autophagy. Mol Med Rep 23(2):135
Feng Y, Xu W, Zhang W et al (2019) LncRNA DCRF regulates cardiomyocyte autophagy by targeting miR-551b-5p in diabetic cardiomyopathy. Theranostics 9(15):4558–4566
Zhuo C, Jiang R, Lin X, Shao M (2017) LncRNA H19 inhibits autophagy by epigenetically silencing of DIRAS3 in diabetic cardiomyopathy. Oncotarget 8(1):1429–1437
Brennan MA, Cookson BT (2000) Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol 38(1):31–40
Shi J, Zhao Y, Wang K et al (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526(7575):660–665
Kayagaki N, Warming S, Lamkanfi M et al (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479(7371):117–121
Kayagaki N, Stowe IB, Lee BL et al (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526(7575):666–671
Orning P, Weng D, Starheim K et al (2018) Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362(6418):1064–1069
Li T, Zheng G, Li B, Tang L (2021) Pyroptosis: a promising therapeutic target for noninfectious diseases. Cell Prolif 54(11):e13137
Broz P, Dixit VM (2016) Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16(7):407–420
Sun P, Zhong J, Liao H et al (2022) Hepatocytes are resistant to cell death from canonical and non-canonical inflammasome-activated pyroptosis. Cell Mol Gastroenterol Hepatol 13(3):739–757
An S, Hu H, Li Y et al (2020) Pyroptosis plays a role in osteoarthritis. Aging Dis 11(5):1146–1157
Downs KP, Nguyen H, Dorfleutner A, Stehlik C (2020) An overview of the non-canonical inflammasome. Mol Aspects Med 76:100924
Yang D, He Y, Muñoz-Planillo R et al (2015) Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 43(5):923–932
Shi J, Zhao Y, Wang Y et al (2014) Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514(7521):187–192
Liu C, Yao Q, Hu T et al (2022) Cathepsin B deteriorates diabetic cardiomyopathy induced by streptozotocin via promoting NLRP3-mediated pyroptosis. Mol Ther Nucleic Acids 30:198–207
Zeng C, Wang R, Tan H (2019) Role of pyroptosis in cardiovascular diseases and its therapeutic implications. Int J Biol Sci 15(7):1345–1357
Xie Y, Huang Y, Ling X et al (2020) Chemerin/CMKLR1 axis promotes inflammation and pyroptosis by activating NLRP3 inflammasome in diabetic cardiomyopathy rat. Front Physiol 11:381
Peng M, Liu Y, Xu Y et al (2021) Cathelicidin-WA ameliorates diabetic cardiomyopathy by inhibiting the NLRP3 inflammasome. Cell Cycle 20(21):2278–2290
Jeyabal P, Thandavarayan RA, Joladarashi D et al (2016) MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem Biophys Res Commun 471(4):423–429
Luo B, Li B, Wang W et al (2014) NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS ONE 9(8):e104771
Luo B, Huang F, Liu Y et al (2017) NLRP3 inflammasome as a molecular marker in diabetic cardiomyopathy. Front Physiol 8:519
Hutchinson KR, Lord KC, West TA et al (2013) Cardiac fibroblast-dependent extracellular matrix accumulation is associated with diastolic stiffness in Type 2 diabetes. PLoS ONE 8:e72080
Kawaguchi M, Takahashi M, Hata T et al (2011) Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123(6):594–604
Gao G, Fu L, Xu Y et al (2022) Cyclovirobuxine D ameliorates experimental diabetic cardiomyopathy by inhibiting cardiomyocyte pyroptosis via NLRP3 in vivo and in vitro. Front Pharmacol 13:906548
Chen Y, Wang L, Pitzer AL et al (2016) Contribution of redox-dependent activation of endothelial Nlrp3 inflammasomes to hyperglycemia-induced endothelial dysfunction. J Mol Med (Berl) 94(12):1335–1347
Xiao W, Zheng D, Chen X et al (2021) Long non-coding RNA MIAT is involved in the regulation of pyroptosis in diabetic cardiomyopathy via targeting miR-214–3p. iScience 24(12):103518
Li X, Du N, Zhang Q et al (2014) MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis 5(10):e1479
Yang L, Cheng CF, Li ZF et al (2023) Berberine blocks inflammasome activation and alleviates diabetic cardiomyopathy via the miR-18a-3p/Gsdmd pathway. Int J Mol Med 51(6):49
Ren L, Chen X, Nie B et al (2022) Ranolazine inhibits pyroptosis via regulation of miR-135b in the treatment of diabetic cardiac fibrosis. Front Mol Biosci 9:806966
Shi P, Zhao XD, Shi KH et al (2021) MiR-21-3p triggers cardiac fibroblasts pyroptosis in diabetic cardiac fibrosis via inhibiting androgen receptor. Exp Cell Res 399(2):112464
Zhao S, Tan Y, Qin J et al (2022) MicroRNA-223–3p promotes pyroptosis of cardiomyocyte and release of inflammasome factors via downregulating the expression level of SPI1 (PU. 1). Toxicology 476:153252
Xiong J, Zhou Q (2023) The lncRNA HOTAIR attenuates pyroptosis of diabetic cardiomyocytes by recruiting FUS to regulate SIRT3 expression. Kaohsiung J Med Sci 39(5):458–467
Wu A, Sun W, Mou F (2021) lncRNA-MALAT1 promotes high glucose-induced H9C2 cardiomyocyte pyroptosis by downregulating miR-141-3p expression. Mol Med Rep 23(4):259
Xu Y, Fang H, Xu Q et al (2020) LncRNA GAS5 inhibits NLRP3 inflammasome activation-mediated pyroptosis in diabetic cardiomyopathy by targeting miR-34b-3p/AHR. Cell Cycle 19(22):3054–3065
Yang F, Qin Y, Wang Y et al (2018) LncRNA KCNQ1OT1 mediates pyroptosis in diabetic cardiomyopathy. Cell Physiol Biochem 50(4):1230–1244
Fu L, Zhang J, Lin Z, Li Y, Qin G (2022) CircularRNA circ_0071269 knockdown protects against from diabetic cardiomyopathy injury by microRNA-145/gasdermin A axis. Bioengineered 13(2):2398–2411
Yang F, Li A, Qin Y et al (2019) A novel circular RNA mediates pyroptosis of diabetic cardiomyopathy by functioning as a competing endogenous RNA. Mol Ther Nucleic Acids 17:636–643
Yuan Q, Sun Y, Yang F et al (2023) CircRNA DICAR as a novel endogenous regulator for diabetic cardiomyopathy and diabetic pyroptosis of cardiomyocytes. Signal Transduct Target Ther 8(1):99
Dixon SJ, Lemberg KM, Lamprecht MR et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Xie Y, Hou W, Song X et al (2016) Ferroptosis: process and function. Cell Death Differ 23(3):369–379
Sun Y, Li Q, Guo H et al (2022) Ferroptosis and iron metabolism after intracerebral hemorrhage. Cells 12(1):90
Galaris D, Barbouti A, Pantopoulos K (2019) Iron homeostasis and oxidative stress: an intimate relationship. Biochim Biophys Acta Mol Cell Res 1866(12):118535
Yu Y, Yan Y, Niu F et al (2021) Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov 7(1):193
Han C, Liu Y, Dai R et al (2020) Ferroptosis and its potential role in human diseases. Front Pharmacol 11:239
Li H, Lin L, Xia YL et al (2022) Research progress on the role of ferroptosis in cardiovascular disease. Front Cardiovasc Med 9:1077332
Chen J, Wang Y, Wu J et al (2020) The potential value of targeting ferroptosis in early brain injury after acute CNS disease. Front Mol Neurosci 13:110
Hou W, Xie Y, Song X et al (2016) Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12(8):1425–1428
Mancias JD, Wang X, Gygi SP et al (2014) Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509(7498):105–109
Fang Y, Chen X, Tan Q et al (2021) Inhibiting ferroptosis through disrupting the NCOA4-FTH1 interaction: a new mechanism of action. ACS Cent Sci 7(6):980–989
Deng L, He S, Guo N et al (2023) Molecular mechanisms of ferroptosis and relevance to inflammation. Inflamm Res 72(2):281–299
Xing L, Liu XY, Zhou TJ et al (2021) Photothermal nanozyme-ignited Fenton reaction-independent ferroptosis for breast cancer therapy. J Control Release 339:14–26
Dodson M, Castro-Portuguez R, Zhang DD (2019) NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol 23:101107
Yang WS, Kim KJ, Gaschler MM et al (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA 113(34):E4966–E4975
Yang WS, SriRamaratnam R, Welsch ME et al (2014) Regulation of ferroptotic cancer cell death by GPX4. Cell 156(1–2):317–331
Liu Y, Gu W (2022) p53 in ferroptosis regulation: the new weapon for the old guardian. Cell Death Differ 29(5):895–910
Altamura S, Müdder K, Schlotterer A et al (2021) Iron aggravates hepatic insulin resistance in the absence of inflammation in a novel db/db mouse model with iron overload. Mol Metab 51:101235
Palomer X, Salvadó L, Barroso E et al (2013) An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int J Cardiol 168(4):3160–3172
Wilson AJ, Gill EK, Abudalo RA et al (2018) Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart 104(4):293–299
Du S, Shi H, Xiong L et al (2022) Canagliflozin mitigates ferroptosis and improves myocardial oxidative stress in mice with diabetic cardiomyopathy. Front Endocrinol (Lausanne) 13:1011669
Wang X, Chen X, Zhou W et al (2022) Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B 12(2):708–722
Ni T, Huang X, Pan S et al (2021) Inhibition of the long non-coding RNA ZFAS1 attenuates ferroptosis by sponging miR-150-5p and activates CCND2 against diabetic cardiomyopathy. J Cell Mol Med 25(21):9995–10007
Jiang M, Jike Y, Liu K et al (2023) Exosome-mediated miR-144-3p promotes ferroptosis to inhibit osteosarcoma proliferation, migration, and invasion through regulating ZEB1. Mol Cancer 22(1):113
Cen J, Liang Y, Feng Z et al (2023) Hsa_circ_0057105 modulates a balance of epithelial-mesenchymal transition and ferroptosis vulnerability in renal cell carcinoma. Clin Transl Med 13(8):e1339
Teng X, Degterev A, Jagtap P et al (2005) Structure-activity relationship study of novel necroptosis inhibitors. Bioorg Med Chem Lett 15(22):5039–5044
Galluzzi L, Vitale I, Aaronson SA et al (2018) Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ 25(3):486–541
Khan I, Yousif A, Chesnokov M et al (2021) A decade of cell death studies: breathing new life into necroptosis. Pharmacol Ther 220:107717
Degterev A, Huang Z, Boyce M et al (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury [published correction appears in Nat Chem Biol. 2005 Sep;1(4):234]. Nat Chem Biol 1(2):112–119
Zhu F, Zhang W, Yang T et al (2019) Complex roles of necroptosis in cancer. J Zhejiang Univ Sci B 20(5):399–413
Feoktistova M, Geserick P, Kellert B et al (2011) cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell 43(3):449–463
McComb S, Cheung HH, Korneluk RG et al (2012) cIAP1 and cIAP2 limit macrophage necroptosis by inhibiting Rip1 and Rip3 activation. Cell Death Differ 19(11):1791–1801
Wang L, Du F, Wang X (2008) TNF-alpha induces two distinct caspase-8 activation pathways. Cell 133(4):693–703
Dondelinger Y, Aguileta MA, Goossens V et al (2013) RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ 20(10):1381–1392
Cho YS, Challa S, Moquin D et al (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137(6):1112–1123
Apaijai N, Jinawong K, Singhanat K et al (2021) Necrostatin-1 reduces cardiac and mitochondrial dysfunction in prediabetic rats. J Endocrinol 251(1):27–39
Cao T, Ni R, Ding W et al (2022) MLKL-mediated necroptosis is a target for cardiac protection in mouse models of type-1 diabetes. Cardiovasc Diabetol 21(1):165
Fang T, Cao R, Wang W et al (2018) Alterations in necroptosis during ALDH2-mediated protection against high glucose-induced H9c2 cardiac cell injury. Mol Med Rep 18(3):2807–2815
Song S, Ding Y, Dai GL et al (2021) Sirtuin 3 deficiency exacerbates diabetic cardiomyopathy via necroptosis enhancement and NLRP3 activation. Acta Pharmacol Sin 42(2):230–241
Chen Y, Li X, Hua Y et al (2021) RIPK3-mediated necroptosis in diabetic cardiomyopathy requires CaMKII activation. Oxid Med Cell Longev 2021:6617816
Zhang T, Zhang Y, Cui M et al (2016) CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med 22(2):175–182
Gao XQ, Liu CY, Zhang YH et al (2022) The circRNA CNEACR regulates necroptosis of cardiomyocytes through Foxa2 suppression. Cell Death Differ 29(3):527–539
Zhang DY, Wang BJ, Ma M, Zhang XW et al (2019) Correction to: MicroRNA-325–3p protects the heart after myocardial infarction by inhibiting RIPK3 and programmed necrosis in mice. BMC Mol Biol. 20(1):18
Qin D, Wang X, Li Y et al (2016) MicroRNA-223-5p and -3p cooperatively suppress necroptosis in ischemic/reperfused hearts. J Biol Chem 291(38):20247–20259
Yang F, Qin Y, Wang Y et al (2019) Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int J Biol Sci 15(5):1010–1019
Qiao S, Hong L, Zhu Y et al (2022) RIPK1-RIPK3 mediates myocardial fibrosis in type 2 diabetes mellitus by impairing autophagic flux of cardiac fibroblasts. Cell Death Dis 13(2):147
Cheng R, Xu H, Hong Y (2021) miR-221 regulates TGF-β1-induced HSC activation through inhibiting autophagy by directly targeting LAMP2. Mol Med Rep 24(5):777
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
ZBR contributed to the conception and design of this manuscript. ZL has critically revised the entire manuscript. WH, ZJW, and CC are responsible for manuscript structure and English grammar. All authors participated in manuscript revision, read and approved the submitted version. All authors revised and approved the final manuscript. “The author(s) read and approved the final manuscript.”
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Wu, H., Zhang, J. et al. The study of the mechanism of non-coding RNA regulation of programmed cell death in diabetic cardiomyopathy. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-023-04909-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04909-7