Abstract
COVID-19 is caused by severe acute respiratory syndrome coronavirus-2, SARS-CoV-2. COVID-19 has changed the world scenario and caused mortality around the globe. Patients who recovered from COVID-19 have shown neurological, psychological, renal, cardiovascular, pulmonary, and hematological complications. In some patients, complications lasted more than 6 months. However, significantly less attention has been given to post-COVID complications. Currently available drugs are used to tackle the complications, but new interventions must address the problem. Phytochemicals from natural sources have been evaluated in recent times to cure or alleviate COVID-19 symptoms. An edible plant, Solanum nigrum, could be therapeutic in treating COVID-19 as the AYUSH ministry of India prescribes it during the pandemic. S. nigrum demonstrates anti-inflammatory, immunomodulatory, and antiviral action to treat the SARS-CoV-2 infection and its post-complications. Different parts of the plant represent a reduction in proinflammatory cytokines and prevent multi-organ failure by protecting various organs (liver, kidney, heart, neuro, and lung). The review proposes the possible role of the plant S. nigrum in managing the symptoms of COVID-19 and its post-COVID complications based on in silico docking and pharmacological studies. Further systematic and experimental studies are required to validate our hypothesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease, commonly known as COVID-19, is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The first confirmed case was reported on December 31, 2019, in Wuhan, China. According to the WHO dashboard, 651,918,402 confirmed cases and 6,656,601 deaths were reported till December 23, 2023, with a 2% mortality rate. The global pandemic has baffled the world with its impact, and eventually, every group of society has been suffering economically, medically, and socially. Clinical manifestations of COVID-19 include fever, cough, fatigue, loss of smell and taste, soreness in the throat, diarrhea, headache, rashes on the skin, and more severe manifestations include difficulty breathing or shortness of breath, chest pain or pressure, and hypoxia [2]. New symptoms are still emerging due to the mutations in the SARS-CoV-2 genome. It takes around 4–6 days for symptoms to appear and lasts up to 14 days [3, 4]. As of now, no particular treatment is available to tackle the disease, and currently available drugs are repurposed to control the disease. Acute respiratory infection is the prime manifestation of COVID-19 [5]. However, non-respiratory and post-treatment manifestations are also reported, suggesting that the virus has a long-term implication on host immunity and organs.
Patients who recovered from COVID-19 experienced multiple symptoms. There is no standardized term coined for that but most commonly, these symptoms are referred to as “post-COVID complications” or “long COVID” [6]. Any abnormal parameter or symptoms that exist 2 to 12 weeks after COVID-19 with negative real-time reverse transcription–polymerase chain reaction (RT-PCR) can be considered an acute post-COVID complications. By comparison, symptoms present beyond 12 weeks can be considered a chronic COVID complications. Many patients worldwide have reported different post-COVID complications through various platforms. Unfortunately, few clinical trials and meta-analyses have been carried out to streamline the different symptoms and abnormalities in clinical parameters. As many as 50 symptoms have been reported regarding post-COVID manifestation [7]. Although no particular guideline or treatment is available to tackle post-COVID complications due to the large and lacking data on symptoms, post-COVID complications have affected patients' quality of life as they are haunted by the fear of significant complications [8]. Patients with post-COVID complications also restrain themself from interacting socially as they cannot carry out day-to-day activities [9].
Available oral antiviral medications, namely remdesivir, lopinavir, and ribavirin, produce multiple adverse effects like hyperuricemia, anemia, bradycardia, and gastrointestinal and hepatic dysfunctions [10]. Recently, on April 22, 2022, WHO strongly recommended “paxlovid” an oral antiviral combination of nirmatrelvir and ritonavir for severe COVID-19 and immunocompromised patients [11]. Besides oral therapeutic drugs, vaccines are being utilized to manage the pandemic, yet mild-to-moderate side effects like nausea, chills, fever, diarrhea, muscle pain, and fatigue are also associated [12, 13]. Among all of the vaccines, ten show good efficacy against SARS-CoV-2 infection and attained emergency use listing (EUL) by WHO in terms of quality, safety, and efficacy [14], Till now, there are 119 vaccines in clinical trials and 49 have attained the final stage. Most vaccines that are approved are in phase three clinical trials and are being utilized in various countries for emergency use [15]. Besides all these successful attempts of COVID-19 rapidly developing vaccines, approximately, 40% of the world population choose to remain unvaccinated, and only 65% have completed their first dose [16]. According to the centers for disease control and prevention (CDC) report, 15% population of the US chooses to remain unvaccinated. A Household pulse survey (HPS) conducted by the US Census Bureau states that 42% of adults do not believe in COVID-19 vaccines [17]. Vaccine breakthrough infections are also happening worldwide as COVID-19 vaccines are not 100% effective [18]. Moreover, variants like delta and omicron also pose challenges to vaccines and therapeutics. Research is ongoing by thousands of scientists worldwide to better understand how new virus mutations and variants affects the effectiveness of different COVID-19 vaccines.
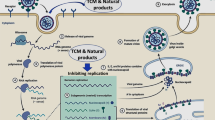
Medicinal plants and their bioactive constituents with high efficacy and minimal side effects play a major role in treating numerous viral diseases [19,20,21,22,23,24]. It is important to find phytochemicals in a single plant possessing antiviral, anti-inflammatory, and immunomodulatory activities. Recent studies suggest multiple medicinal plants and formulations that have been reported to have these activities and could be explored against COVID-19. For instance, Withania somnifera, Adhatoda vasica, Piper longum, Santalum album, Morus alba, and Momordica charantia have been recommended for their immunomodulatory, antiviral, and anti-inflammatory action [25, 26]. Thus, one could say that medicinal plants and phytochemicals from natural sources may show an important role against SARS-CoV-2 infection. Solanum nigrum is an edible plant in India, Australia, Tanzania, Ethiopia, and Uganda [27, 28]. In countries like Australia and Tanzania, people use it as a vegetable, and leaves are boiled and eaten like spinach. In Ethiopia and Uganda, leaves and fruits (black or violet) are edible in their raw form [27]. Traditional practices suggest that various plant parts are useful in ulcers, fever, flatulence, vomiting, dyspepsia, wounds, swelling, and skin-related disorders. Some parts like leaves and roots are used for laxative, cardiotonic, digestive, and diaphoretic action. Moreover, these parts also showed their action against whooping cough, hepatitis, and asthma-related issues particularly in some regions [29, 30]. Decoction of berries is useful in treating pulmonary tuberculosis, bronchitis, diarrhea, hydrophobia, and rat bite [29]. Scientific studies provide pieces of evidence suggesting the medicinal importance of S. nigrum and its part in arthritis [31], cancer [32,33,34], oxidative stress [35], and disease caused by hepatitis C virus (HCV) and human immunodeficiency virus (HIV) [36, 37]. Moreover plant also possess pharmacological activities like anti-ulcer [38,39,40], hepatoprotective [41], and cardioprotective action [42, 43]. Phytochemical studies of S. nigrum show the presence of glycoalkaloids like solanine, solamargine [44], and solasodine [45] as the primary marker compounds (demonstrated in Fig. 1).
Flavonoids, namely quercetin, luteolin, malvidin, pelargonidin, rutin, naringenin, apigenin and kaempferol [46], were reported. Moreover, carbohydrates, saponins [47], and sterols [48] were also reported. A combination of these phytochemicals indicates S. nigrum could be a promising therapeutic option for the treatment of COVID-19 and post-COVID complications. S. nigrum was also included in the guidelines for Ayurveda practitioners for COVID-19 published by the Ministry of AYUSH, where the use of the plant is recommended as a vegetable in the diet for the management of COVID-19 [49]. Moreover, in silico findings of plant phytochemicals against multiple target proteins of SARS-CoV-2 (depicted in Table 1) strengthen our hypothesis.
Furthermore, pharmacological studies suggest the role of the plant as an immunomodulator, anti-inflammatory, and antiviral agent [25, 64] (demonstrated in Table 2).
Thus, the present review features the potentiality of S. nigrum in treating acute and chronic post-COVID-19 complications.
Pathophysiology of COVID-19
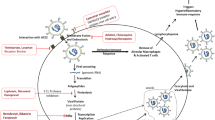
Coronaviruses belong to the group of RNA viruses known to cause mild-to-severe respiratory infections in animals and humans. In the last two decades, three different variants of coronaviruses have caused severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and SARS-CoV-2 in humans [74, 75]. Like other coronaviruses, SARS-CoV-2 consists of spike glycoprotein (S), an envelope protein (E), membrane protein (M), and a nucleocapsid protein (N). S, E, and M proteins make up the virus envelope, and the N protein holds the viral genome. The diameter of SARS-CoV-2 is around 50–200 nm in diameter [76]. It is still unclear about the transmission of the virus to humans, but the most likely hypothesis suggests the cross-transmission of the virus through bats and pangolin [77].
Viral entry and lung damage
SARS-CoV-2 enters the host through the nasal cavity and invades ciliated cells [78]. Angiotensin-converting enzyme 2 (ACE2) receptors are present in most cells, including the lungs, heart, kidney, and liver. The Spike protein of SARS-CoV-2 enters the cells through ACE2 receptors in the lungs. The entire process is carried out with the support of transmembrane protease serine 2 (TMPRSS2) [79]. Damage to the respiratory tract was due to excessive mucus and cell debris present in the respiratory tract, and the lungs are filled with fluid, fibrin, and hyaluronan [80].
In the beginning, the viral load remains high in the lower respiratory tract. The inflammatory response is initiated by infected cells and macrophages present in the alveolar, with the recruitment of neutrophils, lymphocytes, and monocytes [81]. Vasoactive peptide kinins are released with disease progression, activating endothelial kinin receptors, relaxing the vascular smooth muscle, and upregulating vascular permeability [82]. ACE2 controls this entire signaling cascade. Uncontrolled release of proinflammatory markers further results in pulmonary edema entering the alveolar, leading to hyaline membrane formation and acute respiratory distress syndrome (ARDS). The coagulation pathway leads to the formation of the microthrombi [81].
Lung Fibrosis
Pulmonary fibrosis is defined as a decrease in lung function that leads to respiratory failure [83]. Pro-fibrotic factors initiate lung fibrosis, mainly transforming growth factor-β (TGF-β). In a normal scenario, the release of TGF-β from the affected lung leads to tissue repair and damage correction due to an infection. However, excessive infection of COVID-19 leads to higher secretion of TGF-β, ultimately leading to the transition of cells like epithelial-to-mesenchymal transition [84, 85]. This transition leads to thickening of the inner wall of the lungs and eventually loss of efficacy of the lungs [86].
Coagulopathy
Thromboembolism of arteries and veins is one of the critical manifestations of severe COVID-19 infection [87, 88]. The exact cause of this phenomenon is unknown. However, some studies suggest that invasion of lung endothelial cells leads to an increase in D-dimer concentrations (3–50 times higher than normal concentrations), fibrinogen, an increase in prothrombin and thromboplastin time, and thrombocytopenia [89,90,91]. The circulating concentration of D-dimer remains very high in severe COVID-19 than in general pneumonia patients.
Cytokine storm
Production of cytokines mainly depends upon two pathways: The first is direct viral detection by immune cells via pattern-recognition receptors, mainly Toll-like receptors (TLR-3, 7, 8, 9). The second pathway is by activating damage-associated molecular patterns (DAMPS) by damaged epithelial cells [92, 93]. When the injury occurs to different cells like endothelial, epithelial, and parenchymal, they tend to release a variety of inflammatory markers which activate an acute immune response [94]. Some studies showed circulating concentrations of proinflammatory markers like tumor necrosis factor (TNF), interleukin-6 (IL-6), monocyte chemotactic protein 1 (MCP1), and macrophage inflammatory protein-1α (MIP-1α) were higher in patients with COVID-19 [95]. The exact pathophysiology of cytokine storm is debatable, but an increase in proinflammatory markers is proven in COVID-19 infection, although current evidence neither supports nor rejects the fact that proinflammatory response is due to the damage to the lungs only.
Current evidence regarding the pathophysiology of SARS-CoV-2 indicates that SARS-CoV-2 causes cold-like symptoms when present in the upper respiratory tract. When it settles down to the lower respiratory system, it causes severe pneumonia and breathing difficulties. Rather than alveolar infection, endothelial and epithelial infection appears too dominant [96, 97]. The destruction of the alveolar epithelial–endothelial barrier is essential for the development of severe pneumonia and acute respiratory distress syndrome (ARDS). The inflammatory response is atypical; although patients with COVID-19 have elevated circulating proinflammatory cytokines over a longer time than patients with influenza [98], for example, the concentrations seem to be significantly lower than are typical in non-COVID-19-related ARDS [99]. The inflammatory characteristics thus far observed in patients with COVID-19 suggest that either the systemic cytokine component is not a crucial contributor to COVID-19 severity or that the disease features its own unique, poorly understood, yet detrimental inflammatory profile. The inflammatory response remains highly unusual. Though proinflammatory markers are higher in COVID-19 patients, it is much lower than in non-COVID-19 patients with ARDS. The exact pathophysiology is still unknown, and scientists worldwide are working around the clock to understand how coronavirus affects the human body to develop new therapeutic drugs and approaches.
Post-COVID complications of COVID-19
Almost 80% of the patients who have recovered from COVID-19 infection complained about one or more symptoms since the beginning of the pandemic. Due to constant waves of infection shattering healthcare and research, very little importance has been given to post-COVID complications. But now, many prospective and retrospective studies have pointed out the seriousness of post-COVID manifestation. For example, a 6-month cohort study in Wuhan, China, showed that most patients have at least one of the following symptoms: fatigue or muscle weakness, sleep difficulties, and psychological problems, mainly depression. The study also pointed out that female survivors have higher stress levels and anxiety than males. The same study also found other complications such as abnormal pulmonary diffusion, renal dysfunction, newly diagnosed type-II diabetes, and venous thrombolytic diseases [100]. In another prospective single health system, an observational cohort study examining severely ill COVID patients demonstrated that the intensity, frequency, and duration of the shortness of breath worsened after COVID-19. The study also observed severe psychological problems (20%) in patients who live alone [101]. Another meta-analysis showed altered diffusion capacity, restrictive pattern, and obstructive pattern in 39%, 15%, and 7% of patients [102]. The long-term complications that arise after COVID-19 infection are shown in Fig. 2.
Pulmonary complications
Invasion of lung epithelial cells barrier, systemic inflammatory response, and damage to the immune system plays an important role in pulmonary complications. Viral-dependent and independent mechanisms lead to disruption of the endothelial–epithelial layer of alveoli with monocyte and neutrophils infiltration in the alveolar space of the lungs. Fibrosis in the lungs is induced by proinflammatory cytokines such as TNF-α, IL-6, TGF-β, and superficial bacterial infection. Autopsy of lung tissue collected from five severe COVID-19-related pneumonia patients showed single-cell RNA patterns similar to end-stage pulmonary fibrosis without active COVID-19 infection, proving that some patients may suffer from pulmonary fibrosis even after recovering from the active infection. In addition, 20 to 30% of the recovered patients showed micro- and macro-thrombosis, which is higher compared to any other disease condition indicating the role of COVID-19 infection even after the negative RT-PCR.
From dyspnea to pneumonia and lung fibrosis, pulmonary complications have been reported in COVID-19 survivors. After hospital discharge, abnormal pulmonary dysfunction has been reported in severe COVID-19 patients [103]. Severe patients showed a higher prevalence of diffusion capacity of carbon dioxide (DLCO) impairment and lower transfer factor of carbon dioxide (TLC) compared with non-severe patients after 30 days of discharge, indicating that severe patient requires more time to recover from pulmonary dysfunction [104]. A Swiss study has demonstrated that four months after SARS-CoV-2 infection, severe COVID-19 survivors were present with significant functional and radiological abnormalities, possibly due to small airway and lung parenchymal disease [105]. A 6-month follow-up CT study in severe COVID survivors found fibrotic-like changes in the lung within 6 months. Patients with fibrotic-like changes in the lung showed a 63% ARDS, also a predictor of fibrotic-like changes [106]. An almost similar result obtained in an Italian study was that 72% of the patient showed fibrotic-like changes after 6 months of CT[107]. A study on dyspnea and breathlessness in COVID survivors showed that 70% of previously hospitalized COVID-19 survivors experience fatigue and dyspnea 7 months after hospitalization. In addition, 45% of survivors found difficulties in day-to-day activities. It was found that gender difference, preexisting comorbidities, the number of symptoms present at hospital admission, and the number of days at the hospital were potential risk factors associated with fatigue and dyspnea [108]. An 8-month follow-up study in COVID-19 survivors showed that survivors with severe COVID-19 had higher incidences of DLCO impairment, persistent symptoms in daily life, dyspnea, and higher abnormal CT score as compared with mild cases [109]. 1-year prospective cohort study to evaluate lung abnormalities on serial computed tomography (CT) scans found that COVID-19 survivors showed continuous improvement on lung CT scans during 1 year. The study also showed that old age and the severity of COVID infection delay the recovery of lungs and require more comprehensive rehabilitation [110].
Cardiac complications
The mechanism involved in cardiac complications after COVID-19 infection includes downregulation of ACE2 receptors, lung fibrosis, and inflammatory and immune response affecting cardiac muscles, and the heart's conduction system. An autopsy study of 39 COVID-19 patients showed the presence of a virus in the heart. Inflammatory response and cytokine storm may lead to the death of cardiomyocytes and displacement of desmosomal proteins required for the cell adhesion process. Patients who recovered from COVID-19 exhibited more cardiometabolic demand after 6 months, leading to dysfunction of the renin-angiotensin system (RAS) and reduced cardiac reserve. Cytokines such as TNF-α and IL-6 lead to changes in ion exchange in cardiac myocytes leading to arrhythmias and cardiac myopathy.
Cardiovascular complications mainly occur in admitted severe or fatal cases of COVID-19 that have already developed ARDS. A 60-day cohort study on hospitalized COVID survivors found chest pain in 16.6% of patients, while 15.5% required rehospitalization [111]. Different perspectives and retrospective trials demonstrated persistent chest pain ranging from 16 to 21% in severe COVID survivors after 60 days [112,113,114]. A 6-month Chinese cohort study found that 9% of survivors suffered from palpitation, while 5% suffered from chest pain [100]. A clinical study conducted on 287 hospitalized survivors in Egypt reported 28.9% chest discomfort, 1.4% myocarditis, and 0.3% arrhythmia after 20 days [115]. A half-year follow-up of patients recovering from severe COVID-19 in Wuhan, China, demonstrated that around 10% of survivors suffer from chest pain [116]. Based on the trial data, the most common cardiovascular complications were chest pain, dizziness, palpitation, chest tightness, and chest problem on exertion. At the same time, few people were diagnosed with myocarditis and arrhythmia.
Dermatologic complications
The multisystem inflammatory response (MSI) had been documented in many patients who recovered from COVID-19. Those patients who had negative RT-PCR reports and positive antibody status point out that MSI may be due to the host immune response rather than the COVID-19 infection or viral entry and downstream consequence. More study data are required to evaluate the possible mechanism behind the dermatological consequences of long COVID-19.
While the most common symptoms of COVID-19 involve fever, coughing, and cold, skin problems can occur in up to 20.4% of patients after suffering from COVID-19 [117]. Around ten female survivors at Montefiore Medical Center, New York, USA, complained about hair loss after three months. These patients who do not have any history associated with hair loss indicate the possible effect of viral infection [118]. Only 3% of patients reported a skin rash at 6-month follow-up in the post-acute COVID-19 Chinese study [100].
Hematologic complications
Coagulopathy has been documented in around 20–30% of patients with acute COVID-19 infection. The underlying cause of coagulopathy may be an aggressive proinflammatory response and hypercoagulable condition. The possible mechanism that leads to coagulopathy includes injury to the endothelial layer followed by activation of platelet and downstream signaling leading to platelet-leukocyte crosstalk, infiltration of neutrophils, inflammatory response, and disturbance in the coagulation pathway. Coagulation in COVID-19 infection follows a similar pathway as any other thrombotic event. The possible complications from hypercoagulation in the hyperinflammatory state and how long it persists, although very little data are available to conclude this statement.
Venous thromboembolism has always been a critical concern in patients with COVID-19. Patients admitted to COVID-19 frequently developed venous thromboembolism during their hospital stay, especially in critically ill patients. A prospective follow-up study of patients admitted to the hospital due to COVID-19 showed that at 6 weeks after discharge, one (1%) of 102 patients developed asymptomatic venous thromboembolism event by venous ultrasound [119]. A prospective study in China of patients discharged after a hospital stay due to COVID-19 reported no deep vein thrombosis of lower limbs in 390 participants by ultrasonography 6 months after illness onset [100]. In a root cause analysis involving 1877 hospital discharges associated with COVID-19, nine episodes of HA-VTE were diagnosed within 42 days, giving a postdischarge rate of 4.8 per 1000 discharges. In 1 year, 18,159 discharges were associated with a medical admission, out of which, 56 episodes of healthcare-associated venous thromboembolism (HA-VTE) were identified within 42 days (3.1 per 1000 discharges) [120].
Neuropsychiatric complications
The mechanisms involved in neurological and psychiatric complication of COVID-19 includes direct viral infection, systemic inflammation, neuronal inflammation, thrombosis, and stressful event. Till now, no evidence has been found regarding COVID-19 presence in neurons. However, autopsy reports of COVID-19 patients have found a change in parenchyma and vessel of the brain, possibly due to a change in the coagulation system or blood along with persistent inflammation and the blood–brain barrier.
Psychological complications
More than 30% of patients hospitalized with COVID-19 may experience anxiety, depression, and cognitive impairment that last for months after discharge. These symptoms are even more prominent in intensive care patients [121]. A UK-wide surveillance study to assess psychiatric manifestation in 153 COVID-19 patients concluded that 31% of patients had altered mental status, including anxiety and depression encephalopathy, in younger patients [122]. A retrospective cohort study evaluated neurological and psychotic outcomes in 236,379 survivors of COVID-19 at 6 months. After 6 months, the following complications were identified: intracranial hemorrhage (0.56%), ischemic stroke (2.10%), parkinsonism (0.11%), Guillain barre syndrome (0.08%), the myoneural junction (0.45%), encephalitis (0.10%), dementia (0.67%), anxiety/depression (23.59%), substance use disorder (6.58%), and insomnia (5.42%). The study also reveals that the severity of COVID-19 had a clear effect on subsequent neurological diagnoses [123]. An observational study of 58 COVID-19 patients in France reported a confused state of mind, agitation, depression, and anxiety [124].
Neurological complications
A UK-wide surveillance study to assess acute neurological manifestation in 153 COVID-19 patients concluded that 62% of patients suffered from cerebrovascular events [122]. An observational study of 58 COVID-19 patients in France reported encephalopathy and acute ischemic strokes [124]. The risk of ischemic stroke and intracranial hemorrhage was high after COVID-19, with the prevalence of ischemic stroke rising to almost one in ten in patients with encephalopathy. A higher risk of stroke in patients was identified in COVID-19 patients than in those who had a common cold or influenza [125].
Renal complications
Traces of SARS-CoV-2 have been found in renal tissue and necrosis of tubules in necropsies and autopsies of severe COVID-19 patients. As renal failure is associated with a high risk of APOL1 alleles, point out that SARS-CoV-2 can hit only susceptible patients in the same manner as human immunodeficiency virus (HIV). In addition, thrombosis and coagulopathy may also be significant contributors to renal complications.
Kidney involvement is most common in patients with SARS-CoV-2 infection, and chronic inflammation and injury may remain for many months, resulting in a progressive decline in kidney function that leads to kidney failure [126]. A US study from the Veterans Health Administration (VHA) conducted a comprehensive study of long COVID and reported that COVID-19 increased the risk of chronic kidney disease, and the chance was found to be higher who were severely ill [127]. A follow-up study in China found that 35% of patients had reduced kidney function 6 months after COVID-19 hospitalization. Moreover, 13% of patients who did not have AKI during hospitalization showed a reduction in GFR at a 6-month follow-up [100].
Gastrointestinal complications
Mild-to-moderate gastrointestinal complications have been reported in COVID-19 survivors. The presence of viral debris like ribonucleic acid has been documented in fecal after at the 11th day after negative RT-PCR results. COVID-19 can potentially change the gut microflora and lead to the accumulation of bacteria, which may lead to opportunistic infection. The role of gut Microbiota in respiratory illness has been proven in influenza infection. Long COVID-19, patients have experienced irritable bowel syndrome and acid reflux problems. Diarrhea, one of the prominent symptoms of acute COVID-19 infection, typically wears off after 14 days and does not last long. No other serious complications were detected in COVID survivors, indicating no role of COVID in gastric complications.
Hepatic complications
Patients with chronic liver disease and cirrhosis have a low immune response and are more susceptible to COVID-19 infection. Elevated serum levels of ALT and AST were found in hospitalized COVID-19 patients who are not related to increased levels of inflammatory markers or muscle breakdown pointing out that increased liver markers were due to direct liver injury. Patients with very high serum ALT levels also had a high amount of CRP, D-dimer, ferritin, and IL-6. Most of the liver enzymes achieved normal levels after COVID-19 infection. As of now, no particular study is presently focusing on liver complications in long COVID-19. However, an injury sustained during COVID infection may affect the liver in the longer term.
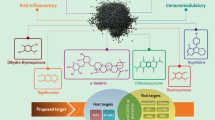
Solanum nigrum in COVID-19 with special emphasis on its immunomodulatory, anti-inflammatory and antiviral action
Numerous medicinal plants and phytochemicals are being recommended to treat COVID-19 based on previous reports against coronaviruses [128,129,130,131,132,133,134,135]. Similarly, during the early pandemic crisis, the Ministry of AYUSH recommends a few Indian medicinal plant-based formulations possessing antiviral, anti-inflammatory, and immunomodulation action [25]. Thus, it is clear that to find a novel therapeutic agent to be effective against COVID-19 from natural sources, a medicinal plant must possess antiviral, anti-inflammatory, and immunomodulatory properties to treat COVID-19 and post-COVID effects as well.
S. nigrum is an edible, herbaceous plant belonging to the family Solanaceae. Commonly, it is known as black nightshade and garden nightshade. The whole plant and its various active constituents exhibit immunomodulatory, anti-inflammatory, and antiviral action, which is the main prerequisite for developing a therapeutic agent for COVID-19 (Table 1).
Immunomodulation
Polysaccharides of the plant S. nigrum are very well-known for immunomodulatory activity[72, 73, 136]. The aqueous and alkali extract of S. nigrum containing polysaccharides at 50, 100, and 200 mg/kg doses reduces interleukin-10 (IL-10), and along with this action, it also shows anticancer potential by inhibiting mouse hepatoma 22 ascitic tumor (H22) cell growth and increases the weight of tumor-bearing mice [72]. In another study, polysaccharides from the S. nigrum stem showed anticancer and immunomodulatory activity. These polysaccharides activate the Toll-like receptor 4-myeloid differentiation primary response 88 (TLR4-MyD88) signaling pathway to promote immune activity and inhibit tumor growth [73]. Similarly, flavonoids like myricetin, rutin, quercetin, and kaempferol are also present in various plants and reported to significant immunomodulatory activity [60, 137]. It is stated that flavonoids can interfere with the mTOR pathway and induce CD4+ and T regulatory cells, thus possessing immunomodulatory action by limiting chronic inflammation [138].
Anti-inflammatory activity
It is investigated that patients with severe COVID-19 are also suffering from acute respiratory distress syndrome due to increased levels of proinflammatory cytokines [139]. In vitro and in vivo studies performed on different plant parts and active constituents of S. nigrum from berries, leaves, and stems prove anti-inflammatory activity [65, 67,68,69,70, 140]. Methanolic extract of S. nigrum fruits at 125, 250, and 275 mg/kg doses showed significant anti-inflammatory activity by inhibiting the edema caused by carrageenan [140]. A similar study performed at a dose of 375 mg/kg also indicated the anti-inflammatory action of plant berries [66]. At acute and sub-acute stages of inflammation, extract of the whole plant of S. nigrum (100 and 200 mg/kg) showed anti-inflammatory potential as compared to indomethacin (10 mg/kg)[69]. Ethanolic extract of the plant berries at 100, 200, and 300 mg/kg concentrations showed 28, 52, and 63% inhibition against carrageenan-induced paw edema. These dose-dependent results confirm the anti-inflammatory potential of plant berries [65]. Solanine, an active glycoalkaloid, was evaluated for anti-inflammatory activity in the croton-oil-induced skin-inflammation model. The alkaloidal fraction containing solanine showed 80% percentage inhibition to reduce ear edema in swiss mice and thus showed anti-inflammatory action [141]. Solasodine at doses of 5, 30, and 75 mg/kg can also produce anti-inflammatory action by inhibiting arachidonic acid, cyclo-oxygenase (COX), and the 5-lipoxygenase pathway, which ultimately reduces inflammation in carrageenan-induced paw edema [142]. Three steroidal glycosides from the plant demonstrated anti-inflammatory potential in LPS-induced RAW 264.7 macrophages. A significant inhibitory effect was seen against LPS-induced nitrous oxide (NO) production and thus proves the anti-inflammatory action of phytoconstituents of S. nigrum [70]. Kaempferol and other glycosides show anti-inflammatory action by inhibiting cytokine storm in SARS-CoV-2 infection [143,144,145,146]. Similarly, other constituents like apigenin, luteolin, rutin, and quercetin showed anti-inflammatory action through various pathways [147]. Moreover, these flavonoids are also included in various Traditional Chinese Medicine (TCM) formulations recommended to treat SARS-CoV-2 infection in China [148,149,150]. Evaluation of three selected TCM formulations through network pharmacology proves that they are beneficial in COVID-19 by reducing IL-6 levels [151]. It has also been proved that IL-6 inhibitors play a major role in the treatment and prevention of COVID-19 [152].
Antiviral activity
Antiviral in vitro culture assay using Huh-7 cell line and real-time quantitative RT-PCR of methanolic and chloroform extract of S. nigrum possesses 37% and 50% inhibition against HCV [37]. However, aqueous extract of aerial parts of S. nigrum at a dose of 100 µg/ml possesses antiviral action against HIV-1 protease and HIV-1 reverse transcriptase by showing 32.6% and 17.4% inhibition, respectively [36]. Both studies demonstrate that plants possess antiviral action against RNA-based viral diseases like HIV and HCV. So, it can be assumed that it could be a potential therapeutic agent against COVID-19 and other RNA-based viral diseases.
Thus, considering the plant's immunomodulatory, antiviral, and anti-inflammatory activities, we proposed that S. nigrum would be a potential candidate for the management of COVID-19 and post-COVID complications.
Role of Solanum nigrum in COVID-19: in silico studies
The major proteins that facilitate the entry mechanism of the novel SARS-CoV-2 virus into the host cell are spike S protein, angiotensin-converting enzyme-2 (ACE2), and human transmembrane serine proteases type-II (TMPRSS2). Similarly, 3C-like main proteases (CLpro) and Papain-like proteases (PLpro) are responsible for viral encoding. However, RNA-dependent RNA polymerase (RdRp) produces multiple copies of RNA and is thus involved in the infection cycle. Some lead molecules from natural sources can be used to target these different proteins of SARS-CoV-2 to control viral infection and post-COVID complications. Previous reports claim that certain phytochemicals such as catechins, polyphenols, and flavonoids are mainly responsible for inhibiting viral entry and replication [153,154,155]. Viral pathology suggests that virus proteins are required for the entry and multiplication of SARS-CoV-2. To identify active constituents of S. nigrum as potential inhibitors of SARS-CoV-2 proteins, multiple in silico studies have been carried out. Molecular docking studies of flavonoids evidenced potent inhibitors of proteins involved in SARS-CoV-2 infection [56, 156,157,158]. For example, luteolin represents its potential against SARS-CoV-2 by binding with a spike (−8.149) (S), TMPRSS-2 (−7.4) ACE2(−10.1), Mpro (−8.17), and RdRp (−8.3) protein through in silico approach [53,54,55, 57, 159]. In silico docking analysis of rutin revealed excellent binding with a spike (−9.2), ACE2 (−11.5), Mpro (−9.2), and RdRp (−9.0) protein that suggest its potentiality towards SARS-CoV-2 [56, 58, 60]. Similarly, molecular docking of kaempferol showed maximum binding affinity towards spike and ACE2 protein with the binding score of − 8.5 and -7.20, respectively [50, 52, 57]. Myricetin, an active flavonoid, docked against multiple SARS-CoV-2 proteins, including ACE2, CLpro, and RdRp, and the result demonstrates a satisfactory binding score, i.e., greater than −7 kcal/mol [50, 61, 62]. Phytoconstituents like solamargine and solanine were also identified through molecular docking, and results revealed good binding affinities towards viral targets like a spike, Mpro, and ACE2, RdRp [58, 62]. Other alkaloids of S. nigrum like solasodine (−8.3), solanine (−9.0), solasurine (−9.1), and α-solamargine (−10.8) have good binding energies against 3CLpro [160]. Active constituents present in S. nigrum targeting various stages of SARS-CoV-2 are shown in Fig. 3
Thus, available literature on molecular docking and other pharmacological reports indicates that S. nigrum could be used for inhibition of SARS-CoV-2 entry, replication, and suppression of COVID-19 symptoms.
Potential role of Solanum nigrum in post-COVID-19 complications
Pulmonary complications
Hospitalized COVID-19 patients suffer from lung fibrosis, pneumonia, chronic cough, and pulmonary embolism [161, 162]. S. nigrum, play a very important role in the treatment of respiratory disorders and the management of cough [163]. Petroleum ether, ethanol, and aqueous extracts of S. nigrum fruit show antiallergic and antihistaminic action by inhibiting contraction caused by histamine and resisting the increased leukocytes. Thus, berries of the plant represent a potential role in the treatment of asthma [164]. Furthermore, phytochemicals of S. nigrum, such as kaempferol, and luteolin, also have their effect on acute lung injury [165, 166], which is a beginning stage of ARDS [167]. Thus, the above findings conclude that fruit extract and phytochemicals of S. nigrum play an important role in controlling acute lung injury and pulmonary disorders, which ultimately suggests its potential in pulmonary complications of COVID-19.
Cardiac complications
Arrhythmia, myocardial infarction, heart failure, chest discomfort, and severe pain in the chest are the common consequences of COVID-19 after recovery. Traditionally, S. nigrum has been reported to have a potential role in treating multiple cardiac disorders [168]. A study conducted on isoproterenol-induced myocardial infarction rats showed the efficacy of S. nigrum as a cardioprotective agent. The hydroalcoholic extract of the whole plant at 75, 150, and 300 mg/kg was given to the albino rats and checked for cardioprotective and antioxidant activity. The in vivo study results have decreased QT interval (duration of ventricular electrical systole) and heart rate due to a decrease in myocardial damage. A reduction in biochemical parameters like triglyceride and cholesterol was also observed by the hydroalcoholic extract of the plant and thus confirms cardioprotective action [169]. Berries of the plant (2.5 and 5.0 mg/kg) also showed cardioprotective and antioxidant properties. The heart of male albino rats was isolated from estimating thiobarbituric acid reactive substances (TBARS), Myocardial Superoxide dismutase (SOD), reduced level of glutathione, and myocardial catalase content (CAT). Results of this study expressed that myocardial TBARS was found to decrease ischemic reperfusion injury, and there was a significant increase in myocardial endogenous antioxidants enzymes (GSH, SOD, and CAT). Thus, fruit extract of S. nigrum shows a protective effect in ischemia–reperfusion-induced oxidative stress, which eventually indicates cardioprotective action [43]. Another study includes an aqueous extract of berries (1 g/kg) for cardioprotective action. In this in vivo study, doxorubicin (20 mg/kg i.p) was used to induce cardiac toxicity in rats, and carvedilol (30 mg/kg) was used as the standard. Results indicate a significant reduction in cardiac serum levels like cardiac serum markers creatinine phosphokinase (CKMB), lactate dehydrogenase (LDH), serum glutamate oxaloacetate transaminase (SGOT), and serum glutamate pyruvate transaminase (SGPT) and thus proved to produce cardioprotective effect [42]. Moreover, S. nigrum fruits have been proven to prevent vascular complications in streptozotocin-induced diabetic rats [170]. Hence, scientific research revealed the cardioprotective action of hydroalcoholic extract of the plant and its fruit that may help later in cardiac complications that arise after COVID-19.
Dermatologic complications
Hair loss is the most common symptom of a patient suffering from COVID-19 recovery. It has been reported that berries of the S. nigrum plant have been traditionally used for alopecia [171]. An herbal hair formulation containing berries extract of S. nigrum (0.5%), the flower of Hibiscus rosa (1%), and Eclipta alba (1%) was prepared on a laboratory scale and evaluated for effective herbal hair therapy. Phytoconstituents in this formulation showed effective action against 5-α-reductase and thus treated alopecia [172]. A recent study stated that S. nigrum could suppress proinflammatory cytokines in a model of 1‑chloro‑2,4‑dinitrobenzene (DNCB)‑induced atopic dermatitis (AD) and in TNF‑α/IFN‑γ‑stimulated HaCaT cells. The in vitro and in vivo studies showed the efficiency of ethanolic extract of the plant in suppressing inflammatory cytokines, mitogen-activated protein kinase (MAPK), and nuclear factor-κB (NF‑κB) in human adult low calcium high temperature (HaCaT) cells. Additionally, serum level of IgE and CD8+ and dermal thickness was also reduced in the DNCB model of AD [173]. A study also showed the efficacy of S. nigrum and Azadirachta indica in skin regeneration [174]. Hair loss is a serious concern after recovery from COVID-19. Furthermore, traditional and scientific reports suggest the plant's probable role in alopecia and other skin-related issues. Thus, S. nigrum could be useful in dermatological complications after COVID-19.
Neurological complications
Plants have been used for neurological disorders for so many years. S. nigrum also has a beneficial role in neurological diseases [175]. Two species of genus Solanum, namely S. nigrum and S. macrocarpon, at 2 mg/kg were given as dietary inclusions to rats for the evaluation of cognitive and neurochemical complications induced by scopolamine in rats, particularly in Alzheimer's disease. Increased level of acetylcholinesterase (AChE), monoamine oxidase (MAO), and butyrylcholinesterase (BChE) is reduced by both species [176]. Hence, this plant could be useful in the prevention of cognitive impairments for Alzheimer's involved after COVID-19 diseases. Leaves of the plant used as dietary inclusion at a dose of 0.1 to 1.0% w/v show a variety of benefits from protecting against aluminum-induced neurotoxicity [177] to preventing cerebral damage and behavioral changes by investigating carbonated alcoholic herbal beverage (CAHB). This in vivo study proves that aqueous leaves extract shows a constructive effect in CAHB-produced cerebellar edema, degeneration of Purkinje cells, vascular stenosis, and ulceration [178]. Phenolic constituents and aqueous leaves extract of the plant S. nigrum were reported to have ameliorative activity against superoxide dismutase (SOD), glutathione-S-transferase (GST), TBARS, and glutathione levels [179]. Thus, results revealed that plant could be used for the prevention of free radicals caused by neurological diseases. Significant results of all the studies indicate the role of the plant in neurological diseases and eventually strengthen its role in the neurological complications of COVID-19.
Psychiatric complications
Although limited data are available to relate plant activity with psychiatric disorders. But a study showed the potential of aqueous extract of the leaves of S. nigrum at 30–60 mg/kg for anti-epileptic action in rats, mice, and chicks. Picrotoxin and pentylenetetrazol were used to induce seizures, and a 10–60 mg/kg dose was given to the animals. The extract showed dose-dependent inhibition and possessed anti-epileptic action [180]. Thus, the anti-epileptic activity of plant suggests that it could be useful in the psychiatric complication of COVID-19.
Renal complications
A report confirms the nephroprotective action of the plant S. nigrum based on its previous studies performed against kidney disorders. Histopathology studies showed that parameters such as ALP, ALT, AST, and bilirubin levels could be normalized by S. nigrum. The study also suggests the safer dose of S. nigrum is up to 5 ml/kg above which plant may be toxic [181]. Fruit extract of S. nigrum (200 mg/kg) shows nephroprotective action by showing significant reductions in blood urea nitrogen and serum creatinine. Results were compared with gentamicin-induced nephrotoxic rats [182]. A similar study performed by the same researcher, along with histopathology, proves the efficacy of plant fruits as a nephroprotective agent [183]. Hence, various renal studies performed on the plant disclose that S. nigrum is very effective in treating kidney disorders which indicates its possible role in the treatment of COVID-19-related renal complications.
Hematologic complications
Although S. nigrum does not play a major role in hematologic complications produced after COVID-19, it has been reported that the aqueous extract of leaves and fruit of the plant possesses hemopoietic and hematinic properties. Phenylhydrazine (10 mg/kg) produces anemia in Wistar rats, and aqueous extract of leaves and fruit of S. nigrum ultimately shows a defensive mechanism against anemia and significantly increases the red blood cell. The study also demonstrated the anti-bacterial and anti-parasitic properties of the aqueous extract of S. nigrum leaves [184].
Gastro complications
S. nigrum has proved its efficacy against ulcers. Methanolic leaves extract of S. nigrum at a dose of 500 mg/kg causes a reduction in ulcer index (77.85% in aspirin and 66.6% in CRS-treated groups) in the cold restraint stress model. The plant extract also showed an ameliorative effect on biochemical parameters and histological changes [38]. Another study on the methanolic extract of S. nigrum fruits proves its efficacy against gastric ulcers at 200 and 400 mg/kg doses [40]. Aqueous and hydroalcoholic extract of leaves and berries also showed a defensive mechanism against gastritis and ulcers in female albino rats. Ethanol and aspirin were used to induce gastritis and gastric ulcers, respectively. Aqueous extract of leaves (80 mg/kg and 250 mg/kg) and berries (50 mg/kg) significantly reduced ulcer index. Also, they showed an anti-gastritis effect by decreasing the concentration of Evans blue [185]. Recently, Althaea officinalis and S. nigrum demonstrated antioxidant and anti-ulcerogenic action on indomethacin-induced gastric ulcerated rats. S. nigrum is a gastro-protective agent as it can reduce TNF-α, IL-1β, and gastric mucosal damage induced by indomethacin. Also, oral administration of plant extract causes a boost in the PG-E2 level [39]. Hence, all these activities prove the efficacy of S. nigrum leaves and berries in gastritis and gastric ulcers which certainly suggests its role in gastric complications of COVID-19.
Hepatic complications
It has been proved that S. nigrum possesses hepatoprotective action in vitro and in vivo methods. Flavonoid-rich extract of the plant at 100, 200, and 300 mg/kg doses showed a dose-dependent effect in CCl4-induced hepatotoxic mice [186]. Similarly, aqueous extract of S. nigrum at the dose of 150 mg/kg also possesses hepatoprotective action against ethanol-induced hepatotoxicity by decreasing liver marker enzymes such as AST, ALT, ALP, and total bilirubin [187]. According to both studies, S. nigrum has proven efficacy against acute hepatic injury induced by CCl4 and ethanol. A polyherbal extract containing three medicinal plants (Andrographis paniculata, Tinospora cordifolia, and S. nigrum) that have been known to treat liver disorders traditionally showed hepatoprotective action by showing reductions in triglycerides (TG) and low-density lipoproteins (LDL) at the dose of 500 mg/kg [188]. Hence, all these activities showed that S. nigrum could show hepatoprotective action, and it could be beneficial against hepatic complications that may arise after COVID-19.
Conclusions and future perspective
COVID-19 has changed the world scenario. Almost every drug failed to conquer the disease, while few drugs are successful only in minimizing or controlling the symptoms. Failure of currently available drugs to destroy the virus leads many scientists worldwide to find alternatives. Scientists successfully developed an mRNA vaccine quickly, but even after the vaccine, the threat of COVID-19 is still present among us. As a result, many critically ill patients are hospitalized for longer. Hospitalized patients had been treated with antiviral, anti-inflammatory, and steroidal drugs. Bombardment of drugs leads to many physiological changes in the body, and patients recovering from COVID-19 face many complications, which were pointed out by many clinical and retrospective studies. The most prominent ones are headache, fatigue, anxiety, depression, difficulty in breathing, and neurological and cardiovascular complications. Post-COVID complications need to be addressed as they are also posing challenges to our day-to-day life.
Many healthcare professionals are working on revisiting the alternatives to standard drugs to tackle long-term complications. Many medicinal plants and their phytoconstituents possess the efficacy to treat the various complications of COVID-19. The traditional report indicates the role of S. nigrum in pulmonary tuberculosis, fever, bronchitis, and asthma-related issues. Moreover, overall literature on molecular docking and reported pharmacological activities confirms that S. nigrum has multiple potentialities in antiviral, anti-inflammatory, and immunomodulatory activity. A combination of these three activities is a necessity to develop a novel therapeutic agent for COVID-19.
Furthermore, developing therapies targeting major proteins in the viral life cycle would be a viable solution, and S. nigrum fulfills the need. Most adults who get SARS-CoV-2 infection experience several long-term symptoms that do not go entirely after several months. These complications are even more challenging than the SARS-CoV-2 infection because they cause diverse adverse effects on multiple organs. Pharmacological studies proved that S. nigrum plays a crucial role in protecting different body organs, i.e., nephroprotective, gastro-protective hepatoprotective, and neuroprotective action that will ultimately help in the post-COVID-19 complications (Fig. 4). However, further focused studies are required in this direction to place this herb as a solution to long-term post-COVID complications.
Data availability
Enquiries about data availability should be directed to the authors.
References
Ma X, Liang M, Ding M, Liu W, Ma H, Zhou X et al (2020) Extracorporeal membrane oxygenation (ECMO) in critically Ill patients with coronavirus disease 2019 (COVID-19) pneumonia and acute respiratory distress syndrome (ARDS). Med Sci Monit. https://doi.org/10.12659/MSM.925364
Alsharif W, Qurashi A (2021) Effectiveness of COVID-19 diagnosis and management tools: a review. Radiography 27(2):682–687. https://doi.org/10.1016/j.radi.2020.09.010
Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, Fotiou D, Migkou M, Tzanninis IG et al (2021) Emerging treatment strategies for COVID-19 infection. Clin Exp Med 21(2):167–179. https://doi.org/10.1007/s10238-020-00671-y
Karim SSA, Karim QA (2021) Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet 398(10317):2126–2128. https://doi.org/10.1016/S0140-6736(21)02758-6
Umakanthan S, Sahu P, Ranade AV, Bukelo MM, Rao JS, Abrahao-Machado LF et al (2020) Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J 96(1142):753–758. https://doi.org/10.1136/postgradmedj-2020-138234
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A et al (2021) More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 11(1):16144. https://doi.org/10.1038/s41598-021-95565-8
Gemelli Against C-P-aCSG (2020) Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res 32(8):1613–1620. https://doi.org/10.1007/s40520-020-01616-x
Yong SJ (2021) Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 53(10):737–754. https://doi.org/10.1080/23744235.2021.1924397
Shanbehzadeh S, Tavahomi M, Zanjari N, Ebrahimi-Takamjani I, Amiri-Arimi S (2021) Physical and mental health complications post-COVID-19: Scoping review. J Psychosom Res. https://doi.org/10.1016/j.jpsychores.2021.110525
Chen P-L, Lee N-Y, Cia C-T, Ko W-C, Hsueh P-R (2020) A Review of treatment of coronavirus disease 2019 (COVID-19): therapeutic repurposing and unmet clinical needs. Front Pharmacol. https://doi.org/10.3389/fphar.2020.584956
Bernstein DI, Wald A, Warren T, Fife K, Tyring S, Lee P et al (2017) Therapeutic vaccine for genital herpes simplex virus-2 Infection: findings from a randomized trial. J Infect Dis 215(6):856–864. https://doi.org/10.1093/infdis/jix004
https://www.who.int/news-room/feature-stories/detail/side-effects-of-covid-19-vaccines
https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued
Wald A, Corey L (2007) Persistence in the population: epidemiology, transmission, Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. In: Arvin A et al (eds) Cambridge University Press. Cambridge
https://worldhealthorg.shinyapps.io/EURO_COVID-19_vaccine_monitor/
https://www.census.gov/library/stories/2021/12/who-are-the-adults-not-vaccinated-against-covid.html
https://covid.cdc.gov/covid-data-tracker/#rates-by-vaccine-status.
Mukhtar M, Arshad M, Ahmad M, Pomerantz RJ, Wigdahl B, Parveen Z (2008) Antiviral potentials of medicinal plants. Virus Res 131(2):111–120. https://doi.org/10.1016/j.virusres.2007.09.008
Lowe H, Steele B, Bryant J, Fouad E, Toyang N, Ngwa W (2021) Antiviral activity of Jamaican medicinal plants and isolated bioactive compounds. Molecules 26(3):607. https://doi.org/10.3390/molecules26030607
Ogbole OO, Akinleye TE, Segun PA, Faleye TC, Adeniji AJ (2018) In vitro antiviral activity of twenty-seven medicinal plant extracts from Southwest Nigeria against three serotypes of echoviruses. Virol J 15(1):110. https://doi.org/10.1186/s12985-018-1022-7
Ben-Shabat S, Yarmolinsky L, Porat D, Dahan A (2020) Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug Deliv Transl Res 10(2):354–367. https://doi.org/10.1007/s13346-019-00691-6
Ogbole OO, Akinleye TE, Segun PA, Faleye TC, Adeniji AJ (2018) In vitro antiviral activity of twenty-seven medicinal plant extracts from Southwest Nigeria against three serotypes of echoviruses. Virol J 15(1):1–8. https://doi.org/10.1186/s12985-018-1022-7
Sharanya C, Sabu A, Haridas M (2021) Potent phytochemicals against COVID-19 infection from phyto-materials used as antivirals in complementary medicines: a review. Future J Pharm Sci 7(1):1–20. https://doi.org/10.1186/s43094-021-00259-7
Ahmad S, Zahiruddin S, Parveen B, Basist P, Parveen A, Gaurav, et al (2021) Indian medicinal plants and formulations and their potential against covid-19–preclinical and clinical research. Front Pharmacol. https://doi.org/10.3389/fphar.2020.578970
Lim XY, Teh BP, Tan TYC (2021) Medicinal Plants in COVID-19: Potential and Limitations. Front Pharmacol. https://doi.org/10.3389/fphar.2021.611408
Edmonds JM, Heller J, and Engels J, Black nightshades. Solanum nigrum L. and related species. 1997.
Patel B, Sharma S, Nair N, Majeed J, Goyal RK, Dhobi M (2021) Therapeutic opportunities of edible antiviral plants for COVID-19. Mol Cell Biochem 476(6):2345–2364. https://doi.org/10.1007/s11010-021-04084-7
Longman O, Indian Medicinal Plants: A Compendium of 500 Species. 1997.
The Ayurvedic Pharmacopoeia of India, M.o.H.a.F.W. Department of AYUSH, Editor.
Alamgeer SA, Uttra AM, Hasan UH (2019) Alkaloids, flavonoids, polyphenols might be responsible for potent antiarthritic effect of Solanum nigrum. J Tradit Chin Med 39(5):632–641
Moglad E, Abdalla O, Koko W, Saadabi A (2014) In vitro Anticancer Activity and Cytotoxicity of Solanum nigrum on Cancers and Normal Cell Lines. Int J Cancer Res 10:74–80. https://doi.org/10.3923/ijcr.2014.74.80
Lai Y-J, Tai C-J, Wang C-W, Choong C-Y, Lee B-H, Shi Y-C et al (2016) Anti-cancer activity of Solanum nigrum (AESN) through suppression of mitochondrial function and epithelial-mesenchymal transition (EMT) in breast cancer cells. Molecules 21(5):553. https://doi.org/10.3390/molecules21050553
Nawaz A, Jamal A, Arif A, Parveen Z (2021) In vitro cytotoxic potential of Solanum nigrum against human cancer cell lines. Saudi J Biol Sci 28(8):4786–4792. https://doi.org/10.1016/j.sjbs.2021.05.004
Campisi A, Acquaviva R, Raciti G, Duro A, Rizzo M, Santagati NA (2019) Antioxidant activities of Solanum nigrum L. leaf extracts determined in in vitro cellular models. Foods 8(2):63. https://doi.org/10.3390/foods8020063
Yu Y-B (2004) The extracts of Solanum nigrum L. for inhibitory effects on HIV-1 and its essential enzymes. Korean J Orient Med 10(1):119–126
Javed T, Ashfaq UA, Riaz S, Rehman S, Riazuddin SJVJ (2011) In-vitro antiviral activity of Solanum nigrum against Hepatitis C Virus. Virol J 8(1):1–7
Saravanan S, Dhasaratha P, Indira V, Venkatrama R (2011) Gastro protective and antioxidant activity of Solanum nigrum Linn. against aspirin and cold restraint stress induced ulcerated rats. Res J Immunol 4:1–11. https://doi.org/10.3923/rji.2011.1.11
Zaghlool SS, Abo-Seif AA, Rabeh MA, Abdelmohsen UR, Messiha BA (2019) Gastro-protective and anti-oxidant potential of Althaea officinalis and Solanum nigrum on pyloric ligation/indomethacin-induced ulceration in rats. Antioxidants 8(11):512. https://doi.org/10.3390/antiox8110512
Jainu M, Devi CSSJJOE (2006) Antiulcerogenic and ulcer healing effects of Solanum nigrum (L.) on experimental ulcer models: possible mechanism for the inhibition of acid formation. J Ethnopharmacol 104(1–2):156–163. https://doi.org/10.1016/j.jep.2005.08.064
Lin H-M, Tseng H-C, Wang C-J, Lin J-J, Lo C-W, Chou F-P (2008) Hepatoprotective effects of Solanum nigrum Linn extract against CCl4-iduced oxidative damage in rats. Chem Biol Interact 171(3):283–293. https://doi.org/10.1016/j.cbi.2007.08.008
Varshney P, Vishwakarma P, Sharma M, Saini M, Bhatt S, Singh G et al (2016) Cardioprotective effect of Solanum nigrum against doxorubicin induced cardiotoxicity-an experimental study. Int J Basic Clin Pharmacol 5(3):748–753. https://doi.org/10.18203/2319-2003.ijbcp20161513
Bhatia N, Maiti P, Kumar A, Tuli A, Ara T, Khan M (2011) Evaluation of cardio protective Activity of Methanolic Extract of Solanum nigrum Linn. Int J Drug Dev Res 3(3):139–147
Tai BH, Van Doan V, Yen PH, Nhiem NX, Cuc NT, Trang DT et al (2018) Two new steroidal alkaloid saponins from the whole plants of Solanum nigrum. Nat Prod Commun. https://doi.org/10.1177/1934578X1801301111
Ding X, Zhu F, Yang Y, Li MJFC (2013) Purification, antitumor activity in vitro of steroidal glycoalkaloids from black nightshade (Solanum nigrum L.). Food chem 141(2):1181–1186. https://doi.org/10.1016/j.foodchem.2013.03.062
Huang H-C, Syu K-Y, Lin J-K (2010) Chemical composition of Solanum nigrum linn extract and induction of autophagy by leaf water extract and its major flavonoids in AU565 breast cancer cells. J Agri Food Chem 58(15):8699–8708. https://doi.org/10.1021/jf101003v
Zhou X, He X, Wang G, Gao H, Zhou G, Ye W et al (2006) Steroidal saponins from Solanum nigrum. J Nat Prod 69(8):1158–1163. https://doi.org/10.1021/np060091z
Khan HJ, Ahmad MK, Khan AR, Rastogi N, Mahdi AA, Ansari JAet al. (2016) Identification of Anticancer and Antioxidant phytoconstituents from chloroform fraction of Solanum nigrum L. berries using GC-MS/MS analysis. Indian J Exp Biol.
Guidelines for Ayurveda Practitioners for COVID 19, Ministry of AYUSH, Govt. of India.
Joshi RS, Jagdale SS, Bansode SB, Shankar SS, Tellis MB, Pandya VK et al (2021) Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease. J Biomol Struct Dyn 39(9):3099–3114. https://doi.org/10.1080/07391102.2020.1760137
Abdul-Jabar RA, SaMJIJOPR A-F (2020) In-Silico Study of the Inhibitory Effect of Some Flavonoids Compounds and their Derivatives on SARS-COV-2. IJPR. https://doi.org/10.31838/ijpr/2020.12.02.360
Tallei TE, Tumilaar SG, Niode NJ, Kepel BJ, Idroes R, Effendi Y et al (2020) (2020) Potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and spike (S) glycoprotein inhibitors: a molecular docking study. Scientifica. https://doi.org/10.1155/2020/6307457
Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, and Soetjipto S (2020) Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints 2020(2020030226)
Alzaabi MM, Hamdy R, Ashmawy NS, Hamoda AM, Alkhayat F, Khademi NN et al (2021) Flavonoids are promising safe therapy against COVID-19. Phytoche Reviews. https://doi.org/10.1007/s11101-021-09759-z
Shawan MM, Khan A, Halder SK, Hasan Md (2021) Luteolin and abyssinone II as potential inhibitors of SARS-CoV-2: An in silico molecular modeling approach in battling the COVID-19 outbreak. Bulletin of the National Research Centre 45(1):1–21. https://doi.org/10.1186/s42269-020-00479-6
Jain AS, Sushma P, Dharmashekar C, Beelagi MS, Prasad SK, Shivamallu C et al (2021) In silico evaluation of flavonoids as effective antiviral agents on the spike glycoprotein of SARS-CoV-2. Saudi J Biol Sci 28(1):1040–1051. https://doi.org/10.1016/j.sjbs.2020.11.049
Abdul-Jabar R, Ali S, Al-Fadal M (2020) In-Silico Study of the Inhibitory Effect of Some Flavonoids Compounds and their Derivatives on SARS- COV-2. Int J Pharma Res. https://doi.org/10.31838/ijpr/2020.12.02.360
Goyal RK, Majeed J, Tonk R, Dhobi M, Patel B, Sharma K et al (2020) Current targets and drug candidates for prevention and treatment of SARS-CoV-2 (COVID-19) infection. Rev Cardiovasc Med 21(3):365–384. https://doi.org/10.31083/j.rcm.2020.03.118
Bhowmik D, Nandi R, Prakash A, Kumar DJH (2021) Evaluation of flavonoids as 2019-nCoV cell entry inhibitor through molecular docking and pharmacological analysis. Heliyon 7(3):e06515
Da Silva AB, Coelho PLC, Das Neves Oliveira M, Oliveira JL, JaO A, Da Silva KC et al (2020) The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav Immun 85:170–185. https://doi.org/10.1016/j.bbi.2019.05.003
Guler HI, Tatar G, Yildiz O, Belduz AO, Kolayli S (2021) Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by molecular docking study. Archives Microbiol 2603(6):3557–3564. https://doi.org/10.1007/s00203-021-02351-1
Teli DM, Shah MB, Chhabria MT (2021) In silico screening of natural compounds as potential inhibitors of SARS-CoV-2 main protease and spike RBD: targets for COVID-19. Front Mol Biosci. https://doi.org/10.3389/fmolb.2020.599079
Nallusamy S, Mannu J, Ravikumar C, Angamuthu K, Nathan B, Nachimuthu K et al (2021) Exploring phytochemicals of traditional medicinal plants exhibiting inhibitory activity against main protease, spike glycoprotein, RNA-dependent RNA polymerase and non-structural proteins of SARS-CoV-2 through virtual screening. Frontiers Pharm. https://doi.org/10.3389/fphar.2021.667704
Goyal RK, Apparsundaram S, Dhobi M, Patel BM (2021) Herbal Formulations for the Treatment of COVID-19, Delineating Health and Health System: Mechanistic Insights into Covid 19 Complications. In: Sobti RC et al (eds) Springer. Singapore, pp 431–447
Ravi V, Mohamed Saleem T, Patel S, Raamamurthy J, Gauthaman K (2009) Anti-inflammatory effect of methanolic extract of Solanum nigrum Linn berries. Int J Appl Res Nat Prod 2(2):33–36
Ravi V, Saleem T, Maiti P, Ramamurthy J (2009) Phytochemical and pharmacological evaluation of Solanum nigrum Linn. Afr J Pharm Pharmacol 3(9):454–457
Yeom Y-E, Kim MA, Kim J, Lee C-M (2019) Anti-inflammatory effects of the extract of Solanum nigrum L. on an acute ear edema mouse model. Mater Technol 34(14):851–857
Arunachalam G, Subramanian N, Perumal Pazhani G, Karunanithi M, Ravichandran V (2009) Evaluation of anti-inflammatory activity of methanolic extract of Solanum nigrum (Solanaceae). Iran J Pharm Sci 5(3):151–156
Aryaa A, Viswanathswamy AH (2017) Effect of Solanum nigrum Linn on acute and sub-acute models of inflammation. J Young Pharm 9(4):566. https://doi.org/10.5530/jyp.2017.9.108
Xiang L, Wang Y, Yi X, He X (2018) Anti-inflammatory steroidal glycosides from the berries of Solanum nigrum L. (European black nightshade). Phytochemistry. https://doi.org/10.1016/j.phytochem.2018.01.019
Li J, Li QW, Gao DW, Han ZS, Lu WZJPR (2009) Antitumor and immunomodulating effects of polysaccharides isolated from Solanum nigrum Linne. Phytother Res 23(11):1524–1530
Ding X, Zhu F, Gao S (2012) Purification, antitumour and immunomodulatory activity of water-extractable and alkali-extractable polysaccharides from Solanum nigrum L. Food Chem 131(2):677–684. https://doi.org/10.1016/j.foodchem.2011.09.060
Pu Y, Liu Z, Zhong C, Zhang X, Bao Y (2020) Immunomodulatory effects of a polysaccharide from Solanum nigrum Linne through TLR4-MyD88 signaling pathway. Int Immunopharmacol 88:106973. https://doi.org/10.1016/j.intimp.2020.106973
Drosten C, Gunther S, Preiser W, Van Der Werf S, Brodt HR, Becker S et al (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348(20):1967–1976. https://doi.org/10.1056/NEJMoa030747
Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367(19):1814–1820. https://doi.org/10.1056/NEJMoa1211721
Chilamakuri R, Agarwal S (2021) COVID-19: characteristics and therapeutics. Cells. https://doi.org/10.3390/cells10020206
Atzrodt CL, Maknojia I, Mccarthy RDP, Oldfield TM, Po J, Ta KTL et al (2020) A guide to COVID-19: a global pandemic caused by the novel coronavirus SARS-CoV-2. FEBS J 287(17):3633–3650. https://doi.org/10.1111/febs.15375
Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH 3rd et al (2020) SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182(2):429–446. https://doi.org/10.1016/j.cell.2020.05.042
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is Blocked by a clinically proven protease inhibitor. Cell 181(2):271–280. https://doi.org/10.1016/j.cell.2020.02.052
Hellman U, Karlsson MG, Engstrom-Laurent A, Cajander S, Dorofte L, Ahlm C et al (2020) Presence of hyaluronan in lung alveoli in severe Covid-19: an opening for new treatment options? J Biol Chem 295(45):15418–15422. https://doi.org/10.1074/jbc.AC120.015967
Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P et al (2020) Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 20(10):1135–1140. https://doi.org/10.1016/S1473-3099(20)30434-5
Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, Lachmann G et al (2021) The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med 9(6):622–642. https://doi.org/10.1016/S2213-2600(21)00218-6
Wilson MS, Wynn TA (2009) Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2(2):103–121. https://doi.org/10.1038/mi.2008.85
Quartuccio L, Semerano L, Benucci M, Boissier MC, De Vita S (2020) Urgent avenues in the treatment of COVID-19: targeting downstream inflammation to prevent catastrophic syndrome. Joint Bone Spine 87(3):191–193. https://doi.org/10.1016/j.jbspin.2020.03.011
Chen W (2020) A potential treatment of COVID-19 with TGF-beta blockade. Int J Biol Sci 16(11):1954–1955. https://doi.org/10.7150/ijbs.46891
Yu M, Liu Y, Xu D, Zhang R, Lan L, Xu H (2020) Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol 21(6):746–755. https://doi.org/10.3348/kjr.2020.0215
Klok FA, Kruip M, Van Der Meer NJM, Arbous MS, Gommers D, Kant KM et al (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191:145–147. https://doi.org/10.1016/j.thromres.2020.04.013
Obi AT, Barnes GD, Napolitano LM, Henke PK, Wakefield TW (2021) Venous thrombosis epidemiology, pathophysiology, and anticoagulant therapies and trials in severe acute respiratory syndrome coronavirus 2 infection. J Vasc Surg Venous Lymphat Disord 9(1):23–35. https://doi.org/10.1016/j.jvsv.2020.08.030
Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M et al (2020) ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 18(5):1023–1026. https://doi.org/10.1111/jth.14810
Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS et al (2020) Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med 8(12):1233–1244. https://doi.org/10.1016/S2213-2600(20)30404-5
Lippi G, Plebani M (2020) Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med 58(7):1131–1134. https://doi.org/10.1515/cclm-2020-0198
Onofrio L, Caraglia M, Facchini G, Margherita V, Placido S, Buonerba C (2020) Toll-like receptors and COVID-19: a two-faced story with an exciting ending. Future Sci OA. https://doi.org/10.2144/fsoa-2020-0091
Totura AL, Whitmore A, Agnihothram S, Schafer A, Katze MG, Heise MT et al (2015) Toll-like receptor 3 signaling via trif contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. MBio 6(3):e00638–e0063815. https://doi.org/10.1128/mBio.00638-15
Merad M, Martin JC (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20(6):355–362. https://doi.org/10.1038/s41577-020-0331-4
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Burkhard-Koren NM, Haberecker M, Maccio U, Ruschitzka F, Schuepbach RA, Zinkernagel AS et al (2021) Higher prevalence of pulmonary macrothrombi in SARS-CoV-2 than in influenza a: autopsy results from ‘Spanish flu’ 1918/1919 in Switzerland to coronavirus disease 2019. J Pathol Clin Res 7(2):135–143. https://doi.org/10.1002/cjp2.189
Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS et al (2020) Extrapulmonary manifestations of COVID-19. Nat Med 26(7):1017–1032. https://doi.org/10.1038/s41591-020-0968-3
Galani IE, Rovina N, Lampropoulou V, Triantafyllia V, Manioudaki M, Pavlos E et al (2021) Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol 22(1):32–40. https://doi.org/10.1038/s41590-020-00840-x
Sinha P, Calfee CS, Cherian S, Brealey D, Cutler S, King C et al (2020) Prevalence of phenotypes of acute respiratory distress syndrome in critically ill patients with COVID-19: a prospective observational study. Lancet Respir Med 8(12):1209–1218. https://doi.org/10.1016/S2213-2600(20)30366-0
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X et al (2021) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397(10270):220–232. https://doi.org/10.1016/S0140-6736(20)32656-8
Weerahandi H, Hochman KA, Simon E, Blaum C, Chodosh J, Duan E et al (2021) Post-discharge health status and symptoms in patients with severe COVID-19. J Gen Intern Med 36(3):738–745. https://doi.org/10.1007/s11606-020-06338-4
Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H et al (2021) Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology 27(4):328–337. https://doi.org/10.1016/j.pulmoe.2020.10.013
Mo X, Jian W, Su Z, Chen M, Peng H, Peng P et al (2020) Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. https://doi.org/10.1183/13993003.01217-2020
Huang Y, Tan C, Wu J, Chen M, Wang Z, Luo L et al (2020) Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res 21(1):163. https://doi.org/10.1186/s12931-020-01429-6
Guler SA, Ebner L, Aubry-Beigelman C, Bridevaux PO, Brutsche M, Clarenbach C et al (2021) Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. https://doi.org/10.1183/13993003.03690-2020
Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M et al (2021) Six-month follow-up Chest CT findings after Severe COVID-19 Pneumonia. Radiology 299(1):E177–E186. https://doi.org/10.1148/radiol.2021203153
Caruso D, Guido G, Zerunian M, Polidori T, Lucertini E, Pucciarelli F et al (2021) Post-acute sequelae of COVID-19 Pneumonia: six-month chest CT follow-up. Radiology 301(2):E396–E405. https://doi.org/10.1148/radiol.2021210834
Fernandez-De-Las-Penas C, Palacios-Cena D, Gomez-Mayordomo V, Palacios-Cena M, Rodriguez-Jimenez J, De-La-Llave-Rincon AI et al (2021) Fatigue and dyspnoea as main persistent post-COVID-19 symptoms in previously hospitalized patients: related functional limitations and disability. Respiration. https://doi.org/10.1159/000518854
Zhang S, Bai W, Yue J, Qin L, Zhang C, Xu S et al (2021) Eight months follow-up study on pulmonary function, lung radiographic, and related physiological characteristics in COVID-19 survivors. Sci Rep 11(1):13854. https://doi.org/10.1038/s41598-021-93191-y
Chen Y, Ding C, Yu L, Guo W, Feng X, Yu L et al (2021) One-year follow-up of chest CT findings in patients after SARS-CoV-2 infection. BMC Med 19(1):191. https://doi.org/10.1186/s12916-021-02056-8
Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC (2021) Sixty-Day outcomes among patients hospitalized with COVID-19. Ann Intern Med 174(4):576–578. https://doi.org/10.7326/M20-5661
Carfi A, Bernabei R, Landi F (2020) Persistent symptoms in patients after acute COVID-19. JAMA 324(6):603–605. https://doi.org/10.1001/jama.2020.12603
Carvalho-Schneider C, Laurent E, Lemaignen A, Beaufils E, Bourbao-Tournois C, Laribi S et al (2021) Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect 27(2):258–263. https://doi.org/10.1016/j.cmi.2020.09.052
Halpin SJ, Mcivor C, Whyatt G, Adams A, Harvey O, Mclean L et al (2021) Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 93(2):1013–1022. https://doi.org/10.1002/jmv.26368
Kamal M, Abo Omirah M, Hussein A, Saeed H (2021) Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract 75(3):e13746. https://doi.org/10.1111/ijcp.13746
Shang YF, Liu T, Yu JN, Xu XR, Zahid KR, Wei YC et al (2021) Half-year follow-up of patients recovering from severe COVID-19: analysis of symptoms and their risk factors. J Intern Med 290(2):444–450. https://doi.org/10.1111/joim.13284
Recalcati S (2020) Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol 34(5):e212–e213. https://doi.org/10.1111/jdv.16387
Mieczkowska K, Deutsch A, Borok J, Guzman AK, Fruchter R, Patel P et al (2021) Telogen effluvium: a sequela of COVID-19. Int J Dermatol 60(1):122–124. https://doi.org/10.1111/ijd.15313
Engelen MM, Vandenbriele C, Balthazar T, Claeys E, Gunst J, Guler I et al (2021) Venous thromboembolism in patients discharged after COVID-19 hospitalization. Semin Thromb Hemost 47(4):362–371. https://doi.org/10.1055/s-0041-1727284
Roberts LN, Whyte MB, Georgiou L, Giron G, Czuprynska J, Rea C et al (2020) Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood 136(11):1347–1350. https://doi.org/10.1182/blood.2020008086
Nakamura ZM, Nash RP, Laughon SL, Rosenstein DL (2021) Neuropsychiatric complications of COVID-19. Curr Psychiatry Rep 23(5):25. https://doi.org/10.1007/s11920-021-01237-9
Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL et al (2020) Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatr 7(10):875–882. https://doi.org/10.1016/S2215-0366(20)30287-X
Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ (2021) 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatr 8(5):416–427. https://doi.org/10.1016/S2215-0366(21)00084-5
Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C et al (2020) Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 382(23):2268–2270. https://doi.org/10.1056/NEJMc2008597
Xie Y, Bowe B, Maddukuri G, Al-Aly Z (2020) Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ 371:m4677. https://doi.org/10.1136/bmj.m4677
Yende S, Parikh CR (2021) Long COVID and kidney disease. Nat Rev Nephrol. https://doi.org/10.1038/s41581-021-00487-3
Al-Aly Z, Xie Y, Bowe B (2021) High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594(7862):259–264. https://doi.org/10.1038/s41586-021-03553-9
Benarba B, Pandiella A (2020) Medicinal plants as sources of active molecules against COVID-19. Front Pharmacol. https://doi.org/10.3389/fphar.2020.01189
Alhazmi HA, Najmi A, Javed SA, Sultana S, Al Bratty M, Makeen HA et al (2021) Medicinal plants and isolated molecules demonstrating immunomodulation activity as potential alternative therapies for viral diseases including COVID-19. Front Immunol. https://doi.org/10.3389/fimmu.2021.637553
Singh RS, Singh A, Kaur H, Batra G, Sarma P, Kaur H et al (2021) Promising traditional Indian medicinal plants for the management of novel Coronavirus disease: a systematic review. Phytother Res 35(8):4456–4484. https://doi.org/10.1002/ptr.7150
Ugwah-Oguejiofor C, Adebisi I (2021) Potential medicinal plant remedies and their possible mechanisms against COVID-19: A review. IIFE J Sci 23(1):161–194. https://doi.org/10.4314/ijs.v23i1.16
Hafez Ghoran S, El-Shazly M, Sekeroglu N, Kijjoa A (2021) Natural products from medicinal plants with anti-human coronavirus activities. Molecules 26(6):1754. https://doi.org/10.3390/molecules26061754
Adhikari B, Marasini BP, Rayamajhee B, Bhattarai BR, Lamichhane G, Khadayat K et al (2021) Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: a review. Phytother Res 35(3):1298–1312. https://doi.org/10.1002/ptr.6893
Chandramouli V, Niraj SK, Nair KG, Joseph J, Aruni W (2021) Phytomolecules repurposed as covid-19 inhibitors: opportunity and challenges. Current Microbiol 78(10):3620–3633. https://doi.org/10.1007/s00284-021-02639-x
Fongnzossie Fedoung E, Biwole AB, Nyangono Biyegue CF, Ngansop Tounkam M, Akono Ntonga P, Nguiamba VP et al (2021) A review of Cameroonian medicinal plants with potentials for the management of the COVID-19 pandemic. Adv Tradit Med. https://doi.org/10.1007/s13596-021-00567-6
Razali FN, Sinniah SK, Hussin H, Abidin NZ, Shuib AS (2016) Tumor suppression effect of Solanum nigrum polysaccharide fraction on Breast cancer via immunomodulation. Int J Biol Macromols 92:185–193. https://doi.org/10.1016/j.ijbiomac.2016.06.079
Ganeshpurkar A, Saluja A (2020) Immunomodulatory effect of rutin, catechin, and hesperidin on macrophage function. Indian J Biochem Biophys 57(1):58–63
Hosseinzade A, Sadeghi O, Naghdipour Biregani A, Soukhtehzari S, Brandt GS, Esmaillzadeh A (2019) Immunomodulatory Effects of flavonoids: possible induction of T CD4+ regulatory cells through suppression of mTOR pathway signaling activity. Front Immunol. https://doi.org/10.3389/fimmu.2019.00051
Tang L, Yin Z, Hu Y, Mei H (2020) Controlling Cytokine Storm Is Vital in COVID-19. Front Immunol. https://doi.org/10.3389/fimmu.2020.570993
R S, KD, and KB, (2018) Evaluation of antioxidant and anti-inflammatory activity of methanolic extract of solanum nigrum fruits. Int J Pharm Educat Res 1(1):1–5. https://doi.org/10.37021/ijper.v1i1.10
Piana M, Camponogara C, Boligon AA, Oliveira SM (2017) Solanum paranense extracts and solanine present anti-inflammatory activity in an acute skin inflammation model in mice. Evid-Based Complement Altern Med. https://doi.org/10.1155/2017/4295680
Pandurangan A, Khosa R, Hemalatha S (2011) Anti-inflammatory activity of an alkaloid from Solanum trilobatum on acute and chronic inflammation models. Nat product Res 25(12):1132–1141. https://doi.org/10.1080/14786410903370783
Liskova A, Samec M, Koklesova L, Samuel SM, Zhai K, Al-Ishaq RK et al (2021) Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2021.111430
Khazdair M, Anaeigoudari A, Agbor G (2021) Anti-viral and anti-inflammatory effects of kaempferol and quercetin and COVID-2019: A scoping review. Asian Pac J Trop Biomed 11(8):327–334. https://doi.org/10.4103/2221-1691.319567
Rowaiye AB, Okpalefe OA, Adejoke OO, Ogidigo JO, Oladipo OH, Ogu AC et al (2021) Attenuating the effects of novel COVID-19 (SARS-CoV-2) infection-induced cytokine storm and the implications. J Inflamm Res. https://doi.org/10.2147/JIR.S301784
Peter AE, Sandeep BV, Rao BG, Kalpana VL (2021) Calming the Storm: Natural Immunosuppressants as Adjuvants to Target the Cytokine Storm in COVID-19. Front Pharmacol. https://doi.org/10.3389/fphar.2020.583777
Funakoshi-Tago M, Nakamura K, Tago K, Mashino T, Kasahara T (2011) Anti-inflammatory activity of structurally related flavonoids, Apigenin. Luteolin Fisetin. Int immunopharmacol 11(9):1150–1159. https://doi.org/10.1016/j.intimp.2011.03.012
Ruan X, Du P, Zhao K, Huang J, Xia H, Dai D et al (2020) Mechanism of Dayuanyin in the treatment of coronavirus disease 2019 based on network pharmacology and molecular docking. Chin Med 15(1):62. https://doi.org/10.1186/s13020-020-00346-6
Wang M, Fu D, Yao L, Li J (2021) Theoretical study of the molecular mechanism of maxingyigan decoction against COVID-19: network pharmacology-based strategy. Comb Chem High Throughput Screen 24(2):294–305. https://doi.org/10.2174/1386207323666200806164635
Zhao J, Tian S, Lu D, Yang J, Zeng H, Zhang F et al (2021) Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine. https://doi.org/10.1016/j.phymed.2020.153315
Niu W-H, Wu F, Cao W-Y, Wu Z-G, Chao Y-C, Peng F et al (2021) Network pharmacology for the identification of phytochemicals in traditional Chinese medicine for COVID-19 that may regulate interleukin-6. Biosci Rep. https://doi.org/10.1042/bsr20202583
Atal S, Fatima Z (2020) IL-6 inhibitors in the treatment of serious COVID-19: a promising therapy? Pharm Med 34(4):223–231. https://doi.org/10.1007/s40290-020-00342-z
Zakaryan H, Arabyan E, Oo A, Zandi K (2017) Flavonoids: promising natural compounds against viral infections. Arch Virol 162(9):2539–2551. https://doi.org/10.1007/s00705-017-3417-y
Muchtaridi M, Fauzi M, Khairul Ikram NK, Mohd Gazzali A, Wahab HA (2020) Natural flavonoids as potential angiotensin-converting enzyme 2 inhibitors for anti-SARS-CoV-2. Molecules 25(17):3980. https://doi.org/10.3390/molecules25173980
Mouffouk C, Mouffouk S, Mouffouk S, Hambaba L, Haba H (2021) Flavonols as potential antiviral drugs targeting SARS-CoV-2 proteases (3CLpro and PLpro), spike protein, RNA-dependent RNA polymerase (RdRp) and angiotensin-converting enzyme II receptor (ACE2). Eur J pharmacol. https://doi.org/10.1016/j.ejphar.2020.173759
Madeswaran A, Brahmasundari S, Midhuna PG (2021) In silico molecular docking studies of certain commercially available flavonoids as effective antiviral agents against spike glycoprotein of SARS-CoV-2. Eur Rev Med Pharmacol Sci 25(21):6741–6744. https://doi.org/10.26355/eurrev_202111_27119
Jo S, Kim S, Kim DY, Kim M-S, Shin DH (2020) Flavonoids with inhibitory activity against SARS-CoV-2 3CLpro. J Enzyme Inhib Med Chem 35(1):1539–1544. https://doi.org/10.1080/14756366.2020.1801672
Cherrak SA, Merzouk H, Mokhtari-Soulimane N (2020) Potential bioactive glycosylated flavonoids as SARS-CoV-2 main protease inhibitors: a molecular docking and simulation studies. PLoS ONE 15(10):e0240653. https://doi.org/10.1371/journal.pone.0240653
Kiran G, Karthik L, Devi MS, Sathiyarajeswaran P, Kanakavalli K, Kumar K et al (2020) In silico computational screening of kabasura kudineer-official Siddha formulation and JACOM against SARS-CoV-2 spike protein. J Ayurveda Integr Med. https://doi.org/10.1016/j.jaim.2020.05.009
Hasan A, Al Mahamud R, Jannat K, Afroze T, Bondhon B-NF, Fariba MH et al (2020) Phytochemicals from Solanum surattense Burm. F. have high binding affinities for C-3 like main protease of COVID-19 (SARS-CoV-2). J Med Plants Stud 8(4):20–26
Esendağli D, Yilmaz A, Akçay Ş, Özlü T (2021) Post-COVID syndrome: pulmonary complications. Turk J Med Sci. https://doi.org/10.3906/sag-2106-238
Jakubec P, Fišerová K, Genzor S, Kolář M (2022) Pulmonary Complications after COVID-19. Life (Basel). 12(3):357. https://doi.org/10.3390/life12030357
Nath V, Buragohain P, and Sharma H (2021) A Review on some medicinal plants of Northeast India used in the treatment of respiratory disorders. Current Trends in Pharmaceutical Research 8(1)
Nirmal S, Patel A, Bhawar S, Pattan S (2012) Antihistaminic and antiallergic actions of extracts of Solanum nigrum berries: possible role in the treatment of asthma. J Ethnopharmacol 142(1):91–97. https://doi.org/10.1016/j.jep.2012.04.019
Yang C, Yang W, He Z, He H, Yang X, Lu Y et al (2020) Kaempferol Improves Lung Ischemia-Reperfusion Injury via Antiinflammation and Antioxidative Stress Regulated by SIRT1/HMGB1/NF-κB Axis. Front Pharmacol. https://doi.org/10.3389/fphar.2019.01635
Rabha DJ, Singh TU, Rungsung S, Kumar T, Parida S, Lingaraju MC et al (2018) Kaempferol attenuates acute lung injury in caecal ligation and puncture model of sepsis in mice. Exp Lung Res 44(2):63–78. https://doi.org/10.1080/01902148.2017.1420271
Cornélio Favarin D, Robison de Oliveira J, Freire J, de Oliveira C, de Paula Rogerio A (2013) Potential effects of medicinal plants and secondary metabolites on acute lung injury. BioMed Res Int. https://doi.org/10.1155/2013/576479
Baharvand-Ahmadi B, Bahmani M, Eftekhari Z, Jelodari M, Mirhoseini M (2015) Overview of medicinal plants used for cardiovascular system disorders and diseases in ethnobotany of different areas in Iran. J HerbMed Pharmacol 5(1):39–44
Shaik SA, Huded S, Fathima A, Preran K, Fathima SJ, Khanum F (2015) Cardio protective effect of Solanum nigrum Linn. In isoproterenol induced myocardial infarction in rat. Sci Tech Arts Res J 4(4):77–82. https://doi.org/10.4314/star.v4i4.11
Sohrabipour S, Kharazmi F, Soltani N, Kamalinejad M (2013) Effect of the administration of Solanum nigrum fruit on blood glucose, lipid profiles, and sensitivity of the vascular mesenteric bed to phenylephrine in streptozotocin-induced diabetic rats. Med sci Mont Basic Res. https://doi.org/10.12659/MSMBR.883892
Patel S, Sharma V, Chauhan N, Mayank Thakur VK, Dixit, (2015) Hair growth: focus on herbal therapeutic agent. Curr Drug Discov Tech 12(1):21–42. https://doi.org/10.2174/1570163812666150610115055
Chakraborty A, Bhattacharjee A, Sodani A, Jain D, Mukhopadhyay G, Sepay N (2016) Herbal Hair Gel Formulation having 5 α-Reductase Inhibitory Activity and its Standardization by HPTLC. J Anal Bioanal Tech 7(341):2. https://doi.org/10.4172/2155-9872.1000341
Hong S, Lee B, Kim JH, Kim EY, Kim M, Kwon B et al (2020) Solanum nigrum Linne improves DNCB-induced atopic dermatitis-like skin disease in BALB/c mice. Mol Med Rep 22(4):2878–2886. https://doi.org/10.3892/mmr.2020.11381
Javed A and Qazi JI (2016) Efficacy of Azadirachta indica and Solanum nigrum for Skin Regeneration in Mice. Pakistan journal of life social sciences 14(3)
Saki K, Bahmani M, Rafieian-Kopaei M, Hassanzadazar H, Dehghan K, Bahmani F et al (2014) The most common native medicinal plants used for psychiatric and neurological disorders in Urmia city, northwest of Iran. Asian Pac J Trop Dis 4:S895–S901. https://doi.org/10.1016/S2222-1808(14)60754-4
Ogunsuyi OB, Ademiluyi AO, Oboh G, Oyeleye SI, Dada AF (2018) Green leafy vegetables from two Solanum spp. (Solanum nigrum L and Solanum macrocarpon L) ameliorate scopolamine-induced cognitive and neurochemical impairments in rats. Food Sci Nutr 6(4):860–870. https://doi.org/10.1002/fsn3.628
Ogunsuyi OB, Olagoke OC, Afolabi BA, Oboh G, Ijomone OM, Barbosa NV et al (2021) Dietary inclusions of Solanum vegetables mitigate aluminum-induced redox and inflammation-related neurotoxicity in Drosophila melanogaster model. Nutr Neurosci. https://doi.org/10.1080/1028415X.2021.1933331
Eze G, Ekechi C, Ekundina V, Akonoafua K (2018) Effect of Aqueous Extract of Blackberry Nightshade (Solanum nigrum) Leaf on Carbonated Alcoholic Herbal Beverage (CAHB)-Induced Cerebellar Damage and Behavioural Changes in Adult Wistar Rats. J Appl Sci Environ Manag 22(11):1801–1806. https://doi.org/10.4314/jasem.v22i11.15
Zaidi SK, Hoda M, Tabrez S, Ansari SA, Jafri MA, Shahnawaz Khan M et al (2014) Protective effect of Solanum nigrum leaves extract on immobilization stress induced changes in rat’s brain. Evi-Based Complement Altern Med. https://doi.org/10.1155/2014/912450
Wannang NN, Anuka JA, Kwanashie HO, Gyang S, and Auta A (2008) Anti-seizure activity of the aqueous leaf extract of Solanum nigrum linn (solanaceae) in experimental animals. African Health Sciences 8(2)
Teklehaimanot H, Gebru G, Tesfay A (2015) Review on Effect of Solanum nigrum L. on Histopathology of Kidneys of Rats. Int J Pharm Sci Res 6:645–649
Kushwaha V, Sharma M, Vishwakarma P, Saini M, Bhatt S, Saxena KK (2016) Evaluation of nephroprotective and nephrocurative activity of Solanum nigrum on gentamicin induced nephrotoxicity in experimental rats. Int J Basic Clin Pharmacol 5(1):74–78. https://doi.org/10.18203/2319-2003.ijbcp20160104
Kushwaha V (2019) Histological evaluation of nephroprotective effect of Solanum nigrum fruit extract against gentamicin induced nephrotoxicity in experimental rats. Int J Basic Clin Pharmacol. https://doi.org/10.18203/2319-2003.ijbcp20194217
Uchechukwu OC, Haemopoeitic and Haematinic property of the leaves and fruit extract of Solanum nigrum L (spp villosum) in anaemic albino wistar rats. 2013, Department of Medical Laboratory Sciences, Faculty of Health Sciences, Faculty of Health Sciences and Technolgy, College of Medicine, University of Nigeria, Enugu Campus NIGERIA.
Rajeswari M, Gurumurthy S, Kamat S (2013) Anti gastritic and antiulcerogenic effects of Solanum nigrum in laboratory animals. Int J Nutr Food Sci 2(6):266–271
Krithika R, Verma RJ (2019) Solanum nigrum confers protection against CCl 4-induced experimental hepatotoxicity by increasing hepatic protein synthesis and regulation of energy metabolism. Clin Phytosci 5(1):1–8
Liu F-P, Ma X, Li M-M, Li Z, Han Q, Li R et al (2016) Hepatoprotective effects of Solanum nigrum against ethanol-induced injury in primary hepatocytes and mice with analysis of glutathione S-transferase A1. J Chin Med Assoc 79(2):65–71. https://doi.org/10.1016/j.jcma.2015.08.013
Singh DP, Awasthi H, Luqman S, Singh S, Mani D (2015) Hepatoprotective effect of a polyherbal extract containing Andrographis paniculata, Tinospora cordifolia and Solanum nigrum against paracetamol induced hepatotoxicity. Pharmacogn Mag 11(Suppl 3):S375. https://doi.org/10.4103/0973-1296.168945
Acknowledgements
We want to thank and acknowledge the Department of Science and Technology for providing a DST-Inspire fellowship to Divya Sharma and Indian Council for Medical Research for providing a Senior Research Fellowship to Mit Joshi.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Conceptualization was done by RKG; designing and supervision were done by BP and MD; material preparation, data collection, and draft preparation were done by DS and MJ; review was done by SA. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest.
Ethical approval
This article does not contain any studies with animal or human participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, D., Joshi, M., Apparsundaram, S. et al. Solanum nigrum L. in COVID-19 and post-COVID complications: a propitious candidate. Mol Cell Biochem 478, 2221–2240 (2023). https://doi.org/10.1007/s11010-022-04654-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04654-3