Abstract
Background
Hepatotoxicity induced by carbon tetrachloride (CCl4) is used as an experimental model to screen phytochemicals with liver protecting activity. Solanum nigrum (SN) is a shrub which is widely distributed throughout India. Traditionally the plant has been used for curing various ailments related to gastroenterology and hepatology. The present study was performed to evaluate the hepatoprotective property of S. nigrum extract standardized for its flavonoid content against CCl4-induced hepatotoxicity.
Methods
The liver protecting property of SN was evaluated by means of various biochemical parameters and histopathologically. S. nigrum was administered to Swiss strain female albino mice with either 100/ 200/ 300 mg/kg body weight/day for 30 days along with CCl4 which is a well established model to induce hepatotoxicity.
Results
Administration of CCl4 for 30 days caused a significant increase in liver marker enzymes and a decrease in hepatic DNA, RNA and protein levels which was effectively mitigated by treatment with the plant extract in a dose dependent manner. Similarly co-treatment of the extract along with the hepatotoxin improved hepatic energy status by increasing the activities of succinate dehydrogenase (SDH) and adenosine triphosphatase (ATPase). Histopathological findings indicated severe vacuolization and necrotic changes after CCl4 treatment which was mitigated by the co-administration of SN extract in a dose-dependent manner.
Conclusions
Carbon tetracholoride is known to exert hepatotoxicty by forming adducts with tissue macromolecules through covalent interactions. S. nigrum extract was found to effectively mitigate CCl4-induced changes in hepatic macromolecular content and energy status of liver tissue. The present study has identified the standardized S. nigrum as an effective hepatoprotective agent probably due to its role in improving the protein and energy levels in the hepatic tissue.
Similar content being viewed by others
Background
Carbon tetrachloride (CCl4) once used as an industrial solvent, is presently used as an experimental agent to induce hepatotoxicity. This agent has been used successfully studies both in vitro and in vivo in many species to produce liver injury. It is well established that CCl4 is metabolically activated in the liver to produce intermediates which are then dealt by the hepatic mixed function oxidases and some related enzyme systems. Metabolism of CCl4 by mixed function cytochrome P450 oxygenase system of the endoplasmic reticulum and especially by CYP2E1 generates trichloromethyl free radical (CCl3.) which has the capacity to interact with critical target biomolecules like DNA, lipids and proteins via covalent bond formation. The reductive metabolism of CCl4 to the CCl3. free radical thus results in the production of covalently bound adducts and have been reported to be responsible for CCl4-induced hepatic necrosis [1]. It is widely accepted that CCl4-induced experimental hepatotoxicity greatly mimics viral hepatitis [2]. Moreover CCl4 can also induce hepatotoxicity via lipid peroxidation mechanism through trichloromethyl peroxy radical (CCl3OO.) which is formed when CCl3. reacts with O2 [3].
Consumption of herbal medications against diseases is on a high and a very sharp rise in its usage has been reported by patients suffering from liver diseases [4]. Medicinal plants can act as supreme agents against any form of liver ailments. Thus treating liver diseases with plant derived compounds seems to be very lucrative option especially considering the fact that it does not require any laborious or vigorous process for production or development and is also cost effective and hence can be considered as supreme liver protecting agents. Hence treatment of liver diseases with plant-derived compounds which are highly accessible and do not require laborious pharmaceutical synthesis seems highly attractive.
S. nigrum has been commonly used as a traditional medicine for treating various diseases. It has been used to treat inflammation, oedema and other related disorders since time immemorial. It is commonly known as ‘Black nightshade’ and has been extensively used in traditional medicine in India and other parts of world to cure liver disorders, chronic skin ailments (psoriasis and ringworm), inflammatory conditions, painful periods, fevers, diarrhea, eye diseases, hydrophobia and as a folk medicine for treatment of peptic ulcer [5]. In traditional medicine, the plant has also been used as an anti-diarrhoeal, in eye diseases and hydrophobia [6]. Chinese medicine employs the juice of the leaves to alleviate pain in inflammation of the kidney and bladder and internally for cardialgia [5].
Many previous studies have been carried out to determine the hepatoprotective effect of different extracts of S. nigrum against different toxicants. The anti-fibrotic role of S. nigrum against thioacetamide-induced toxicity has been was reported earlier [7]. Raju et al. 2003, have reported that the dried fruits of S. nigrum possess anti-hepatotoxic potential when administered for 7 days on a pre-treatment basis against a single oral dose of CCl4 [8]. Similarly S. nigrum when administered for 10 days on a co-treatment basis was found to ameliorate the drastic increase in hepatic markers and was able to reduce the histopathological anomalies and restore back normalcy in haematological parameter induced by CCl4 in rats [9]. Mir et al. 2010, have evaluated the hepatoprotective property of S. nigrum against CCl4-induced acute toxicity in rats [10]. Hepatotoxicity was induced by administrating four oral dose of CCl4 within 24 h interval period and subsequent administration of 4 oral doses of S. nigrum extract (500 mg/kg body weight) resulted in marked reduction in liver enzymes and improved histopathological changes especially the fatty changes and prevented liver enlargement.
The protective effect of water extract of S. nigrum against CCl4-induced chronic hepatotoxicity in rats has been evaluated [11]. S. nigrum was found to reduce CCl4-induced lipid peroxidation by modulating the antioxidative defence mechanism. Elshater et al. 2013, have reported that rats treated with S. nigrum extract for 30 days after challenge with CCl4 (1 mg/kg body weight dose thrice per week for2 weeks) resulted in significant reduction in liver marker enzymes, lipid peroxidation and an elevation in enzymatic and non-enzymatic antioxidative defence system [12].
Pre-treatment with S. nigrum extract for 7 days of was shown to reduce the levels of liver marker enzymes, decrease lipid peroxidation and improve histopathological features in rats administered with a single ip dose of CCl4 [13]. The hepatoprotective potential of aqeous extract of S. nigrum on pre-treatment basis for 10–30 days against toxicity induced by a single dose of CCl4 has been studied which indicated that treatment S. nigrum extract with CCl4 caused marked elevation in the levels of ALT, ALP and bilirubin along with distortion in histopathological structure [14]. S. nigrum is thus known to exert its effect by curtailing lipid peroxidation due to its antioxidative property Sivgami et al. 2012 [15].
The present study was carried out to evaluate the mitigatory effect of standardized extract of S. nigrum against CCl4-induced changes in hepatic biomolecules via adduct formation and deterioration of hepatic energy status. S. nigrum was administered on a co-treatment basis in the present study against CCl4-induced sub-chronic hepatic toxicity to address the shortcomings of most of the previous studies performed to evaluate the protective action of the plant extract in which hepatotoxicity was induced by a single dose of CCl4 after pre-treatment with the extract.
Methods
Animals
The experiments were performed in inbred Swiss strain female albino mice (Mus musculus) weighing between 32 and 35 g (6–8 weeks old) obtained from Zydus Research Centre (Ahmedabad, India). All animals were maintained under controlled conditions of temperature (25 ± 2 °C) and relative humidity (50–55%) and under a 12 h light / dark cycle in the Animal House of Zoology Department, Gujarat University (Ahmedabad, India). Animals were fed certified rodent diet supplied by Amrut Feeds, Pranav Agro Industries Limited (Pune, India) and water ad libitum. Formal approval was taken from “The Committee for the Purpose of Control and Supervision of Experiment on Animals” (Reg – 167/ 1999/ CPCSEA), New Delhi, India for performing the experiments and the animals were cared strictly abiding to the guidelines of imposed by Prevention of Cruelty to Animals Act, 1960 (59 of 1960; Government of India, New Delhi).
Chemicals
All chemicals used in the entire study were procured from standard agency. HPLC grade CCl4 and other solvents were obtained from Merck Specialities Pvt. Ltd. (Mumbai, India) and olive oil from Figaro (Madrid, Spain).
Experimental protocol
Plant material
The aerial parts of S. nigrum (SN) bearing ripe fruits were obtained from Botanical Garden of Gujarat University (Ahmedabad, India). The identity of the plant materials was confirmed by Dr. A. K. S. Rawat, Scientist and Head, Pharmacognosy and Ethnopharmacology Division, National Botanical Research Institute (NBRI), Lucknow, India. The aerial parts were shade dried, powdered and soaked with ethanol: water (3 × 1000 mL, 50:50, v/v) at room temperature. The solvent was evaporated under reduced pressure in a rotary evaporator at 40 °C. The S. nigrum extract was standardized for its flavonoid content before its administration to experimental animals. The total flavonoid content (%) of the SN extract calculated in terms of quercetin equivalence by plotting a calibration curve was measured at 510 nm in triplicates using quercetin standard [16].
Animal groupings
The mice were divided into seven groups consisting of ten animals each. Group 1 mice were marked as untreated control and were given free access to feed and drinking water. Animals of group 2 (vehicle control) received 0.2 mL of olive oil which was used as the vehicle to dissolve CCl4. Animals of groups 3 was given S. nigrum (SN) extract standardized on flavonoid content (300 mg /kg body weight, p.o.) which served as plant control group. Group 4 animals were administered with CCl4 (826 mg/0.2 mL olive oil/kg body weight/day, p. o.). Groups 5–7 were orally treated with CCl4 along with 100, 200 and 300 mg/kg body weight/day of standardized SN extract.
Treatment was continued for 30 days in all groups and mice were also observed for any behavioural or clinical changes during the course of treatment. The body weights of mice were individually recorded after completion of treatment and mean weights were calculated. Blood was collected by cardiac puncture on 31st day and centrifuged at 1000 x g for 10 min to obtain serum. Once the animals were sacrificed by cervical dislocation the liver was dissected out quickly, blotted free of blood and used for histopathological examinations and biochemical analysis.
Histopathological examination
Tissues for histopathological examination were preserved in 10% neutral buffered formalin immediately after autopsy. Standard technique for hematoxylin and eosin (H & E) staining was followed by dehydrating the tissues in ascending grades of alcohol, clearing them in xylene and embedding in paraffin wax. 5 μm thick sections were cut on a rotary microtome and stained in H & E, treated with alcohol, cleared in xylene and mounted in DPX and examined under a light microscope.
Assessment of liver function
The alanine transaminase (ALT) activity and aspartate transaminase (AST) in liver homogenate and serum were determined [17]. The alkaline phosphatase (ALP) activity in both liver and serum was estimated [18]. Alkaline phosphatase at optimum pH 10.5 catalyzes the hydrolysis of p-nitrophenyl phosphate (disodium salt) to p-nitrophenol and inorganic phosphate. The liberated p-nitrophenol combines with sodium hydroxide to form a yellow coloured complex which was measured at 410 nm. Similarly the activity of acid phosphatase (ACP) was assayed in liver and serum by the method as described in Sigma Technical Bulletin [19]. Acid phosphatase at optimum pH 4.8 catalyzes the hydrolysis of p-nitrophenyl phosphate (disodium salt) to p-nitrophenol and inorganic phosphate. The liberated p-nitrophenol combines with sodium hydroxide to form a yellow coloured complex which was measured at 420 nm.
Serum lactate dehydrogenase (LDH) activity was determined by the method of King (1965) [20]. The method is based on the ability of LDH to convert lactate to pyruvate with the help of coenzyme nicotinamide adenine dinucleotide (NAD+). The pyruvate formed is made to react with 2, 4–dinitrophenyl hydrazine in hydrochloric acid. The hydrazone formed in alkaline medium was read at 540 nm. The γ–glutamyl transpeptidase (γ-GT) activity in serum was also estimated following the method of Orlowski and Meister (1965) [21]. This enzyme catalyzes transfer of gamma glutamyl groups from gamma glutamyl peptides to suitable acceptor. The enzymatic reaction in the presence of substrate γ-glutamyl-p-nitroaniline results in the formation of p–nitroaniline whose release was monitored by noting increase in absorbance at 410 nm.
Estimation of nucleic acids and proteins content
A known weight of liver was homogenized in 5 mL of cold 5% TCA and homogenate was kept at 0–4 °C for 30 min and subsequently processed in alcohol: ether (1:3, v/v) and centrifuged to obtain lipid free pellet which was dissolved in of 0.1 N KOH and incubated at 37 °C for 16–18 h to will 6 N HCl and 10% TCA were added and centrifuged. The supernatant was used for RNA estimation by the method of Schneider (1945) using orcinol reagent to give a greenish colour, which was read at 670 nm [22]. To the pellet 5% TCA was added and heated at 90 °C for 15 min. The supernatant was then separated by centrifugation (10 min at 1000 x g) and used for DNA estimation by the method of Giles and Meyer (1965) [23]. The DNA in the supernatant reacts with diphenylamine to give blue coloured complex whose optical density was read at 620 nm. The protein content of the liver was estimated by the method of Lowry et al. (1951) [24].
Estimation of hepatic succinate dehydrogenase (SDH) and adenosine triphosphatase (ATPase) activity
The liver succinate dehydrogenase (SDH) activity was measured by the method of Beatty et al. 1966, using 2-(4-iodophenyl)-3(4-nitrophenyl)-5-phenyl-2H-tetrazolium chloride (INT) as an electron acceptor [25]. The electrons released by the enzyme from the substrate are taken up by INT, which was reduced to a red coloured formazon. This was extracted in ethyl acetate and measured at 420 nm. The hepatic adenosine triphosphatase (ATPase) activity was assayed by using the method of Quinn and White (1968) [26]. ATPase causes hydrolysis of adenosine triphosphate (ATP) into adenosine diphosphate (ADP) and inorganic phosphate (i.p.). The liberated inorganic phosphorus was estimated by the method of Fiske and Subbarow (1925) [27]. The optical density was read at 660 nm.
Statistical analysis
The results were analyzed by one-way analysis of variance (ANOVA) followed by least significant difference (LSD) multiple comparison post-hoc tests using the 19th version of SPSS software. The level of significance was accepted with *p < 0.001.
Results
Total flavonoid content
The total flavonoid content (%) of the standardized S. nigrum extract was determined to be 1.75 ± 0.17
Changes in body weight
Table 1 represents the result of plant extracts treatment on CCl4–induced changes in the body weight of mice. No significant difference in body weight was observed between different control groups (Groups 1–3). However, CCl4 treatment for 30 days (Group 4), as compared to vehicle control (group 2), caused a significant (p < 0.001) reduction in body weight. Oral administration of CCl4 along-with hydroalcoholic extracts of SN (Groups 5–7) caused significant increase (p < 0.001) in body weight, as compared to CCl4 alone treated animals (Group 4) in a dose–dependent manner. The 300 mg/kg body weight dose was the most effective in increasing the body weight of mice.
Histopathology
Liver sections (H & E stained) of vehicle control mice (Fig. 1a) showed normal structure of central vein with radially arranged normal hepatocytes. No apparent histopathological changes were observed in the liver of plant extracts alone treated mice (Fig. 1b). Oral administration of HD of CCl4 for 30 days caused hepatocellular necrosis and cytoplasmic vacuolization (Fig. 1c). On the other hand oral administration of SN alongwith CCl4 caused significant, dose-dependent protection against the changes induced by the hepatotoxin (Fig. 1 d-e). Dilation of central vein and distortion of hepatocytes became less apparent in liver of SN300 plus CCl4 (Fig. 1f) administered mice. Fatty degenerative changes were also less pronounced in SN300 and CCl4-treated mice (Figs. 1f).
Light micrographs (H & E stained) of liver (a) vehicle control mice showing normal architecture with radially arranged central vein (200 x) (b) S. nigrum control mice showing normal structure and absence of vacuolization and fatty degenerative changes (200x) (c) CCl4-treated indicating extensive cytoplasmic vacuolization and fatty infiltration (200x) (d) S. nigrum 100 mg/kg bwt plus CCl4-treated mice showing moderate necrotic changes and fatty degenerative changes and ballooning of hepatocytes (e) S. nigrum 200 mg/kg bwt plus CCl4-treated mice showing mild centrilobular necrosis degenerative changes (200x) (f) S. nigrum 300 mg/kg bwt plus CCl4-treated mice showing normal arrangement of hepatocytes and almost similar to vehicle control mice (400 x)
Liver biochemical analysis
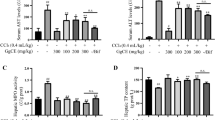
Table 2 represents the results of CCl4–induced changes in activities of some liver enzymes of mice with and without plant extract treatment. Results revealed no significant changes in the activities of ALT, AST, ALP and ACP between different control groups (Groups 1–3). However, CCl4 treatment for 30 days (Group 4), as compared to vehicle control (group 2), caused significant (p < 0.001) increase in all these parameters. Oral administration of three different doses of SN alongwith CCl4 (Groups 5–7) significantly (p < 0.001) ameliorated the CCl4-induced changes. The effect was dose-dependent and maximum at the dose regimen of 300 mg/kg body weight.
Serum biochemical parameters
Table 3 shows the results of plant extract treatment on CCl4–induced changes in the activities of marker enzymes in serum of mice. No significant difference in the activities of ALT, AST, ALP, ACP, LDH and γ-GT were observed among all control group animals (Groups 1–3). It was observed that activities of all serum marker enzymes were significantly (p < 0.001) increased after oral administration of HD CCl4 (Group 4) as compared to vehicle control (Group 2) which was effectively reversed in a dose–dependent manner when SN extract was given alongwith the toxin (Groups 5–7).
Hepatic nucleic acid, protein content and energy status
Table 4 presents the results of mitigatory effects of plant extracts on biochemical changes induced by CCl4 in liver of mice. No significant change was observed in DNA, RNA and protein contents in different control groups (Groups 1–3). Conversely, oral administration of HD of CCl4 (Group 4) caused a significant (p < 0.001) decrease in these parameters. Results revealed that co-treatment of SN alongwith CCl4 (Groups 5–7), significantly (p < 0.001) ameliorated the toxin-induced changes in DNA, RNA and protein content in a dose–dependent manner.
Table 5 depicts the results of CCl4–induced changes in energy metabolism. No significant changes were observed in the activities of SDH and ATPase in liver of control mice (Groups 1–3). However, a significant reduction was noted in the activities of SDH and ATPase in CCl4-treated animals (Group 4). The percent decrease in the SDH activity was more prominent as compared to ATPase. Results revealed a significant amelioration in the activities of SDH and ATPase when SN was concurrently administered with CCl4 (Groups 5–7). The effects were dose-dependent.
Discussion
Oral administration of CCl4 for 30 days caused a significant reduction in the body weight of mice (Table 1). Mice administered with CCl4 were observed to be dull with reduced feed consumption which probably contributed to the decrease in the body weight observed. A similar loss in body weight, dullness and anorexic behaviour in rats treated with CCl4 has been reported by Abuelgasim et al. (2008) [28]. Plant extracts alone treated mice (group 3) did not show any significant difference in body weight as compared to vehicle control (group 2). Results indicated a significant and dose-dependent increase in body weight of plant extract plus CCl4-treated mice. Lin et al. 2008, have reported a significant decrease in the body weight of rats administered with 0.5 mL of 20% (v/v) solution CCl4 in corn oil, which was significantly mitigated by treatment with S. nigrum extract [11]. Hsieh et al. 2008, have reported a similar increase in body weight of thioacetamide-induced fibrotic mice after administration of S. nigrum extract [7].
Histopathological (H & E) examinations revealed severe cytoplasmic vacuolization and fatty degenerative changes in the liver of CCl4-treated mice (Fig. 1 c). Earlier reports have confirmed the disruption of lattice nature of hepatocytes, degenerated nuclei, ballooning of liver cells, disintegrated central vein and fibrotic changes in CCl4-treated animals [29]. However, administration of the plant extracts alongwith the hepatotoxin caused reversal of the histopathological alterations induced by the oral administration of the toxin in a dose-dependent manner (Fig. 1 d-f). Lin et al. 2008, have reported that administration of S. nigrum extract to CCl4-intoxicated rats resulted in reduced incidence of hepatocyte swelling, lymphocyte infiltration and fibrous tissue proliferation [11].
The results of plant extract treatment on CCl4- induced changes in activities of some liver enzymes are depicted in Table 2. Table 3 shows the effect of SN treatment on various hepatic marker enzymes in serum of mice. Results indicate a marked increase in activities of these enzymes after treatment with CCl4 which was significantly and dose dependently mitigated by the administration of the plant extract. CCl4 intoxication results in severe damage to hepatic tissue membranes results in leakage of these enzymes into circulation. [30]. Raju et al. 2003, have reported that ethanolic extract of S. nigrum possessed remarkable hepatoprotective activity against CCl4-induced hepatic damage in rats by evaluating biochemical parameters such as AST, ALT, ALP and total bilirubin [8]. The histopathological changes of liver sample of extract-treated rats were normal and resembled control animals. Lee and Lim (2009) have investigated the potential of glycine and proline-rich glycoprotein isolated from S. nigrum in decreasing the leakage of LDH in 1, 2-dimethylhydrazine-treated mice [31].
In the present study carbon tetrachloride treatment for 30 days caused a significant and dose-dependent reduction in hepatic DNA, RNA and protein levels (Table 4). The Metabolic activation of CCl4 results in production of free radicals which form covalent bonds with tissue macromolecules like DNA, RNA and proteins. It is well known that administration of CCl4 results in dislocation of ribonucleoproteins from rough endoplasmic membranes causing an inhibition in protein synthesis [32]. Omar et al. 2006, have found out that CCl4 to diminish the levels of DNA in liver by using Feulgen histochemical staining technique [33]. Voronova et al. 1976, have reported decrease in RNA content after CCl4 administration [34]. Treatment of S. nigrum extract alongwith CCl4 during the present study significantly and dose-dependently ameliorated the toxin-induced changes in all these biochemical parameters and enhanced the levels of DNA, RNA and stabilizing the protein synthesizing function of the liver. The liver protecting property of Liv.52 which includes S. nigrum as a major ingredient against CCl4-mediated liver damage was evaluated in rats by [35]. Their results revealed that CCl4 caused significant reduction in the liver microsomal protein and RNA. However rats pre-treated with the indigenous drug Liv-52 and subjected to challenge with the hepatotoxin showed complete recovery.
Table 5 indicates the results of activities of SDH and ATPase of different doses of plant extract with and without CCl4 treatment. Reduction in activity of SDH due to CCl4 treatment indicates damage to mitochondria and hence results in overall reduction in energy status of the system [36]. Similarly uncoupling of oxidative phosphorylation and a decrease in ATPase activity due to CCl4 treatment has also been reported [37]. Treatment with S. nigrum extract resulted in restoration of SDH and ATPase kevels thus improving the energy status of the hepatic system.
The present study has reported a very potent hepatoprotective property of standardized flavonoid extract of S. nigrum. Flavonoids are considered to be the most frequently occurring polyphenolic Phytochemical with high medicinal properties. Many previous studies have reported the liver protecting property of S. nigrum against CCl4-induced acute toxicity. In the present study S. nigrum was found to confer significant dose-dependent hepatoprotection against CCl4-induced sub-chronic toxicity by improving the DNA, RNA and protein levels and also by increasing the energy levels of the hepatic tissue.
Conclusion
The present study has identified S. nigrum to be a potent hepatoprotective plant and it was found to confer hepatoprotection probably due to its high flavonoid content by preventing CCl4-induced adduct formations in tissue macromolecules. More systematic studies are required to clearly understand the mechanistic aspect and also the molecular mechanism responsible for the potent hepatoprotective property of the plant S. nigrum.
Abbreviations
- CCl4 :
-
carbon tetracholoride
- SN:
-
Solanum nigrum
References
Recknagel RO, Glende EA. Carbon tetrachloride hepatotoxicity: an example of lethal cleavage. CRC Crit Rev Toxicol. 1973;2:263–97.
Yadav NP, Pal A, Shanker K, Bawankule DU, Gupta AK, Darokar MP, Khanuja SP. Synergistic effect of silymarin and standardized extract of Phyllanthus amarus against CCl4-induced hepatotoxicity in Rattus norvegicus. Phytomedicine. 2008;15:1053–61.
Mico BA, Pohl LR. Reductive oxidation of carbon tetrachloride: Trichloromethylperoxyl radical as a possible intermediate in the conversion of carbon tetrachloride to electrophilic chlorine. Arch Biochem Biophys. 1983;225:596–609.
Kessler RC, Davis RB, Foster DF, Van Rompay MI, Walters EE, Wilkey SA, Kaptchuk TJ, Eisenberg DM. Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann Int Med. 2001;135:262–8.
Kirtikar KR, Basu BD. Indian Medicinal Plants, Allahabad. Lalit Mohan Basu Publisher. 1935;2:457.
Hussain A, Virmani OP, Popli SP, Misra LN, Gupta MM, Srivastava GN, Abraham Z, Singh AK. Dictionary of Indian Medicinal Plants. Lucknow: Central Institute of Medicinal and Aromatic plants; 1992. p. 35.
Hsie CC, Fang HL, Lina WC. Inhibitory effect of Solanum nigrum on thioacetamide-induced liver fibrosis in mice. J Ethnopharmacol. 2008;119:117–21.
Raju K, Anbuganapathi G, Gokulakrishnan V, Rajkapoor B, Jayakar B, Manian S. Effect of dried fruits of Solanum nigrum Linn. Against CCl4-induced hepatic damage in rats. Biol Pharma Bull. 2003;26:1618–9.
Elhag RAM, SMA EB, Bakhiet AO, Galal M. Hepatoprotective activity of Solanum nigrum extracts on chemically induced liver damage in rats. J Vet Med Ani Health. 2011;3:45–50.
Mir A, Anjum F, Riaz N, Iqbal H, Wahedi HM, Khattak JZK, Khan MA, Malik SA. Carbon tetrachloride (CCl4) - induced hepatotoxicity in rats: curative role of Solanum nigrum. J Med Plants Res. 2010;4:2525–32.
Lin HM, Tseng HC, Wang CJ, Lin JJ, Lo CW, Chou FP. Hepatoprotective effects of Solanum nigrum Linn extract against CCl4-induced oxidative damage in rats. Chem Biol Interact. 2008;171:283–93.
Elshater A, Salman MAA, Mohamed SA. The hepato-ameliorating effect of Solanum nigrum against CCl4 induced liver toxicity in albino rats. Egypt Acad J Biolog Sci. 2013;5:59–66.
Subash KR, Ramesh KS, Charian BV, Britto F, Rao JN, Vijaykumar S. Study of Hepatoprotective activity of Solanum nigrum and Cichorium intybus. Int J Pharmacol. 2011;7:504–9.
Kumar V, Sharma S, Modi PK. Exploration of hepatoprotective activity of aqueous extract of solanum nigrum - an experimental study. Int J Pharma Sci Res. 2013;4:464–70.
Sivgami S, Gayathri P, Ramapriya R. The antioxidant potential of two selected varieties of Solanum nigrum. J Pharm Res. 2012;5:2221–3.
Mohy-ud-din A, Khan Z, Ahmad M, Kashmiri MA, Yasmin S, Mazhar H. Chemotaxonomic significance of flavonoids in the solanum nigrum complex. J Chil Chem Soc. 2009;54:486–90.
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic acid and glutamic pyruvate transaminases. Am J Clin Pathol. 1957;28:56–63.
Bessey OA, Lowry OH. Brick NJ. A method for the rapid determination of alkaline phosphatase in 5 cu mm of serum. J Biol Chem. 1946;164:321–9.
Anon. The colorimertric determination of phosphatase- Sigma Technical Bulletin. St. Louis: Sigma Chemicals Co.; 1963. p. 104.
King J. The dehydrogenase of oxido-reductase lactate dehydrogenase. In: Van D, editor. Practical clinical enzymology. London: Nostrand; 1965. p. 83–93.
Orlowski M, Meister A. Isolation of γ-Glutamyl transpeptidase from hog kidney. J Biol Chem. 1965;240:338–47.
Schneider WC. Phosphorus compounds in animal tissues I. extraction and estimation of desoxypentose nucleic acid and of pentose nucleic acid. J Biol Chem. 1945;161:293–303.
Giles KW, Meyer A. An improved diphenylamine method for the estimation of DNA. Nature. 1965;206:93–4.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin-phenol reagent. J Biol Chem. 1951;193:265–75.
Beatty CH, Basinger GM, Dully CC, Bocek RM. Comparison of red and white voluntary skeletal muscle of several species of primates. J Histochem Cytochem. 1966;14:590–600.
Quinn PJ, White IG. Distributions of adenosine triphosphatase activity in ram and bull spermatozoa. J Reprod Fertil. 1968;15:449–52.
Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400.
Abuelgasim AI, Nuha HS, Mohammed AH. Hepatoprotective effect of Lepidium sativum against carbon tetrachloride-induced damage in rats. Res J Anim Vet Sci. 2008;3:20–3.
Dhanasekaran M, Ignacimuthu S, Agastian P. Potential hepatoprotective activity of ononitol monohydrate isolated from Cassia tora L. on carbon tetrachloride-induced hepatotoxicity in wistar rats. Phytomedicine. 2009;16:891–5.
Ahn TH, Yang YS, Lee JC, Moon CJ, Kim SH, Jun W, Park SC, Kim JC. Ameliorative effects of pycnogenol on carbon tetrachloride-induced hepatic oxidative damage in rats. Phytother Res. 2007;21:1015–9.
Lee SJ, Lim KT. Glycine- and proline-rich glycoprotein regulates the balance between cell proliferation and apoptosis for ACF formation in 1,2-dimethylhydrazine-treated A/J mice. Mol Cell Biochem. 2009;325:187–97.
Smuckler EA, Iseri OA, Benditt EP. An intracellular defect in protein synthesis induced by carbon tetrachloride. J Exp Med. 1962;116:55–72.
Omar ME, Salam A, Sleem AA, Hassan NS, Sharaf HA, Gy M. Capsaicin ameliorates hepatic injury caused by carbon tetrachloride in the rats. J Pharmacol Toxicol. 2006;1:147–56.
Voronova LA, Ivanov SD, Zabezhinski MA. Effect of carbon tetrachloride on RNA metabolism in the rat liver. Bull Exp Biol Med. 1976;81:677–80.
Subbarao VV, Gupta ML. Effect of Liv-52 and carbon tetrachloride on the liver protein and nucleic acids. IRCS Med Sci. 1979;7:499–500.
Gao H, Zhou YW. Anti-lipid peroxidation and protection of liver mitochondria against injuries by picroside II. World J Gastroenterol. 2005;11:3671–4.
Jadon A, Bhadauria M, Shukla S. Protective effect of Terminalia belerica Roxb. and gallic acid against carbon tetrachloride-induced damage in albino rats. J Ethnopharmacol. 2007;109:214–8.
Acknowledgements
The fellowship assistance to Dr. Krithika Rajesh from Rameshwardasji Birla Smarak Kosh, Mumbai, India is acknowledged with thanks. Dr. krithika Rajesh also acknowledges Department of Science and Technology, Government of India for financial support vide reference no SR/WOS-A/LS-04/2013 under Women Scientist Scheme to carry out some work presented in this paper.
Funding
Rameshwardasji Birla Smarak Kosh, Mumbai, India and Department of Science and Technology, Government of India for financial support vide reference no SR/WOS-A/LS-04/2013 under Women Scientist Scheme.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
KR designed the study, performed the experiments and acquired the data and drafted the manuscript; RJV designed and supervised the study; both authors critically revised and approved the manuscript.
Corresponding author
Ethics declarations
Authors’ information
Department of Zoology, Biomedical Technology and Human Genetics, University School of Sciences, Gujarat University, Ahmedabad – 380,009, India.
Ethics approval and consent to participate
The study was approved by “The Committee for the Purpose of Control and Supervision of Experiment on Animals” (Reg – 167/ 1999/ CPCSEA), New Delhi, India.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Krithika, R., Verma, R.J. Solanum nigrum confers protection against CCl4-induced experimental hepatotoxicity by increasing hepatic protein synthesis and regulation of energy metabolism. Clin Phytosci 5, 1 (2019). https://doi.org/10.1186/s40816-018-0096-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-018-0096-5