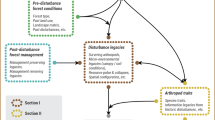
Abstract
Context
Evidence for declines in insect populations is growing with climate change being one suspected driver. Forests, however, are still underrepresented in the relevant research. Recent droughts (2018–2020) have severely affected forests in Central Europe and have been linked to declines in carabid abundance, biomass as well as changes in species traits at the local scale.
Objective
We tested drought effects on forest carabids at regional scale. We additionally investigated whether variability in drought effects could be explained with the initial community composition and the local environmental context.
Methods
We used generalized linear mixed models to compare data from 1999 to 2001 and 2020 to 2022 across eleven old beech forest sites of high conservation interest in North-East Germany and investigated changes in carabid abundance, biomass, Hill numbers and selected species traits. We then tested additional community-related and environmental predictors to explain spatial variability in changes in biomass.
Results
We found significant declines in biomass of 65% and in abundance of 51%. There were no significant changes in Hill numbers. We found consistent evidence that declines affected especially larger and less mobile species. Declines and changes in species traits also occurred in strictly protected old-growth beech forests. Among environmental predictors, landscape composition explained local variability in biomass declines best with stronger decline at forest sites with less forest area in their vicinity.
Conclusions
Our findings reveal large-scale declines in forest carabids in the context of recent droughts and highlight the exceptional role of landscape composition in this regard. Future insect conservation strategies need to incorporate the landscape context and potential exposure to extreme weather.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There have been concerning reports about declines in insect populations affecting a variety of taxonomic groups and habitats (e.g. Dalton et al. 2023; Seibold et al. 2019; van Klink et al. 2020; and see Didham et al. 2020a for a selection of further studies). Moreover, several suspected drivers contributing to declines have moved into the focus of research (Habel and Schmitt 2018; Wagner et al. 2021; Weisser et al. 2023). For instance, recent studies have linked insect trends to weather anomalies (Bauerfeind and Fischer 2014; Ewald et al. 2015; Evans et al. 2022; Welti et al. 2022; Müller et al. 2023). Consequently, climate change is among the suspected drivers of population declines (Didham et al. 2020a; Halsch et al. 2021; Wagner et al. 2021; Harvey et al. 2022), especially in the context of recent droughts and heatwaves in Europe (2018–2020), which have been unprecedented for centuries (Hari et al. 2020; Rakovec et al. 2022; Schumacher et al. 2022; Zachariah et al. 2023; Treydte et al. 2023).
Despite increasing research efforts, drivers and long-term trends of insect populations in forest ecosystems remain largely understudied (Staab et al. 2023; Blüthgen et al. 2023). Some of the few available studies thus far found notable declines (Seibold et al. 2019; Harris et al. 2019; Barendregt et al. 2022; Staab et al. 2023). Therefore, there remains an urgent need to learn more about exactly where and why certain groups of insects are declining to better understand the nuances in population trends. Some of the suspected causes of declines in open landscapes, such as industrial agriculture or urbanization, play a lesser role in European forests. However, the effects of anthropogenic climate change on forests are becoming increasingly clear (Oakes et al. 2014; Seidl et al. 2017; Senf et al. 2018). For instance, recent periods of drought have severely affected forests in Europe, leading to growth declines and increased mortality (Senf and Seidl 2021; Schnabel et al. 2022; Spiecker and Kahle 2023). Recent droughts have probably also had severe effects on forest insects (Pureswaran et al. 2018; Cours et al. 2023; Blüthgen et al. 2023), but these have hardly been investigated to date.
Large proportions of the insect biodiversity and biomass of temperate forests can be found in the litter and upper soil layers (Schowalter 2017). Here, carabid beetles (Coleoptera: Carabidae), a family of mostly predatory beetles, make up a considerable proportion of the epigeic fauna and play an essential role in invertebrate food webs (Magura 2002). They are sensitive to environmental changes and thus are often used as bio-indicators (Rainio and Niemelä 2003; Kotze et al. 2011). Many of the species found in temperate forests are adapted to relatively stable, cool and humid environments (Fitzgerald et al. 2021; Irmler 2007; Koivula et al. 1999; Müller-Motzfeld 2001). In line with this, several experimental studies (Williams et al. 2014; Jouveau et al. 2022) and observational studies (Šustek et al. 2017; Šiška et al. 2020) found a high sensitivity of forest carabids towards droughts. A recent study by Weiss et al. (2024) revealed declines in carabid abundance in a small forest area in North-East Germany, which were linked to the recent period of drought. They found even greater declines in biomass and larger, less mobile, and predatory species seemed to be particularly affected by these declines. However, there remains a general lack of knowledge about how recent droughts have affected forest insects such as carabids on a larger spatial scale. Investigating this knowledge gap would also improve the understanding of environmental factors for insect trends in general.
In this study, we investigated changes in forest carabids in the context of recent severe droughts (2018–2020) at the regional scale. For that purpose, we analyzed data from 1999 to 2001 and 2020 to 2022, collected at eleven old lowland beech forest sites in North-East Germany, the same region from which the findings of Weiss et al. (2024) originate (Fig. 1). Drought conditions similar to those described by Weiss et al. (2024) also prevailed at the regional level, suggesting that there might have been declines in forest carabid communities exceeding the local scale (Fig. S1, Table S1, Method S1). We therefore investigated if there had been regional-scale declines in carabid abundance, biomass and diversity as well as changes in the relative abundance of species traits, expecting changes corresponding with the findings of Weiss et al. (2024). In addition, we examined the variability of potential biomass declines at the local scale as the effects of drought on the carabid community might vary among sites. Forests have the capacity to buffer heat and retain moisture (Haesen et al. 2021; Gohr et al. 2021; Floriancic et al. 2023). However, this capacity can be influenced by factors such as local water availability, stand structure, forest fragmentation, or edge effects (Davis et al. 2019; Koelemeijer et al. 2022; Mann et al. 2023). Moreover, disturbance effects in insect communities are often determined by their initial composition (Kotiaho et al. 2005), which might additionally vary among different sites.
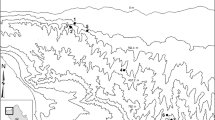
Map of the study area. Study sites are shown as filled circles and DWD stations used to source meteorological data as squares (four stations outside the shown area). The study site of Weiss et al. (2024) is highlighted as star. Land cover classes are based on Pflugmacher et al. (2019). Only 9 of 14 meteorological stations of which we used data are located within the mapped area
We tested the following hypotheses at the regional scale:
-
1)
Severe drought leads to declines in carabid abundance, biomass, and taxonomic diversity; effects on carabid biomass are most pronounced.
-
2)
Larger, predatory species with poorer dispersal abilities are affected more strongly by declines.
-
3)
Drought effects (i.e. changes in biomass) are variable due to differences in the initial community composition and the local environmental context.
Methods
Study area
The study area is located in the North-East German Lowland (Fig. 1). It represents the center of the natural distribution range of lowland beech (Fagus sylvatica) forests (Bohn et al. 2000). The eleven study sites of this study were chosen as representative selection of managed and unmanaged beech forests of old stand age and comparable environmental site conditions (Winter 2005). Today, all sites have a stand age of approximately 120–140 years or older (Fig. S2, Table S2) and mainly fall under the definition of old-growth beech forests (Dieter et al. 2020; Bolte et al. 2022). Five of the sites are strict reserves with no timber use before or during the period of study and meet the stricter definition for old-growth beech forests (> 180 years) used by the Biodiversity Strategy of the European Union (European Commission 2020; Meyer et al. 2023). Two of these sites (Serrahn and Grumsin) have been declared UNESCO World Heritage Sites, two others (Heilige Hallen and Fauler Ort) represent the oldest known lowland beech forests without timber use in Germany (Winter 2005). The remaining six areas are extensively managed to promote natural processes and structures according to the guidelines of Winter et al. (2020). The majority of the sites (seven, including Grumsin) are part of the Schorfheide-Chorin Biosphere Reserve, while Serrahn is part of the Müritz National Park. There were no general changes in the landscape at regional level and at individual sampling sites, where the share of agricultural areas and forests in the surrounding landscape remained stable during the period of study (Fig. S3). In the period after 1999, the annual precipitation and mean temperature ranged from 386 to 868 mm and from 7.3 to 10.9 °C, respectively (Weigel et al. 2023).
Beetle sampling
In 1999–2001 Winter (2005) sampled carabid beetles at the eleven forest sites described above. We relocated the original sampling locations (with a tolerance of < 2 m) using the GPS locations and tree survey maps of Begehold et al. (2016). From 2020 to 2022 we re-sampled the original locations using the exact same methods, which we describe in the following: Single pitfall traps were placed at five to ten sampling locations at each of the eleven sampling sites resulting in 79 trap locations in total. The number of sampling locations per site varied among sites and sampling years due to logistical constrains and conservation reasons. The sampling locations were initially (1999–2001) determined by randomly selecting intersection of a 100 × 100 m grid. Only at one site were traps placed closer together maintaining a minimum distance of 30 m, which can still be considered sufficient to ensure spatial independence of the individual traps (Zhao et al. 2013). Sampling was conducted from mid-April to early November each year. In all years, pitfall traps were emptied fortnightly, yielding 15 samples per trap and sampling year. In a few cases, traps were collected after a shorter or longer exposure time for practical reasons. Each of the eleven study sites was sampled for at least one whole season (April–November) during each period (1999–2001 and 2020–2022), while nine of the sites were sampled for two or three season during at least one of the periods to better account for interannual variation during modeling (Fig. S4).The pitfall traps consisted of a 400 ml honey jar featuring an extended PVC rim plate for increased standardization and reduction of the ‘digging-in effect’ (Digweed et al. 1995; Boetzl et al. 2018). They were placed in a piece of PVC pipe and covered with a metal roof leaving a gap of approximately 2 cm. Each trap contained 200 ml of trapping fluid (50% solution of monoethylene glycol and water). We provide an illustration of the trap design in the Supporting Information (Fig. S5).
After collection, the carabid beetles were determined to the species level according to Müller-Motzfeld (2006 and earlier editions) by T.K. and D.P. in 1999–2001 and by F.W. and T.K. in 2020–2022. We calculated the biomass for all the samples using the size-weight equations of Szyszko (1983) and Booij et al. (1994) following the approach proposed by Weiss and Linde (2022) based on the mean sizes provided in Müller-Motzfeld (2006). We took information for the other selected species traits (wing morphology and feeding guild) from carabids.org (Homburg et al. 2014a, b, accessed in 2020). In some cases, we complemented unavailable trait information based on Müller-Motzfeld (2006). We provide the compiled trait information in the Supporting Information (Table S3).
In 2020–2022, a considerable number of traps were affected by disturbances caused by wild boars (Sus scrofa), invasive racoons (Procyon lotor) and invasive slugs (Arion vulgaris), all possibly searching for sources of water during drought. If minor disturbances were visible but traps contained insects (14% of samples in 2020–2022), samples were considered for pooling community samples for diversity metrics, but excluded for modeling quantitative trends (e.g. abundance, biomass). This discrimination was not made for data from 1999 to 2001, as these sources of disturbance were neglectable then. We generally excluded all samples from traps heavily disturbed by rainfall or destroyed by wildlife. This affected approximately 8% of all samples in 1999–2001 and 4% in 2020–2022.
Meteorological data
We extracted publicly available meteorological data from the German Weather Service (DWD) for 14 meteorological stations in and around the study area from 1974 to 2022 (DWD 2023, Table S4). Data on daily precipitation and temperature were only available for eight stations for the whole period. We used ordinary least squares (OLS) regression with measured data from these eight available stations (continuous) and ‘month’ (categorical, only in temperature models) as predictors to back-cast periodically missing daily precipitation sums and daily mean temperatures for the remaining six stations. In the next step, we used inverse distance weighting (IDW) (‘gstat’ package, Pebesma and Graeler 2023) to interpolate daily precipitation and mean temperatures for the eleven study sites.
Statistical analyses
To investigate changes in insect communities, it is usually preferable to use time series that provide longitudinal data from consecutive years without gaps. In this study, we investigated changes in the carabid community by comparing data from 1999 to 2001 and 2020 to 2022. Generally, comparing data from relatively short periods is prone to time selection bias and so-called ‘snapshot effects’, especially when interannual fluctuations are strong (Didham et al. 2020b). Comparing two periods of multiple years avoids this problem and allows for more reliable trend estimates (Schuch et al. 2012; Harris et al. 2019). All the statistical analyses were conducted using R v.4.4.0 (R Core Development Team 2024). The ‘glmmTMB’ package (Brooks et al. 2022) was used for fitting generalized linear mixed models (GLMMs), and the ‘DHARMa’ framework (Hartig and Lohse 2022) was used for evaluating the model fit. We used the ‘ggeffects’ package (Lüdecke et al. 2023) to predict and plot all the effects.
Modeling changes in abundance and biomass
We used GLMMs of the negative-binomial family (O’Hara and Kotze 2010; Stoklosa et al. 2022) and of the zero-inflated gamma family to model abundance and biomass, respectively (Eq. S1 and S2). We included data from only undisturbed fortnightly samples to ensure maximum quantitative standardization and to minimize false zeros (see Blasco‐Moreno et al. 2019). This resulted in 1071 samples from 1999 to 2001 and 1350 samples from 2020 to 2022 collected. The abundance and biomass of single samples served as response variables, and period (1999–2001 or 2020–2022) was the main categorical predictor. We used the mean temperature (continuous) and sum of precipitation (continuous) of each fortnightly sampling interval as additional fixed effects to account for sampling errors due to short-term weather fluctuations (Saska et al. 2013). The values were scaled separately within each sampling interval to avoid collinearity with sampling interval. The sampling year, sampling interval and trap location nested in site were used as crossed random intercepts. For the biomass GLMM, we used period as an additional zero-inflation term. Relative changes for abundance and biomass were additionally bootstrapped (1000 iterations) to estimate confidence intervals.
Modeling changes in taxonomic diversity
To investigate changes in taxonomic diversity, we aggregated community samples by pooling data per individual trap and year for all sampling locations where data was available throughout the whole season (April–November). We also included data from samples that had a longer or shorter exposure or were disturbed but still yielded a viable sample (Fig. S6). In some cases, gaps in sampling occurred due to missing samples. However, Sapia et al. (2006) showed that a ‘pulsating’ sampling scheme with gaps between sampling but coverage throughout the whole season still yields accurate estimates of taxonomic diversity. Single metrics often fail to detect changes in taxonomic diversity reliably (Pozsgai et al. 2016; Hillebrand et al. 2018; Edmonds et al. 2024). We therefore used the framework of Hill numbers (Hill 1973) to describe taxonomic diversity using species richness (q0) and the inverse Simpson (q2), which is less sensitive to rarer species than is q0, and evenness (q2/q0 Jost 2010). Hill numbers were calculated for standardized coverage (Chao and Jost 2012) with the functions iNEXT and estimateD from the ‘iNEXT’ package (Hsieh et al. 2016, 2022). We excluded all community samples with an abundance of ≤ 15 individuals and standardized them to an estimated coverage of 72% to limit extrapolation to < 2*sample size (Chao et al. 2014). This resulted in 70 community samples from 1999 to 2001 and 79 community samples from 2020 to 2022 collected at 55 different locations that were sampled in both periods. We then fit Gaussian GLMMs for q0 and q2 and a beta GLMM for evenness (Geissinger et al. 2022) with period (categorical) as a single fixed effect (Eq. S3–S5). We included crossed random effects for sampling year and trap location nested within the site.
Modeling changes in species traits
We assessed changes in community composition using different species traits. For this purpose, we assessed each trait for separate samples based on individuals and for community samples based on occurring species. The data selection process for the two approaches was analogous to that used for modeling abundance/biomass and taxonomic diversity, respectively. We calculated the community weighted mean (CWM) of size for each sample serving as a model response and fitted a Gaussian GLMM with period (categorical) as a single predictor (Eq. S6). We included crossed random intercepts for sampling year, sampling interval and trap location nested in site (analogous to the abundance/biomass models). We calculated community means (CM) for each community sample (pooled for trap and year; see previous section) and based on species occurrence. This served as a response variable in a Gaussian GLMM with period (categorical) as a single predictor (Eq. S7). Here, we included crossed random intercepts for sampling year and trap location nested in site (analogous to the taxonomic diversity models). Wing morphology was coded as a binary response variable (1 = winged/0 = shortwinged or dimorphic). We then fit a binomial GLMM modeling the probability of ability to fly (1 = winged) for individuals of each sample and for occurring species of each community sample (Eq. S8 and S9). The fixed and random effects were analogous to the GLMMs for size. The same approach was used to model changes in feeding guild (1 = predator/0 = herbivore or omnivore) (Eq. S10 and S11).
Exploring spatial heterogeneity of trends and testing potential predictors for variability in local drought effects
In addition, we fit all the above-described GLMMs with ‘site’ (categorical) as an additional fixed effect (instead of a random intercept), including an interaction with period, to assess site-level changes (Eq. S12). We used this approach to explore the variability of local trends as well as to identify sites with extreme changes that could have disproportionate influence on estimated regional-scale trends. Moreover, we tested potential correlations (Spearman) among site-level changes and geographic coordinates to test for any spatial patterns in the changes that might bias the mean estimates. Blowes et al. (2022) showed that metrics such as abundance, species richness and evenness are often related and show related trends at local scale, we therefore also tested for correlations (Pearson) among the different investigated metrics at site-level.
To test how the local context might determine the severity of drought effects we then tested a set of variables representing the initial carabid community (1999–2001) and environmental conditions as additional predictors in the biomass model (Table 1 and S5). Variables regarding the carabid community were initial CWM of size and initial probabilities of sampling a winged or predatory individual as estimated by the respective GLMMs. Environmental variables featured canopy cover of the main tree layer (plot and site scale), forest edge (plot and site scale), landscape composition (i.e. share of forest and wetlands), mean annual precipitation and protection status (all at site scale). We provide references for justification and information about the calculation for each variable in Table 1. We scaled all variables except protection, which was binary, and then added these variables individually as additional fixed effect with interaction with temporal change (period) (Eq. S13). We compared the resulting models regarding effect size of the interaction, corrected Akaike’s Information Criterion (AICc) compared to the initial biomass model (∆AICc) and calculated Akaike’s weights based on AICc for all model candidates. We tested for correlations (Spearman) among all additionally tested predictors.
Results
Abundance and biomass
We sampled 10,799 carabid beetles (6113 in 1999–2001 and 4686 in 2020–2022). We found that mean abundance across sites decreased significantly (p = 0.018) by 51% [0.95 CI (− 73%, − 9%)] between the two periods (Fig. 2, Table S6). There was substantial variability between sites, with local declines ranging from 17 to 74%. Biomass decreased significantly (p = 0.003) by 65% [0.95 CI (− 81%, − 36%)], with site-specific declines ranging from 51 to 76% (Fig. 2, Table S7). Specifically in the two UNESCO World Heritage sites Serrahn and Grumsin we found respective declines of 38% and 18% in abundance and 55% and 72% in biomass (Table S17). There were no strong associations (i.e. − 0.7 < r < 0.7) between changes in abundance and biomass as well as with changes in the other metrics (Fig. S18). We found no indication for spatial patterns in the changes in abundance and biomass that might bias the mean estimates (Fig. S19).
Carabid abundance (top) and biomass (bottom): Estimated changes between 1999 and 2001 and 2020 and 2022 across all sites (left) and for specific study sites (right). Prediction were made with all other (scaled) fixed effects set to 0 and for marginal effects only. Dots represent estimated means, error bars (left plots) represent 0.95 confidence intervals. Significance codes: **p = 0.001–0.01, *p = 0.01–0.05, n.s. p > 0.05
Taxonomic diversity
The samples contained 58 carabid species: 49 species in 1999–2001 and 41 species in 2020–2022 (Table S3). 32 species were caught in both periods. There were no significant changes in Hill numbers, between 1999 and 2001 and 2020 and 2022 across sites (Fig. 3, Tables S8–S10). However, site-specific changes appeared to be very heterogeneous ranging from − 4.2 to + 2 for q0, − 2 to + 1.9 for q2 and − 0.1 to + 0.2 for evenness (Fig. 3, Table S17). Generally, there were strong correlations among local changes of all Hill numbers (Fig. S18).
Hill numbers: Estimated changes between 1999 and 2001 and 2020 and 2022 in q0 (top), q2 (middle) and evenness (bottom) across all sites (left) and for specific study sites (right). Dots represent estimated means, error bars (left plots) represent 0.95 confidence intervals. Significance codes: **p = 0.001–0.01, *p = 0.01–0.05, n.s. p > 0.05
Species traits
Across sites, we found a significant (p = 0.031) decrease in mean individual size (CWM) of 1.7 mm [− 9.8%, 0.95 CI (− 18.2%, − 0.7%)] (Fig. 4, Table S11). There was substantial variation in site-specific changes, ranging from a decrease of 5.2 mm to an increase of 2.2 mm (Table S17). However, there were only two sites where the CWM increased. CWM size was the only metric, for which local changes showed an association with geographic location (latitude, r = − 0.66, Fig. S19). The probability of sampling a winged individual was generally low (< 0.1) but increased significantly (p = 0.045) by a mean of 0.02 for 2020–2022 (Fig. 4, Table S13). Site-specific changes represented mostly increases (with four exceptions) but revealed one site with an extreme increase of 0.17, which most likely affected across-site estimates. The probability of sampling a predatory individual was generally very high (~ 0.995) and did not change significantly across sites (Fig. 4, Table S15). This was largely reflected in the estimated site-specific changes. However, there were two sites with strong increases. Changes in species-based traits generally reflected changes also observed in individual-based traits, but with lower significance—most likely due to a lower sample size in species-based metrics (Tables S12, S14 and S16).
Species traits: Estimated changes between 1999 and 2001 and 2020 and 2022 in Size (top), wing morphology/ability to fly (middle) and feeding guild/predators (bottom), each based on individuals and occurring species and across all sites and for specific study sites. Dots represent estimated means, error bars (left plots) represent 0.95 confidence intervals. Significance codes: *p = 0.01–0.05, n.s. p > 0.05
Potential predictors of variability in local drought effects
Three of the tested predictors (initial CWM size, edge length, landscape composition; see Table 1) led to an improvement in AICc by − 8.8, − 1.9 and − 17.6, respectively. (Fig. 5). The inclusion of forest edge length led to an improvement in AICc, likely due to a significant correlation of forest edge length and landscape composition (r = − 0.62, Fig. S20). Moreover, AICc mainly supported the model accounting for landscape composition, while giving some support to the model accounting for initial CWM size (i.e. ∆AICc compared to the best model < 10, Burnham and Anderson 2004). This corresponded with Akaike’s weights, which identified the model including landscape composition as the likeliest (0.988), followed by the model including initial CWM size (0.012) and giving no weight to all other models. A greater share of forest and wetlands mitigated declines to some extent, while larger initial CWM size led to stronger declines in biomass. We provide summary tables for all model candidates in Supporting Information (Tables S18–S27).
Results from testing additional predictors and their effects on biomass trends. ‘Scale’ states the spatial resolution of the predictors. The points indicate the respective variable’s effect on biomass trends expressed as prevalence ratio (i.e. 1 = no effect). Bars highlight the 0.95 confidence interval of the respective effect on biomass trends. ΔAICc values state the respective model’s difference in AICc compared to the initial biomass GLMM and Akaike’s weights were calculated based on AICc
Discussion
We found strong evidence for regional-scale declines in carabid communities of old lowland beech forests in the North-East of Germany between 1999 and 2001 and 2020 and 2022, which we attribute to recent periods of drought. Biomass declined more strongly and consistently than abundance, and declines were generally more pronounced for larger, less mobile species. On the local scale, the severity of biomass declines did not depend on the protection status of the individual sampling site but was significantly influenced by landscape composition and the initial CWM size of the carabid community in 1999–2001.
Regional declines in carabid abundance and biomass, but not taxonomic diversity
Comparing the periods 1999–2001 and 2020–2022, we observed declines of 51% [0.95 CI (− 73%, − 9%)] in carabid abundance and 65% [0.95 CI (− 81%, − 36%)]] in biomass at the regional level. We found substantial heterogeneity when considering changes at the study sites, but they were consistently negative for both metrics (Fig. 2). Having only two narrow periods to compare data generally limits detailed insights about trends and raises the question of potential confounding factors. On the other hand, there were no regional-scale changes in the landscape, such as land-use change or increasing fragmentation, which could explain these changes (Fig. S3). Management regimes of the study sites did not change during the period of study (compare Winter 2005). While natural forest succession and smaller management interventions at some sites represent potential confounding factors, these would have contributed to the variability in local changes but could not have caused consistent declines at such a spatial scale. This leaves climatic factors that affected the whole region, such as the recent drought period, as the likeliest cause.
Our results generally support the findings of several other studies of drought effects on forest carabids (Williams et al. 2014; Šustek et al. 2017; Šiška et al. 2020) and highlight declines in biomass over those in abundance as also found by Weiss et al. (2024). Generally, the declines in biomass described in this study might still be underestimating true declines. We calculated biomass based on average beetle sizes reported in the literature using the same sizes in 1999–2001 and 2020–2022. Sizes of adult beetles, however, largely depend on conditions during larval development with heat and drought leading to smaller adult beetles (Huk and Kühne 1999; Tseng et al. 2018).
We did not find consistent changes in taxonomic diversity, which contrasts the findings of other studies with regard to drought responses of forest carabid communities (Williams et al. 2014; Šustek et al. 2017; Jouveau et al. 2022). This might be because most of these studies did not standardize diversity metrics. Accordingly, Weiss et al. (2024) who also used Hill numbers, did not find a clear link between drought conditions and taxonomic diversity of forest carabids. Morecroft et al. (2002) observed that during a drought, a similar number of carabid species decreased and increased, offsetting the effects on taxonomic diversity.
Larger species with poorer dispersal abilities disproportionately affected
We observed a significant decrease in the CWM of size and a marginally significant increase in flying individuals at the regional level. Our findings indicate changes in the relative abundance of these traits and generally support the idea that recent droughts especially affected larger, less mobile carabid species. Furthermore, we found stronger evidence for changes in individual-based traits than in species-based traits (i.e. lower p-values, greater consistency at the local level). This suggests that changes in trait composition are due to changes in abundance of more common species rather than species turnover. Larger, poorly dispersing species are typically associated with stable habitats such as old forests (Müller-Motzfeld 2001) and have been reported to be generally more sensitive to environmental changes and disturbances (Rainio and Niemelä 2003; Homburg et al. 2014b; Qiu et al. 2023). While large carabid species might be affected through a change in prey availability and size (Rudolf 2012), species with poorer dispersal ability (i.e. short-winged) might take more time to re-colonize areas after disturbance (Homburg et al. 2013; Nardi et al. 2022). In carabid species, adult beetle size and wing morphology are often correlated (e.g. Homburg et al. 2019). The more distinctive results regarding size, suggest that these might also drive changes regarding the dispersal ability in this study. We did not find significant consistent changes in feeding guilds, as proposed by other studies (Brandmayr and Pizzolotto 2016; Kirichenko-Babko et al. 2020; Jouveau et al. 2022; Weiss et al. 2024). However, there were generally few non-predatory (i.e., omnivorous, herbivorous) species, and their abundance was very low at the study sites during both periods.
Landscape composition as strong predictor for local changes in biomass
We tested if differences in the initial community composition and the local environmental context could explain the local variability of drought effects on carabid beetles. Above all, we found landscape composition to be a meaningful predictor for the severity of biomass declines observed in this study. Sites that featured less forest (incl. waterbodies and wetlands) within a 1 km-radius showed greater declines in biomass (Fig. 5). Gohr et al. (2021) reported that these types of landscape cover effectively offset extreme temperatures at a similar spatial scale. According to Mann et al. (2023), the size of the forested areas, which is indirectly reflected in our landscape composition variable, also plays a decisive role in this mitigation capacity. Several studies agree that canopy cover (Davis et al. 2019; De Frenne et al. 2021; Haesen et al. 2021) and edge effects (Arroyo-Rodríguez et al. 2017; De Frenne et al. 2021; Koelemeijer et al. 2022) determine forest microclimate. Consequently, these parameters could also regulate drought effects on carabids. Yet, we found little to no indication that canopy cover or forest edge determined changes in carabid biomass at plot or site scale. Protection status could not explain difference in local declines of carabid biomass. However, it is worth noting that we only sampled data from extensively managed or strictly protected sites of old stand age and that drought impacts on carabids in intensively managed beech forest sites of younger stand age may be different.
Community responses to disturbances such as drought may vary with the respective community’s trait composition (Kotiaho et al. 2005). We observed that initial CWM size of carabids in 1999–2001 could explain some of the spatial variability of biomass trends indicating stronger declines at sites that initially featured larger carabid beetles. This finding supports the notion that declines especially affect larger species (Brandmayr and Pizzolotto 2016; Nolte et al. 2019; Qiu et al. 2023; Weiss et al. 2024). Again, this suggests that size, rather than wing morphology, may be the key trait for the effects of drought in ground beetles.
Finally, it is crucial to note that the number of independent sites (n = 11) in this study was relatively low and that these were not initially selected to cover consistent gradients of the selected variables. Therefore, estimated effects, CIs and p values should be interpreted with caution. Although our results provide important indication, we emphasize the importance of further research. Future studies should further investigate how local pre-conditions affect drought effects on forest insects (hypothesis 3) with the aim to quantify and disentangle effects using a targeted study design.
Underlying ecological processes
It remains difficult to determine exactly through which processes carabid beetles are affected by drought. Carabids could be affected directly through changes in microclimate. For instance, some carabid species have been found to be negatively affected by low soil moisture (Tyszecka et al. 2023). A higher risk of desiccation might present a direct threat to carabid beetles of all species and life stages, but especially to eggs and larvae (Huk and Kühne 1999; Pozsgai and Littlewood 2014; Tseng et al. 2018; Magura et al. 2021). In addition, higher temperatures can reduce the fertility and reproductive success of insects (Sales et al. 2021). Exposure to drought as larvae can lead to morphological deformations in adult beetles ultimately affecting reproductive success (Huk and Kühne 1999; Tyszecka et al. 2023). In accordance with those observations, we also found morphological deformations in numerous adult carabids of different species in 2020–2022, which could potentially result from droughts (Fig. S21). However, as we did not collect any respective quantitative data, further research is required to confirm this link.
Moreover, carabid beetles could be indirectly affected via trophic interactions (Cours et al. 2023). Wise and Lensing (2019) proposed that reduced soil moisture first affects fungal communities and subsequently cascades up trophic levels. Carabids might respond to lower abundances of drought-sensitive prey such as Gastropoda (Irmler 2007; Šiška et al. 2020). At the same time, process could additionally work top-to-bottom; for example, when omnivorous mammals and birds increasingly search the relatively soft soil and litter layers in deciduous forests for invertebrates during drought periods (Baubet et al. 2003). Ultimately, the observed declines and size shifts in the carabid community could both indicate and lead to broader changes in food webs with potentially far-reaching consequences (Rudolf 2012; Eisenhauer et al. 2023).
Implications for conservation and management
Beech forests are the natural vegetation for large parts of Europe (Bohn et al. 2000; Giesecke et al. 2007). Especially old beech forests play an important role in biodiversity conservation (Springer et al. 2024 [preprint]). They are, as most forests in Central Europe, under increasing pressure due to climate change (Martinez del Castillo et al. 2022; Leuschner et al. 2023) and have been particularly affected by recent droughts (Meyer et al. 2020; Rukh et al. 2023; Weigel et al. 2023). This study, together with that of Weiss et al. (2024), provides first evidence that the insect communities in these forests were also severely affected. These results emphasize climate change with increasing weather extremes as one major threat for insect populations of temperate forests (Harris et al. 2019; Evans et al. 2022; Blüthgen et al. 2023). Furthermore, this study found declines in extensively managed as well as in some of Germany’s most protected beech forest areas, such as UNESCO World Heritage sites. This underlines potential limitations of local protection for the conservation of insects under progressing climate change (Warren et al. 2018; Rashid et al. 2023). Future efforts to protect insects should take even greater account of the landscape context and exposure to potential drivers of decline. Without climate change mitigation and adapted conservation approaches, progressing climate change will most likely lead to further declines and changes in the carabid communities of lowland beech forests.
Data availability
Data and metadata are available at PubData (https://doi.org/10.48548/pubdata-118).
Code availability
R code is available at https://gitlab.com/fabioweiss/beech_carabids_landscapeecology .
References
Arroyo-Rodríguez V, Saldaña-Vázquez RA, Fahrig L, Santos BA (2017) Does forest fragmentation cause an increase in forest temperature? Ecol Res 32:81–88
Barendregt A, Zeegers T, van Steenis W, Jongejans E (2022) Forest hoverfly community collapse: abundance and species richness drop over four decades. Insect Conserv Divers 15:510–521
Baubet E, Ropert-Coudert Y, Brandt S (2003) Seasonal and annual variations in earthworm consumption by wild boar (Sus scrofa scrofa L.). Wildl Res 30:179–186
Bauerfeind SS, Fischer K (2014) Simulating climate change: temperature extremes but not means diminish performance in a widespread butterfly. Popul Ecol 56:239–250
Begehold H, Rzanny M, Winter S (2016) Patch patterns of lowland beech forests in a gradient of management intensity. For Ecol Manag 360:69–79
Blasco-Moreno A, Pérez-Casany M, Puig P, Morante M, Castells E (2019) What does a zero mean? Understanding false, random and structural zeros in ecology. Methods Ecol Evol 10:949–959
Blowes SA, Daskalova GN, Dornelas M, Engel T, Gotelli NJ, Magurran AE, Martins IS, McGill B, McGlinn DJ, Sagouis A, Shimadzu H, Supp SR, Chase JM (2022) Local biodiversity change reflects interactions among changing abundance, evenness, and richness. Ecology 103:e3820
Blüthgen N, Dicks LV, Forister ML, Outhwaite CL, Slade EM (2023) Insect declines in the anthropocene. Nat Rev Earth Environ 4:683–686
Boetzl FA, Ries E, Schneider G, Krauss J (2018) It’s a matter of design—how pitfall trap design affects trap samples and possible predictions. PeerJ 6:e5078
Bohn U, Gollup G, Hettwer C, Neuhäuslova Z, Raus T, Schlüter H, Weber H (2000) Map of the Natural vegetation of Europe. Scale 1: 2 500 000. Bundesamt für Naturschutz
Bolte A, Kroiher F, Rock J, Dieter M, Bösch M (2022) Einschlagstopp in alten, naturnahen Buchenwäldern im öffentlichen Besitz: Definition, Vorkommen, Inventur-Kennzahlen, Gefährdung und ökonomische Bewertung
Booij K, den Nijs L, Heijermann T, Jorritsma I, Lock C, Noorlander J (1994) Size and weight of carabid beetles: ecological applications. Proc Exper Appl Entomol 5:93–98
Brandmayr P, Pizzolotto R (2016) Climate change and its impact on epigean and hypogean carabid beetles. Period Biol 118:147–162
Brooks M, Bolker B, Kristensen K, Maechler M, Magnusson A, McGillycuddy M, Skaug H, Nielsen A, Berg C, van Bentham K, Sadat N, Lüdicke D, Lenth R, O’Brien J, Geyer C, Jagan M, Wiernik B, Stouffer D (2022) R package glmmTMB: linear mixed-effects models using Eigen and S4, version 1.1-27.1.
Burnham KP, Anderson DR (2004) Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res 33:261–304
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93:2533–2547
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67
Cours J, Bouget C, Barsoum N, Horák J, Le Souchu E, Leverkus AB, Pincebourde S, Thorn S, Sallé A (2023) Surviving in changing forests: abiotic disturbance legacy effects on arthropod communities of temperate forests. Curr Forestry Rep 9:189–218
Dalton RM, Underwood NC, Inouye DW, Soulé ME, Inouye BD (2023) Long-term declines in insect abundance and biomass in a subalpine habitat. Ecosphere 14:e4620
Davis KT, Dobrowski SZ, Holden ZA, Higuera PE, Abatzoglou JT (2019) Microclimatic buffering in forests of the future: the role of local water balance. Ecography 42:1–11
De Frenne P, Lenoir J, Luoto M, Scheffers BR, Zellweger F, Aalto J, Ashcroft MB, Christiansen DM, Decocq G, De Pauw K, Govaert S, Greiser C, Gril E, Hampe A, Jucker T, Klinges DH, Koelemeijer IA, Lembrechts JJ, Marrec R, Meeussen C, Ogée J, Tyystjärvi V, Vangansbeke P, Hylander K (2021) Forest microclimates and climate change: importance, drivers and future research agenda. Glob Change Biol 27:2279–2297
Didham RK, Barbero F, Collins CM, Forister ML, Hassall C, Leather SR, Packer L, Saunders ME, Stewart AJA (2020a) Spotlight on insects: trends, threats and conservation challenges. Insect Conserv Divers 13:99–102
Didham RK, Basset Y, Collins CM, Leather SR, Littlewood NA, Menz MHM, Müller J, Packer L, Saunders ME, Schönrogge K, Stewart AJA, Yanoviak SP, Hassall C (2020b) Interpreting insect declines: seven challenges and a way forward. Insect Conserv Divers 13:103–114
Dieter M, Weimar H, Iost S, Englert H, Fischer R, Günter S, Morland C, Roering H-W, Schier F, Seintsch B, Schweinle J, Zhunusova E (2020) Abschätzung möglicher Verlagerungseffekte durch Umsetzung der EU-KOM-Vorschläge zur EU-Biodiversitätsstrategie auf Forstwirtschaft und Wälder in Drittstaaten
Digweed SC, Currie CR, Carcamo HA, Spence JR (1995) Digging out the “‘digging-in effect’” of pitfall traps: Influences depletion and disturbance on catches of ground beetles (Coleoptera: Carabidae). Pedobiologia 39:561–576
DWD (2023) Daily climate data. https://opendata.dwd.de/climate_environment/CDC/observations_germany/climate/daily/. Accessed 27 July 2023
Edmonds MV, Bufford JL, Godsoe W (2024) A mismatch between community assembly and abundance-based diversity indices. Ecography 2024:e06987
Eisenhauer N, Ochoa-Hueso R, Huang Y, Barry KE, Gebler A, Guerra CA, Hines J, Jochum M, Andraczek K, Bucher SF, Buscot F, Ciobanu M, Chen H, Junker R, Lange M, Lehmann A, Rillig M, Römermann C, Ulrich J, Weigelt A, Schmidt A, Türke M (2023) Ecosystem consequences of invertebrate decline. Curr Biol 33:4538-4547.e5
European Commission (2020) Biodiversity strategy for 2030. https://environment.ec.europa.eu/strategy/biodiversity-strategy-2030_en. Accessed 14 Dec 2023
Evans MJ, Barton P, Niwa S, Soga M, Seibold S, Tsuchiya K, Hisano M (2022) Climate-driven divergent long-term trends of forest beetles in Japan. Ecol Lett 25:2009–2021
Ewald JA, Wheatley CJ, Aebischer NJ, Moreby SJ, Duffield SJ, Crick HQP, Morecroft MB (2015) Influences of extreme weather, climate and pesticide use on invertebrates in cereal fields over 42 years. Glob Change Biol 21:3931–3950
Fitzgerald JL, Stuble KL, Nichols LM, Diamond SE, Wentworth TR, Pelini SL, Gotelli NJ, Sanders NJ, Dunn RR, Penick CA (2021) Abundance of spring- and winter-active arthropods declines with warming. Ecosphere 12:e03473
Floriancic MG, Allen ST, Meier R, Truniger L, Kirchner JW, Molnar P (2023) Potential for significant precipitation cycling by forest-floor litter and deadwood. Ecohydrology 16:e2493
Frey SJK, Hadley AS, Johnson SL, Schulze M, Jones JA, Betts MG (2016) Spatial models reveal the microclimatic buffering capacity of old-growth forests. Sci Adv 2:e1501392
Fuller RJ, Oliver TH, Leather SR (2008) Forest management effects on carabid beetle communities in coniferous and broadleaved forests: implications for conservation. Insect Conserv Divers 1:242–252
Geissinger EA, Khoo CLL, Richmond IC, Faulkner SJM, Schneider DC (2022) A case for beta regression in the natural sciences. Ecosphere 13:e3940
Giesecke T, Hickler T, Kunkel T, Sykes MT, Bradshaw RHW (2007) Towards an understanding of the Holocene distribution of Fagus sylvatica L. J Biogeogr 34:118–131
Gohr C, Blumröder JS, Sheil D, Ibisch PL (2021) Quantifying the mitigation of temperature extremes by forests and wetlands in a temperate landscape. Ecol Inf 66:101442
Habel JC, Schmitt T (2018) Vanishing of the common species: empty habitats and the role of genetic diversity. Biol Conserv 218:211–216
Haesen S, Lembrechts JJ, De Frenne P, Lenoir J, Van Meerbeek K et al (2021) ForestTemp—sub-canopy microclimate temperatures of European forests. Glob Change Biol 27:6307–6319
Halsch CA, Shapiro AM, Fordyce JA, Nice CC, Thorne JH, Waetjen DP, Forister ML (2021) Insects and recent climate change. Proc Natl Acad Sci 118:e2002543117
Hari V, Rakovec O, Markonis Y, Hanel M, Kumar R (2020) Increased future occurrences of the exceptional 2018–2019 Central European drought under global warming. Sci Rep 10:12207
Harris JE, Rodenhouse NL, Holmes RT (2019) Decline in beetle abundance and diversity in an intact temperate forest linked to climate warming. Biol Conserv 240:108219
Hartig F, Lohse L (2022) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package v.0.4.6.
Harvey JA, Tougeron K, Gols R, Heinen R, Chown SL et al (2022) Scientists’ warning on climate change and insects. Ecol Monogr 93:e1553
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432
Hillebrand H, Blasius B, Borer ET, Chase JM, Downing JA, Eriksson BK, Filstrup CT, Harpole WS, Hodapp D, Larsen S, Lewandowska AM, Seabloom EW, Van de Waal DB, Ryabov AB (2018) Biodiversity change is uncoupled from species richness trends: consequences for conservation and monitoring. J Appl Ecol 55:169–184
Homburg K, Schuldt A, Drees C, Assmann T (2013) Broad-scale geographic patterns in body size and hind wing development of western Palaearctic carabid beetles (Coleoptera: Carabidae). Ecography 36:166–177
Homburg K, Homburg N, Schäfer F, Schuldt A, Assmann T (2014a) Carabids.org—a dynamic online database of ground beetle species traits (Coleoptera, Carabidae). Insect Conserv Divers 7:195–205
Homburg K, Brandt P, Drees C, Assmann T (2014b) Evolutionarily significant units in a flightless ground beetle show different climate niches and high extinction risk due to climate change. J Insect Conserv 18:781–790
Homburg K, Drees C, Boutaud E, Nolte D, Schuett W, Zumstein P, von Ruschkowski E, Assmann T (2019) Where have all the beetles gone? Long-term study reveals carabid species decline in a nature reserve in Northern Germany. Insect Conserv Divers 12:268–277
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456
Hsieh TC, Ma KH, Chao A (2022) iNEXT: Interpolation and extrapolation for species diversity. R package v.3.0.0.
Huk T, Kühne B (1999) Substrate selection by Carabus clatratus (Coleoptera, Carabidae) and its consequences for offspring development. Oecologia 121:348–354
Irmler U (2007) Long-term fluctuations of ground beetles in a wood-agrarian landscape of Northern Germany (Coleoptera: Carabidae). Entomol General 30:13–32
Jost L (2010) The relation between evenness and diversity. Diversity 2:207–232
Jouveau S, Poeydebat C, Castagneyrol B, van Halder I, Jactel H (2022) Restoring tree species mixtures mitigates the adverse effects of pine monoculture and drought on forest carabids. Insect Conserv Divers 15:725–738
Kirichenko-Babko M, Danko Y, Musz-Pomorksa A, Widomski MK, Babko R (2020) The impact of climate variations on the structure of ground beetle (Coleoptera: Carabidae) assemblage in forests and wetlands. Forests 11:1074
Koelemeijer IA, Ehrlén J, Jönsson M, De Frenne P, Berg P, Andersson J, Weibull H, Hylander K (2022) Interactive effects of drought and edge exposure on old-growth forest understory species. Landsc Ecol 37:1839–1853
Koivula M, Punttila P, Haila Y, Niemelä J (1999) Leaf litter and the small-scale distribution of carabid beetles (Coleoptera, Carabidae) in the boreal forest. Ecography 22:424–435
Kotiaho JS, Kaitala V, Komonen A, Päivinen J (2005) Predicting the risk of extinction from shared ecological characteristics. Proc Natl Acad Sci 102:1963–1967
Kotze DJ, Brandmayr P, Casale A, Dauffy-Richard E, Dekoninck W, Koivula MJ, Lövei GL, Mossakowski D, Noordijk J, Paarmann W, Pizzolotto R, Saska P, Schwerk A, Serrano J, Szyszko J, Taboada A, Turin H, Venn S, Vermeulen R, Zetto T (2011) Forty years of carabid beetle research in Europe—from taxonomy, biology, ecology and population studies to bioindication, habitat assessment and conservation. Zookeys 100:55–148
Leuschner C, Weithmann G, Bat-Enerel B, Weigel R (2023) The future of European beech in northern Germany—climate change vulnerability and adaptation potential. Forests 14:1448
Lövei GL, Ferrante M, Möller D, Möller G, Vincze É (2023) The need for a (non-destructive) method revolution in entomology. Biol Conserv 282:110075
Lüdecke D, Aust F, Crawley S, Ben-Shachar MS (2023) ggeffects: create tidy data frames of marginal effects for “ggplot” from model outputs. R package v.1.2.2.
Magura T (2002) Carabids and forest edge: spatial pattern and edge effect. For Ecol Manag 157:23–37
Magura T, Ködöböcz V, Tóthmérész B (2001) Effects of habitat fragmentation on carabids in forest patches. J Biogeogr 28:129–138
Magura T, Mizser S, Horváth R, Nagy DD, Tóth M, Csicsek R, Lövei GL (2021) Differences in life history traits in rural vs. urban populations of a specialist ground beetle Carabus convexus. InSects 12:540
Mann D, Gohr C, Blumröder JS, Ibisch PL (2023) Does fragmentation contribute to the forest crisis in Germany? Front for Glob Change 6:1099460
Martinez del Castillo E, Zang CS, Buras A, Hacket-Pain A, Esper J, Serrano-Notivoli R, Hartl C, Weigel R, Klesse S, Resco de Dios V, Scharnweber T, Dorado-Liñán I, van der Maaten-Theunissen M, van der Maaten E, Jump A, Mikac S, Banzragch B-E, Beck W, Cavin L, Claessens H, Čada V, Čufar K, Dulamsuren C, Gričar J, Gil-Pelegrín E, Janda P, Kazimirovic M, Kreyling J, Latte N, Leuschner C, Longares LA, Menzel A, Merela M, Motta R, Muffler L, Nola P, Petritan AM, Petritan IC, Prislan P, Rubio-Cuadrado Á, Rydval M, Stajić B, Svoboda M, Toromani E, Trotsiuk V, Wilmking M, Zlatanov T, de Luis M (2022) Climate-change-driven growth decline of European beech forests. Commun Biol 5:1–9
Meyer BF, Buras A, Rammig A, Zang CS (2020) Higher susceptibility of beech to drought in comparison to oak. Dendrochronologia 64:125780
Meyer P, Lindner M, Bauhus J, Müller J, Farwig N, Lang F, Dieter M, Endres E, Hafner A, Kätzel R, Knoke T, Kleinschmit B, Schraml U, Seeling U, Weber-Blaschke G (2023) Zum Umgang mit alten, naturnahen Laubwäldern in Deutschland im Spannungsfeld zwischen Biodiversitätsschutz, Klimaschutz und Anpassung an den Klimawandel: Stellungnahme des Wissenschaftlichen Beirates für Waldpolitik—Dezember 2023. Berichte über Landwirtschaft - Zeitschrift für Agrarpolitik und Landwirtschaft
Morecroft MD, Bealey CE, Howells O, Rennie S, Woiwod IP (2002) Effects of drought on contrasting insect and plant species in the UK in the mid-1990s. Glob Ecol Biogeogr 11:7–22
Müller J, Hothorn T, Yuan Y, Seibold S, Mitesser O, Rothacher J, Freund J, Wild C, Wolz M, Menzel A (2023) Weather explains the decline and rise of insect biomass over 34 years. Nature (in press)
Müller-Motzfeld G (2001) Laufkäfer in Wäldern Deutschlands. Angew Carabidol 2:9–20
Müller-Motzfeld G (2006) Bd. 2 Adephaga 1: Carabidae (Laufkäfer) (revised reprint of the 2 edition 2004). In: Freude H, Harde KW, Lohse GA, Klausnitzer B (eds) Die Käfer Mitteleuropas. Spektrum Akademischer Verlag, Heidelber
Nardi D, Giannone F, Marini L (2022) Short-term response of ground-dwelling arthropods to storm-related disturbances is mediated by topography and dispersal. Basic Appl Ecol 65:86–95
Nolte D, Boutaud E, Kotze DJ, Schuldt A, Assmann T (2019) Habitat specialization, distribution range size and body size drive extinction risk in carabid beetles. Biodivers Conserv 28:1267–1283
Oakes LE, Hennon PE, O’Hara KL, Dirzo R (2014) Long-term vegetation changes in a temperate forest impacted by climate change. Ecosphere 5:art135
O’Hara RB, Kotze DJ (2010) Do not log-transform count data. Methods Ecol Evol 1:118–122
Pebesma E, Graeler B (2023) gstat: spatial and spatio-temporal geostatistical modelling, prediction and simulation
Pflugmacher D, Rabe A, Peters M, Hostert P (2019) Mapping pan-European land cover using Landsat spectral-temporal metrics and the European LUCAS survey. Remote Sens Environ 221:583–595
Pozsgai G, Littlewood NA (2014) Ground beetle (Coleoptera: Carabidae) population declines and phenological changes: is there a connection? Ecol Indic 41:15–24
Pozsgai G, Baird J, Littlewood NA, Pakeman RJ, Young MR (2016) Long-term changes in ground beetle (Coleoptera: Carabidae) assemblages in Scotland. Ecol Entomol 41:157–167
Pureswaran DS, Roques A, Battisti A (2018) Forest insects and climate change. Curr for Rep 4:35–50
Qiu T, Bell AJ, Swenson JJ, Clark JS (2023) Habitat–trait interactions that control response to climate change: North American ground beetles (Carabidae). Glob Ecol Biogeogr 32:987–1001
R Core Development Team (2024) R: A language and environment for statistical computing v.4.4.0. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rainio J, Niemelä J (2003) Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers Conserv 12:487–506
Rakovec O, Samaniego L, Hari V, Markonis Y, Moravec V, Thober S, Hanel M, Kumar R (2022) The 2018–2020 multi-year drought sets a new benchmark in Europe. Earth’s Future 10:e2021EF002394
Rashid S, Wessely J, Moser D, Rumpf SB, Kühn I, Fiedler K, Hülber K, Dullinger S (2023) Threatened European butterflies concentrate in areas of strong climatic change and atmospheric deposition pressure. Biol Conserv 288:110352
Rudolf VHW (2012) Seasonal shifts in predator body size diversity and trophic interactions in size-structured predator–prey systems. J Anim Ecol 81:524–532
Rukh S, Sanders TGM, Krüger I, Schad T, Bolte A (2023) Distinct responses of European beech (Fagus sylvatica L.) to drought intensity and length—a review of the impacts of the 2003 and 2018–2019 drought events in Central Europe. Forests 14:248
Sales K, Vasudeva R, Gage MJG (2021) Fertility and mortality impacts of thermal stress from experimental heatwaves on different life stages and their recovery in a model insect. R Soc Open Sci 8:201717
Sapia M, Lövei G, Elek Z (2006) Effects of varying sampling effort on the observed diversity of carabid (Coleoptera: Carabidae) assemblages in the Danglobe Project, Denmark. Entomol Fenn 17:345–350
Saska P, van der Werf W, Hemerik L, Luff ML, Hatten TD, Honek A (2013) Temperature effects on pitfall catches of epigeal arthropods: a model and method for bias correction. J Appl Ecol 50:181–189
Schnabel F, Purrucker S, Schmitt L, Engelmann RA, Kahl A, Richter R, Seele-Dilbat C, Skiadaresis G, Wirth C (2022) Cumulative growth and stress responses to the 2018–2019 drought in a European floodplain forest. Glob Change Biol 28:1870–1883
Schowalter T (2017) Arthropod diversity and functional importance in old-growth forests of North America. Forests 8:97
Schuch S, Wesche K, Schaefer M (2012) Long-term decline in the abundance of leafhoppers and planthoppers (Auchenorrhyncha) in Central European protected dry grasslands. Biol Conserv 149:75–83
Schumacher DL, Zachariah M, Otto F (2022). High temperatures exacerbated by climate change made 2022 Northern Hemisphere droughts more likely. World Weather Attribution (in press)
Seibold S, Gossner MM, Simons NK, Blüthgen N, Müller J, Ambarlı D, Ammer C, Bauhus J, Fischer M, Habel JC, Linsenmair KE, Nauss T, Penone C, Prati D, Schall P, Schulze E-D, Vogt J, Wöllauer S, Weisser WW (2019) Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574:671–674
Seidl R, Thom D, Kautz M, Martin-Benito D, Peltoniemi M, Vacchiano G, Wild J, Ascoli D, Petr M, Honkaniemi J, Lexer MJ, Trotsiuk V, Mairota P, Svoboda M, Fabrika M, Nagel TA, Reyer CPO (2017) Forest disturbances under climate change. Nat Clim Change 7:395–402
Senf C, Seidl R (2021) Persistent impacts of the 2018 drought on forest disturbance regimes in Europe. Biogeosciences 18:5223–5230
Senf C, Pflugmacher D, Zhiqiang Y, Sebald J, Knorn J, Neumann M, Hostert P, Seidl R (2018) Canopy mortality has doubled in Europe’s temperate forests over the last three decades. Nat Commun 9:4978
Šiška B, Eliašová M, Kollár J (2020) Carabus population response to drought in lowland oak hornbeam forest. Water 12:3284
Spiecker H, Kahle H-P (2023) Climate-driven tree growth and mortality in the Black Forest, Germany—long-term observations. Glob Change Biol 29:5908–5923
Springer K, Manning P, Boesing A, Ammer C, Fiore-Donno AM, Fischer M, Goldmann K, Le Provost G, Overmann J, Ruess L, Schöning I, Seibold S, Sikorski J, Neyret M (2024) Old, deciduous stands support both high biodiversity and carbon storage in German forests. bioRxiv preprint
Staab M, Gossner MM, Simons NK, Achury R, Ambarlı D, Bae S, Schall P, Weisser WW, Blüthgen N (2023) Insect decline in forests depends on species’ traits and may be mitigated by management. Commun Biol 6:1–13
Stoklosa J, Blakey RV, Hui FKC (2022) An overview of modern applications of negative binomial modelling in ecology and biodiversity. Diversity 14:320
Šustek Z, Vido J, Škvareninová J, Škvarenina J, Šurda P (2017) Drought impact on ground beetle assemblages (Coleoptera, Carabidae) in Norway spruce forests with different management after windstorm damage—a case study from Tatra Mts. (Slovakia). J Hydrol Hydromech 65:333–342
Szyszko J (1983) Methods of macrofauna investigations. In: Szyszko J (ed) The process of forest soil macrofauna formation after afforestation of farmland. Agricultural University Press, Warsaw, pp 10–16
Treydte K, Liu L, Padrón RS, Martínez-Sancho E, Loader NJ et al (2023) Recent human-induced atmospheric drying across Europe unprecedented in the last 400 years. Nat Geosci 17:58–65
Tseng M, Kaur KM, Pari SS, Sarai K, Chan D, Yao CH, Porto P, Toor A, Toor HS, Fograscher K (2018) Decreases in beetle body size linked to climate change and warming temperatures. J Anim Ecol 87:647–659
Tyszecka K, Zając K, Kadej M (2023) Habitat preferences of the mountain population of the endangered beetle Carabus variolosus ssp. variolosus indicate its vulnerability to climate change. GlobEcol Conserv 46:e02601
van Klink R, Bowler DE, Gongalsky KB, Swengel AB, Gentile A, Chase JM (2020) Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368:417–420
Vicente-Serrano SM, Beguería S, López-Moreno JI (2010) A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J Clim 23:1696–1718
Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D (2021) Insect decline in the anthropocene: death by a thousand cuts. PNAS 118:e2023989118
Warren R, Price J, VanDerWal J, Cornelius S, Sohl H (2018) The implications of the United Nations Paris Agreement on climate change for globally significant biodiversity areas. Clim Change 147:395–409
Weigel R, Bat-Enerel B, Dulamsuren C, Muffler L, Weithmann G, Leuschner C (2023) Summer drought exposure, stand structure, and soil properties jointly control the growth of European beech along a steep precipitation gradient in northern Germany. Glob Change Biol 29:763–779
Weiss F, Linde A (2022) How to estimate carabid biomass?—an evaluation of size-weight models for ground beetles (Coleoptera: Carabidae) and perspectives for further improvement. J Insect Conserv 26:537–548
Weiss F, Von Wehrden H, Linde A (2024) Long-term drought triggers severe declines in carabid beetles in a temperate forest. Ecography (in press)
Weisser W, Blüthgen N, Staab M, Achury R, Müller J (2023) Experiments are needed to quantify the main causes of insect decline. Biol Lett 19:20220500
Welti EAR, Zajicek P, Frenzel M, Ayasse M, Bornholdt T, Buse J, Classen A, Dziock F, Engelmann RA, Englmeier J, Fellendorf M, Förschler MI, Fricke U, Ganuza C, Hippke M, Hoenselaar G, Kaus-Thiel A, Kerner J, Kilian D, Mandery K, Marten A, Monaghan MT, Morkel C, Müller J, Puffpaff S, Redlich S, Richter R, Rojas-Botero S, Scharnweber T, Scheiffarth G, Yáñez PS, Schumann R, Seibold S, Steffan-Dewenter I, Stoll S, Tobisch C, Twietmeyer S, Uhler J, Vogt J, Weis D, Weisser WW, Wilmking M, Haase P (2022) Temperature drives variation in flying insect biomass across a German malaise trap network. Insect Conserv Divers 15:168–180
Williams RS, Marbert BS, Fisk MC, Hanson PJ (2014) Ground-dwelling beetle responses to long-term precipitation alterations in a hardwood forest. Southeast Nat 13:138–155
Winter S (2005) Ermittlung von Struktur-Indikatoren zur Abschätzung des Einflusses forstlicher Bewirtschaftung auf die Biozönosen von Tiefland-Buchenwäldern
Winter S, Begehold H, Herrmann M, Lüderitz M, Möller G, Rzanny M, Flade M (2020) Best practice handbook-nature conservation in beech forests used for timber: nature conservation objectives and management recommendations for mature beech forests in north-eastern Germany. Ministry of Agriculture, Environment and Climate Protection of the Federal State of Brandenburg
Wise DH, Lensing JR (2019) Impacts of rainfall extremes predicted by climate-change models on major trophic groups in the leaf litter arthropod community. J Anim Ecol 88:1486–1497
Zachariah M, Philip S, Pinto I, Vahlberg M, Singh R, Otto F, Barnes C, Kimutai J (2023) Extreme heat in North America, Europe and China in July 2023 made much more likely by climate change. Grantham Institute for Climate Change. 10044/1/105549
Zhao Z-H, Shi P-J, Hui C, Ouyang F, Ge F, Li B-L (2013) Solving the pitfalls of pitfall trapping: a two-circle method for density estimation of ground-dwelling arthropods. Methods Ecol Evol 4:865–871
Acknowledgements
Benjamin Herold and Martin Flade of Schorfheide-Chorin Biosphere Reserve contributed to developing early ideas of this study. Jenny Hammerich, Uli Gräbener, Pierre Ibisch, and Vera Luthardt helped acquiring funding for F.W.'s work and the data collection in 2020-2022. Benjamin Herold, Martin Flade, Gundula Wernicke, Matthias Schwabe and Silke Oldorff of Naturpark Ruppiner Land supported the process of obtaining research and access permits. Gundula Wernicke and Matthias Schwabe of Müritz National Park supported fieldwork at Müritz National Park. Naturwacht Barnim supported fieldwork at Schorfheide-Chorin Biosphere Reserve. Johanna Herzog, Janosch Becker, Andrea Brini, Jakob Weinert, Rebecca Arnrich, Nancy Müller, Jakob Jilg, Florian Kukulka and Kai Conrad assisted with field- and labwork. Henrik von Wehrden, Paula Casanovas and Alba Cervantes Loreto provided comments on statistical methods. Xavier Pochon provided office space for F.W.'s work. We would like to thank two anonymous reviewers for their constructive reviews and helpful comments.
Funding
Open Access funding enabled and organized by Projekt DEAL. Data collection in 1999–2001 and the work of S.W. was funded through a ‘Research and Development Project’ by the Federal Agency for Nature Conservation (BfN). Data collection in 2020–2022 and the work of F.W. was funded through the Biosphere Reserves Institute and the Innovation and Career Center “ProBio-LaB” by the Ministry of Science, Research and Culture of the federal state of Brandenburg (MWFK). Open Access funding was enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
F.W., S.W. and A.L. conceived the research. F.W., S.W., D.P. and T.K. collected the data. F.W., S.W. and D.P. curated, processed and validated the data. F.W., S.W., D.P. and T.K. developed and refined the methodology. F.W. conducted all statistical analysis and created all figures. S.W. acquired funding in 1999–2001, A.L. and F.W. acquired funding in 2020–2022. S.W. administrated the project in 1999–2001. F.W. administrated the project in 2020–2022. A.L. supervised the research and provided resources. F.W. wrote the initial manuscript draft and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Insect research should always carefully consider the use of lethal methods (see Lövei et al. 2023). For reasons of quantitative comparability, this study, however, relied on lethal sampling methods. All carabid species are protected under German law and some of the study sites have limited accessibility due to their conservation status. We sampled Carabid beetles and accessed sites under special permits of the environmental agency of Mecklenburg West-Pomerania (permit 661.34.4.5.7-10/20), the environmental agency of Brandenburg (permit SNN1-FF 31.03.20), and the Müritz National Park administration. Bycatch was minimized, where possible, and kept for educational purposes.
Competing interests
The authors declare to not have any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weiss, F., Winter, S., Pflugmacher, D. et al. Evidence for regional-scale declines in carabid beetles in old lowland beech forests following a period of severe drought. Landsc Ecol 39, 123 (2024). https://doi.org/10.1007/s10980-024-01920-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10980-024-01920-1