Abstract
Gaps in our understanding of muscle mechanics demonstrate that the current model is incomplete. Increasingly, it appears that a role for titin in active muscle contraction might help to fill these gaps. While such a role for titin is increasingly accepted, the underlying molecular mechanisms remain unclear. The goals of this paper are to review recent studies demonstrating Ca2+-dependent interactions between N2A titin and actin in vitro, to explore theoretical predictions of muscle behavior based on this interaction, and to review experimental data related to the predictions. In a recent study, we demonstrated that Ca2+ increases the association constant between N2A titin and F-actin; that Ca2+ increases rupture forces between N2A titin and F-actin; and that Ca2+ and N2A titin reduce sliding velocity of F-actin and reconstituted thin filaments in motility assays. Preliminary data support a role for Ig83, but other Ig domains in the N2A region may also be involved. Two mechanical consequences are inescapable if N2A titin binds to thin filaments in active muscle sarcomeres: (1) the length of titin’s freely extensible I-band should decrease upon muscle activation; and (2) binding between N2A titin and thin filaments should increase titin stiffness in active muscle. Experimental observations demonstrate that these properties characterize wild type muscles, but not muscles from mdm mice with a small deletion in N2A titin, including part of Ig83. Given the new in vitro evidence for Ca2+-dependent binding between N2A titin and actin, it is time for skepticism to give way to further investigation.

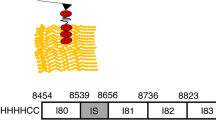
Reproduced with permission from Nishikawa (2016). Copyright 2016, The Company of Biologists Ltd

Reproduced with permission from Dutta et al. (2018)

Reproduced with permission from Dutta et al. (2018)

Reproduced with permission from Dutta et al. (2018)


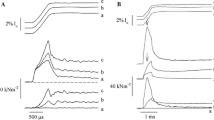
Reproduced with permission from Powers et al. (2016). Copyright 2016, The Company of Biologists Ltd

Reproduced with permission from Nishikawa (2016). Copyright 2016, The Company of Biologists Ltd

Reproduced with permission from Monroy et al. (2017). Copyright 2017, The Company of Biologists Ltd

Similar content being viewed by others
References
Abbott BC, Aubert XM (1952) The force exerted by active striated muscle during and after change of length. J Physiol 117:77–86
Ahn AN, Monti RJ, Biewener AA (2003) In vivo and in vitro heterogeneity of segment length changes in the semimembranosus muscle of the toad. J Physiol 549:877–888. https://doi.org/10.1113/jphysiol.2002.038018
Allen DG, Kentish JC (1985) The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol 17:821–840
Baguet F, Gillis JM (1968) Energy cost of tonic contraction in a lamellibranch catch muscle. J Physiol 198:127–143. https://doi.org/10.1113/jphysiol.1968.sp008597
Bianco P, Nagy A, Kengyel A, Szatmari D, Martonfalvi Z, Huber T, Kellermayer MS (2007) Interaction forces between F-actin and titin PEVK domain measured with optical tweezers. Biophys J 93:2102–2109. https://doi.org/10.1529/biophysj.107.106153
Bigland-Ritchie B, Woods JJ (1976) Integrated electromyogram and oxygen uptake during positive and negative work. J Physiol 260:267–277. https://doi.org/10.1113/jphysiol.1976.sp011515
Brynnel A et al (2018) Downsizing the molecular spring of the giant protein titin reveals that skeletal muscle titin determines passive stiffness and drives longitudinal hypertrophy. Elife 7:150. https://doi.org/10.7554/eLife.40532
Butler TM, Siegman MJ (2010) Mechanism of catch force: tethering of thick and thin filaments by twitchin. J Biomed Biotechnol 2010:725207. https://doi.org/10.1155/2010/725207
Butler TM, Mooers SU, Li C, Narayan S, Siegman MJ (1998) Regulation of catch muscle by twitchin phosphorylation: effects on force, ATPase, and shortening. Biophys J 75:1904–1914. https://doi.org/10.1016/S0006-3495(98)77631-3
Butler TM, Mooers SU, Narayan SR, Siegman MJ (2010) The N-terminal region of twitchin binds thick and thin contractile filaments: redundant mechanisms of catch force maintenance. J Biol Chem 285:40654–40665. https://doi.org/10.1074/jbc.M110.166041
Chow JW, Darling WG (1999) The maximum shortening velocity of muscle should be scaled with activation. J Appl Physiol 86:1025–1031. https://doi.org/10.1152/jappl.1999.86.3.1025
Cornachione AS, Leite F, Bagni MA, Rassier DE (2016) The increase in non-cross-bridge forces after stretch of activated striated muscle is related to titin isoforms. Am J Physiol Cell Physiol 310:C19–C26. https://doi.org/10.1152/ajpcell.00156.2015
Dick TJM, Biewener AA, Wakeling JM (2017) Comparison of human gastrocnemius forces predicted by Hill-type muscle models and estimated from ultrasound images. J Exp Biol 220:1643–1653. https://doi.org/10.1242/jeb.154807
Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S (2000) How animals move: an integrative view. Science 288:100–106
Dos Remedios C, Gilmour D (2017) An historical perspective of the discovery of titin filaments. Biophys Rev 9:179–188. https://doi.org/10.1007/s12551-017-0269-3
Dutta S et al (2018) Calcium increases titin N2A binding to F-actin and regulated thin filaments. Sci Rep 8:14575. https://doi.org/10.1038/s41598-018-32952-8
DuVall M (2015) Titin regulation of active and passive force in skeletal muscle. Ph.D. Dissertation, University of Calgary, Canada
DuVall MM, Jinha A, Schappacher-Tilp G, Leonard TR, Herzog W (2017) Differences in titin segmental elongation between passive and active stretch in skeletal muscle. J Exp Biol 220:4418–4425. https://doi.org/10.1242/jeb.160762
Edman KA (1978) Maximum velocity of shortening in relation to sarcomere length and degree of activation of frog muscle fibres. J Physiol 278:9P–10P
Fenn WO (1923) A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J Physiol 58:175–203. https://doi.org/10.1113/jphysiol.1923.sp002115
Freiburg A, Trombitas K, Hell W, Cazorla O, Fougerousse F, Centner T, Kolmerer B, Witt C, Beckmann JS, Gregorio CC, Granzier H, Labeit S (2000) Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res 86:1114–1121
Freundt JK, Linke WA (2018) Titin as a force generating muscle protein under regulatory control. J Appl Physiol 126(5):1474–1482. https://doi.org/10.1152/japplphysiol.00865.2018
Funabara D, Kanoh S, Siegman MJ, Butler TM, Hartshorne DJ, Watabe S (2005) Twitchin as a regulator of catch contraction in molluscan smooth muscle. J Muscle Res Cell Motil 26:455–460. https://doi.org/10.1007/s10974-005-9029-2
Furst DO, Osborn M, Nave R, Weber K (1988) The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol 106:1563–1572
Garvey SM, Rajan C, Lerner AP, Frankel WN, Cox GA (2002) The muscular dystrophy with myositis (mdm) mouse mutation disrupts a skeletal muscle-specific domain of titin. Genomics 79:146–149. https://doi.org/10.1006/geno.2002.6685
Gautel M, Djinovic-Carugo K (2016) The sarcomeric cytoskeleton: from molecules to motion. J Exp Biol 219:135–145. https://doi.org/10.1242/jeb.124941
George NT, Sponberg S, Daniel TL (2012) Temperature gradients drive mechanical energy gradients in the flight muscle of Manduca sexta. J Exp Biol 215:471–479. https://doi.org/10.1242/jeb.062901
Gilliver SF, Jones DA, Rittweger J, Degens H (2011) Variation in the determinants of power of chemically skinned type I rat soleus muscle fibres. J Comp Physiol A 197:311–319. https://doi.org/10.1007/s00359-010-0613-6
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184:170–192
Granzier HL, Labeit S (2004) The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res 94:284–295. https://doi.org/10.1161/01.RES.0000117769.88862.F8
Gregorio CC et al (1998) The NH2 terminus of titin spans the Z-disc: its interaction with a novel 19-kD ligand (T-cap) is required for sarcomeric integrity. J Cell Biol 143:1013–1027
Gregorio CC, Granzier H, Sorimachi H, Labeit S (1999) Muscle assembly: a titanic achievement. Curr Opin Cell Biol 11:18–25
Hanson J (1968) Recent x-ray diffraction studies of muscle. Q Rev Biophys 1:177–216
Hanson J, Huxley HE (1953) Structural basis of the cross-striations in muscle. Nature 172:530–532
Herzog W (2017) Skeletal muscle mechanics: questions, problems and possible solutions. J Neuroeng Rehabil 14:98. https://doi.org/10.1186/s12984-017-0310-6
Herzog W, Schachar R, Leonard TR (2003) Characterization of the passive component of force enhancement following active stretching of skeletal muscle. J Exp Biol 206:3635–3643
Hessel AL, Nishikawa KC (2017) Effects of a titin mutation on negative work during stretch-shortening cycles in skeletal muscles. J Exp Biol 220:4177–4185. https://doi.org/10.1242/jeb.163204
Hill AV (1922) The maximum work and mechanical efficiency of human muscles, and their most economical speed. J Physiol 56:19–41. https://doi.org/10.1113/jphysiol.1922.sp001989
Hitchcock-DeGregori SE, Irving TC, Hugh E (2014) Huxley: the compleat biophysicist. Biophys J 107:1493–1501. https://doi.org/10.1016/j.bpj.2014.07.069
Hooper SL, Hobbs KH, Thuma JB (2008) Invertebrate muscles: thin and thick filament structure; molecular basis of contraction and its regulation, catch and asynchronous muscle. Prog Neurobiol 86:72–127. https://doi.org/10.1016/j.pneurobio.2008.06.004
Horowits R, Podolsky RJ (1987) The positional stability of thick filaments in activated skeletal muscle depends on sarcomere length: evidence for the role of titin filaments. J Cell Biol 105:2217–2223
Horowits R, Kempner ES, Bisher ME, Podolsky RJ (1986) A physiological role for titin and nebulin in skeletal muscle. Nature 323:160–164. https://doi.org/10.1038/323160a0
Houmeida A, Holt J, Tskhovrebova L, Trinick J (1995) Studies of the interaction between titin and myosin. J Cell Biol 131:1471–1481
Hoyle G (1967) Diversity of striated muscle. Am Zool 7:435–449
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
Huxley HE (1964) Structural arrangements and the contraction mechanism in striated muscle. Proc R Soc Lond B 160:442–448. https://doi.org/10.1098/rspb.1964.0054
Huxley AF (1973) A note suggesting that the cross-bridge attachment during muscle contraction may take place in two stages. Proc R Soc Lond B 183:83–86. https://doi.org/10.1098/rspb.1973.0006
Huxley H, Hanson J (1954) Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature 173:973–976
Huxley AF, Simmons RM (1971a) Mechanical properties of the cross-bridges of frog striated muscle. J Physiol 218(Suppl):59P–60P
Huxley AF, Simmons RM (1971b) Proposed mechanism of force generation in striated muscle. Nature 233:533–538
Irving M (2017) Regulation of contraction by the thick filaments in skeletal muscle. Biophys J 113:2579–2594. https://doi.org/10.1016/j.bpj.2017.09.037
Irving T, Wu Y, Bekyarova T, Farman GP, Fukuda N, Granzier H (2011) Thick-filament strain and interfilament spacing in passive muscle: effect of titin-based passive tension. Biophys J 100:1499–1508. https://doi.org/10.1016/j.bpj.2011.01.059
Jewell BR (1959) The nature of the phasic and the tonic responses of the anterior byssal retractor muscle of Mytilus. J Physiol 149:154–177. https://doi.org/10.1113/jphysiol.1959.sp006332
Jin JP (1995) Cloned rat cardiac titin class I and class II motifs: expression, purification, characterization, and interaction with F-actin. J Biol Chem 270:6908–6916
Jin JP (2000) Titin-thin filament interaction and potential role in muscle function. Adv Exp Med Biol 481:319–333 discussion 334–315
Josephson RK (1985) Mechanical power output from striated muscle during cyclic contraction. J Exp Biol 114:493–512
Kellermayer MS, Granzier HL (1996) Calcium-dependent inhibition of in vitro thin-filament motility by native titin. FEBS Lett 380:281–286
Kenny PA, Liston EM, Higgins DG (1999) Molecular evolution of immunoglobulin and fibronectin domains in titin and related muscle proteins. Gene 232:11–23
Konhilas JP, Irving TC, de Tombe PP (2002) Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing. Circ Res 90:59–65
Kulke M et al (2001) Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ Res 89:874–881
Labeit S, Kolmerer B (1995) Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science 270:293–296
Labeit S, Gautel M, Lakey A, Trinick J (1992) Towards a molecular understanding of titin. EMBO J 11:1711–1716
Labeit D et al (2003) Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci USA 100:13716–13721. https://doi.org/10.1073/pnas.2235652100
Lappin AK, Monroy JA, Pilarski JQ, Zepnewski ED, Pierotti DJ, Nishikawa KC (2006) Storage and recovery of elastic potential energy powers ballistic prey capture in toads. J Exp Biol 209:2535–2553. https://doi.org/10.1242/jeb.02276
Lee HD, Herzog W, Leonard T (2001) Effects of cyclic changes in muscle length on force production in in situ cat soleus. J Biomech 34:979–987
Lee SS, Arnold AS, Miara Mde B, Biewener AA, Wakeling JM (2013) Accuracy of gastrocnemius muscles forces in walking and running goats predicted by one-element and two-element Hill-type models. J Biomech 46:2288–2295. https://doi.org/10.1016/j.jbiomech.2013.06.001
Leonard TR, Herzog W (2010) Regulation of muscle force in the absence of actin-myosin-based cross-bridge interaction. Am J Physiol Cell Physiol 299:C14–C20. https://doi.org/10.1152/ajpcell.00049.2010
Li Q, Jin JP, Granzier HL (1995) The effect of genetically expressed cardiac titin fragments on in vitro actin motility. Biophys J. 69:1508–1518. https://doi.org/10.1016/S0006-3495(95)80021-4
Li Y, Lang P, Linke WA (2016) Titin stiffness modifies the force-generating region of muscle sarcomeres. Sci Rep 6:24492. https://doi.org/10.1038/srep24492
Li Y, Unger A, von Frieling-Salewsky M, Rivas Pardo JA, Fernandez JM, Linke WA (2018) Quantifying the titin contribution to muscle force generation using a novel method to specifically cleave the titin springs in situ. Biophys J 114:645a. https://doi.org/10.1016/j.bpj.2017.11.3480
Linari M, Woledge RC, Curtin NA (2003) Energy storage during stretch of active single fibres from frog skeletal muscle. J Physiol 548:461–474. https://doi.org/10.1113/jphysiol.2002.032185
Lindstedt S, Nishikawa K (2017) Huxleys’ missing filament: form and function of titin in vertebrate striated muscle. Ann Rev Physiol 79:145–166. https://doi.org/10.1146/annurev-physiol-022516-034152
Linke WA, Ivemeyer M, Olivieri N, Kolmerer B, Ruegg JC, Labeit S (1996) Towards a molecular understanding of the elasticity of titin. J Mol Biol 261:62–71
Linke WA (2018) Titin gene and protein functions in passive and active muscle. Annu Rev Physiol 80:389–411. https://doi.org/10.1146/annurev-physiol-021317-121234
Linke WA, Ivemeyer M, Labeit S, Hinssen H, Ruegg JC, Gautel M (1997) Actin-titin interaction in cardiac myofibrils: probing a physiological role. Biophys J 73:905–919. https://doi.org/10.1016/S0006-3495(97)78123-2
Linke WA, Ivemeyer M, Mundel P, Stockmeier MR, Kolmerer B (1998a) Nature of PEVK-titin elasticity in skeletal muscle. Proc Natl Acad Sci USA 95:8052–8057
Linke WA, Stockmeier MR, Ivemeyer M, Hosser H, Mundel P (1998b) Characterizing titin’s I-band Ig domain region as an entropic spring. J Cell Sci 111:1567–1574
Linke WA, Rudy DE, Centner T, Gautel M, Witt C, Labeit S, Gregorio CC (1999) I-band titin in cardiac muscle is a three-element molecular spring and is critical for maintaining thin filament structure. J Cell Biol 146:631–644
Linke WA et al (2002) PEVK domain of titin: an entropic spring with actin-binding properties. J Struct Biol 137:194–205. https://doi.org/10.1006/jsbi.2002.4468
Locker RH, Leet NG (1975) Histology of highly-stretched beef muscle. I. The fine structure of grossly stretched single fibers. J Ultrastruct Res 52:64–75
Lombardi V, Piazzesi G (1990) The contractile response during steady lengthening of stimulated frog muscle fibres. J Physiol 431:141–171
Lombardi V, Piazzesi G, Ferenczi MA, Thirlwell H, Dobbie I, Irving M (1995) Elastic distortion of myosin heads and repriming of the working stroke in muscle. Nature 374:553–555. https://doi.org/10.1038/374553a0
Ma W, Gong H, Irving T (2018a) Myosin head configurations in resting and contracting murine skeletal muscle. Int J Mol Sci 19:2643. https://doi.org/10.3390/ijms19092643
Ma W, Gong H, Kiss B, Lee EJ, Granzier H, Irving T (2018b) Thick-filament extensibility in intact skeletal muscle. Biophys J 115:1580–1588. https://doi.org/10.1016/j.bpj.2018.08.038
Maruyama K (1976) Connectin, an elastic protein from myofibrils. J Biochem 80:405–407
Maruyama K (1995) Birth of the sliding filament concept in muscle contraction. J Biochem 117:1–6
Maruyama K, Natori R, Nonomura Y (1976) New elastic protein from muscle. Nature 262:58–60
Maruyama K, Hu DH, Suzuki T, Kimura S (1987) Binding of actin filaments to connectin. J Biochem 101:1339–1346
Mijailovich SM, Stojanovic B, Nedic D, Svicevic M, Geeves MA, Irving TC, Granzier HL (2019) Nebulin and titin modulate cross-bridge cycling and length-dependent calcium sensitivity. J Gen Physiol 151:680–704. https://doi.org/10.1085/jgp.201812165
Monroy JA, Powers KL, Pace CM, Uyeno T, Nishikawa KC (2017) Effects of activation on the elastic properties of intact soleus muscles with a deletion in titin. J Exp Biol 220:828–836. https://doi.org/10.1242/jeb.139717
Murayama T, Nakauchi Y, Kimura S, Maruyama K (1989) Binding of connectin to myosin filaments. J Biochem 105:323–326
Nagy A, Cacciafesta P, Grama L, Kengyel A, Malnasi-Csizmadia A, Kellermayer MS (2004) Differential actin binding along the PEVK domain of skeletal muscle titin. J Cell Sci 117:5781–5789. https://doi.org/10.1242/jcs.01501
Neagoe C, Opitz CA, Makarenko I, Linke WA (2003) Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness. J Muscle Res Cell Motil 24:175–189
Nishikawa K (2016) Eccentric contraction: unraveling mechanisms of force enhancement and energy conservation. J Exp Biol 219:189–196. https://doi.org/10.1242/jeb.124057
Nishikawa KC, Monroy JA, Uyeno TE, Yeo SH, Pai DK, Lindstedt SL (2012) Is titin a ‘winding filament’? A new twist on muscle contraction. Proc Biol Sci 279:981–990. https://doi.org/10.1098/rspb.2011.1304
Nishikawa K, Lindstedt SL, LaStayo PC (2018a) Basic science and clinical use of eccentric contractions: history and uncertainties. J Sport Health Sci 7:265–274
Nishikawa K, Tahir U, Monroy JA (2018b) Muscle function from organisms to molecules. Int Comp Biol 58:194–206
Obermann WM, Gautel M, Steiner F, van der Ven PF, Weber K, Furst DO (1996) The structure of the sarcomeric M band: localization of defined domains of myomesin, M-protein, and the 250-kD carboxy-terminal region of titin by immunoelectron microscopy. J Cell Biol 134:1441–1453
Ortega JO, Lindstedt SL, Nelson FE, Jubrias SA, Kushmerick MJ, Conley KE (2015) Muscle force, work and cost: a novel technique to revisit the Fenn effect. J Exp Biol 218:2075–2082. https://doi.org/10.1242/jeb.114512
Pfuhl M, Gautel M (2012) Structure, interactions and function of the N-terminus of cardiac myosin binding protein C (MyBP-C): who does what, with what, and to whom? J Muscle Res Cell Motil 33:83–94. https://doi.org/10.1007/s10974-012-9291-z
Piazzesi G, Lombardi V (1995) A cross-bridge model that is able to explain mechanical and energetic properties of shortening muscle. Biophys J 68:1966–1979. https://doi.org/10.1016/S0006-3495(95)80374-7
Powers K, Schappacher-Tilp G, Jinha A, Leonard T, Nishikawa K, Herzog W (2014) Titin force is enhanced in actively stretched skeletal muscle. J Exp Biol 217:3629–3636. https://doi.org/10.1242/jeb.105361
Powers K, Nishikawa K, Joumaa V, Herzog W (2016) Decreased force enhancement in skeletal muscle sarcomeres with a deletion in titin. J Exp Biol 219:1311–1316. https://doi.org/10.1242/jeb.132027
Rassier DE (2017) Sarcomere mechanics in striated muscles: from molecules to sarcomeres to cells. Am J Physiol Cell Physiol 313:C134–C145. https://doi.org/10.1152/ajpcell.00050.2017
Rivas-Pardo JA, Eckels EC, Popa I, Kosuri P, Linke WA, Fernandez JM (2016) Work done by titin protein folding assists muscle contraction. Cell Rep 14:1339–1347. https://doi.org/10.1016/j.celrep.2016.01.025
Romano M, Cifelli RL (2015) Plate tectonics: continental-drift opus turns 100. Nature 526:43. https://doi.org/10.1038/526043e
Sanger JW, Sanger JM (2001) Fishing out proteins that bind to titin. J Cell Biol 154:21–24
Schappacher-Tilp G, Leonard T, Desch G, Herzog W (2015) A novel three-filament model of force generation in eccentric contraction of skeletal muscles. PLoS ONE 10:e0117634. https://doi.org/10.1371/journal.pone.0117634
Shelud’ko NS, Matusovskaya GG, Permyakova TV, Matusovsky OS (2004) Twitchin, a thick-filament protein from molluscan catch muscle, interacts with F-actin in a phosphorylation-dependent way. Arch Biochem Biophys 432:269–277. https://doi.org/10.1016/j.abb.2004.10.006
Shimamoto Y, Suzuki M, Mikhailenko SV, Yasuda K, Ishiwata S (2009) Inter-sarcomere coordination in muscle revealed through individual sarcomere response to quick stretch. Proc Nat Acad Sci USA 106:11954–11959
Sjostrand FS (1962) The connections between A- and I-band filaments in striated frog muscle. J Ultrastruct Res 7:225–246
Soteriou A, Clarke A, Martin S, Trinick J (1993) Titin folding energy and elasticity. Proc Biol Sci 254:83–86. https://doi.org/10.1098/rspb.1993.0130
Sugi H, Suzuki S (1978) Ultrastructural and physiological studies on the longitudinal body wall muscle of Dolabella auricularia. I. Mechanical response and ultrastructure. J Cell Biol 79:454–466
Sugi H, Akimoto T, Kobayashi T, Suzuki S, Shimada M (2000) Possible contribution of titin filaments to the compliant series elastic component in horseshoe crab skeletal muscle fibers. Adv Exp Med Biol 481:371–380 discussion 381–372
Tahir U, Monroy JA, Rice NA, Nishikawa K (2019) Effects of a titin mutation on force enhancement and force depression in mouse soleus muscles. J Exp Biol
Takahashi M, Sohma H, Morita F (1988) The steady state intermediate of scallop smooth muscle myosin ATPase and effect of light chain phosphorylation. A molecular mechanism for catch contraction. J Biochem 104:102–107
Tameyasu T, Sugi H (1976) The series elastic component and the force-velocity relation in the anterior byssal retractor muscle of Mytilus edulis during active and catch contractions. J Exp Biol 64:497–510
Tatsumi R, Maeda K, Hattori A, Takahashi K (2001) Calcium binding to an elastic portion of connectin/titin filaments. J Muscle Res Cell Motil 22:149–162
Trinick J (1996) Interaction of titin/connectin with the thick filament. Adv Biophys 33:81–90
Trombitas K, Granzier H (1997) Actin removal from cardiac myocytes shows that near Z line titin attaches to actin while under tension. Am J Physiol 273:C662–C670
von Castelmur E et al (2008) A regular pattern of Ig super-motifs defines segmental flexibility as the elastic mechanism of the titin chain. Proc Natl Acad Sci USA 105:1186–1191. https://doi.org/10.1073/pnas.0707163105
Wakabayashi K, Sugimoto Y, Tanaka H, Ueno Y, Takezawa Y, Amemiya Y (1994) X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J 67:2422–2435. https://doi.org/10.1016/S0006-3495(94)80729-5
Wang K, McClure J, Tu A (1979) Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci USA 76:3698–3702
Yamada A, Yoshio M, Kojima H, Oiwa K (2001) An in vitro assay reveals essential protein components for the “catch” state of invertebrate smooth muscle. Proc Natl Acad Sci USA 98:6635–6640. https://doi.org/10.1073/pnas.111585098
Yamasaki R et al (2001) Titin-actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/S100A1. Biophys J 81:2297–2313. https://doi.org/10.1016/S0006-3495(01)75876-6
Yarom R, Meiri U (1971) N lines in striated muscle: a site of intracellular Ca2+. Nat New Biol 234:254–256
Zhukarev V, Sanger JM, Sanger JW, Goldman YE, Shuman H (1997) Distribution and orientation of rhodamine-phalloidin bound to thin filaments in skeletal and cardiac myofibrils. Cell Motil Cytoskelet 37:363–377. https://doi.org/10.1002/(SICI)1097-0169(1997)37:4%3c363:AID-CM7%3e3.0.CO;2-5
Acknowledgements
We thank Stan Lindstedt for helpful comments on earlier versions of this manuscript. This work was supported by the National Science Foundation [IOS-0732949, IOS-1025806, IOS-1456868], the W. M. Keck Foundation, and the Technology Research Initiative Fund of Northern Arizona University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishikawa, K., Dutta, S., DuVall, M. et al. Calcium-dependent titin–thin filament interactions in muscle: observations and theory. J Muscle Res Cell Motil 41, 125–139 (2020). https://doi.org/10.1007/s10974-019-09540-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-019-09540-y