Abstract
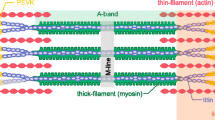
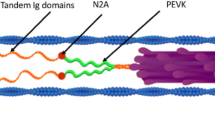
When skeletal muscles are stretched during activation in the absence of myosin-actin interactions, the force increases significantly. The force remains elevated throughout the activation period. The mechanism behind this non-crossbridge force, referred to as static tension, is unknown and generates debate in the literature. It has been suggested that the static tension is caused by Ca2+-induced changes in the properties of titin molecules that happens during activation and stretch, but a comprehensive evaluation of such possibility is still lacking. This paper reviews the general characteristics of the static tension, and evaluates the proposed mechanism by which titin may change the force upon stretch. Evidence is presented suggesting that an increase in intracellular Ca2+ concentration leads to Ca2+ binding to the PEVK region of titin. Such binding increases titin stiffness, which increases the overall sarcomere stiffness and causes the static tension. If this form of Ca2+-induced increase in titin stiffness is confirmed in future studies, it may have large implications for understating of the basic mechanisms of muscle contraction.




Similar content being viewed by others
References
Abbott BC, Aubert X (1952) The force exerted by active striated muscle during and after change of length. J Physiol 117(1):77–86
Bagni MA, Cecchi G, Schoenberg M (1988) A model of force production that explains the lag between crossbridge attachment and force after electrical stimulation of striated muscle fibers. Biophys J 54(6):1105–1114
Bagni MA, Cecchi G, Colomo F, Garzella P (1992) Are weakly binding bridges present in resting intact muscle fibers? Biophys J 63(5):1412–1415
Bagni MA, Cecchi G, Colomo F, Garzella P (1994) Development of stiffness precedes cross-bridge attachment during the early tension rise in single frog muscle fibres. J Physiol 481:273–278
Bagni MA, Cecchi G, Colombini B, Colomo F (2002) A non-cross-bridge stiffness in activated frog muscle fibers. Biophys J 82(6):3118–3127
Bagni MA, Colombini B, Geiger P, Berlinguer PR, Cecchi G (2004) Non-cross-bridge calcium-dependent stiffness in frog muscle fibers. Am J Physiol Cell Physiol 286(6):C1353–C1357
Bagni MA, Colombini B, Colomo F, Palmini RB, Cecchi G (2005) Non cross-bridge stiffness in skeletal muscle fibres at rest and during activity. Adv Exp Med Biol 565:141–154
Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S (2001) The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res 89(11):1065–1072
Baylor SM, Hollingworth S (2003) Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibers of mouse muscle. J Physiol 551(1):125–138
Brenner B, Schoenberg M, Chalovich JM, Greene LE, Eisenberg E (1982) Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci USA 79(23):7288–7291
Campbell KS, Moss RL (2002) History-dependent mechanical properties of permeabilized rat soleus muscle fibers. Biophys J 82(2):929–943
Cecchi G, Griffiths PJ, Taylor S (1982) Muscular contraction: kinetics of crossbridge attachment studied by high-frequency stiffness measurements. Science 217(4554):70–72
Cecchi G, Griffiths PJ, Bagni MA, Ashley CC, Maeda Y (1991) Time-resolved changes in equatorial X-ray diffraction and stiffness during rise of tetanic tension in intact length-clamped single muscle fibers. Biophys J 59(6):1273–1283
Chalovich JM, Chock PB, Eisenberg E (1981) Mechanism of action of troponin. tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J Biol Chem 256(2):575–578
Colombini B, Benelli G, Nocella M, Musaro A, Cecchi G, Bagni MA (2009) Mechanical properties of intact single fibres from wild-type and MLC/mIgf-1 transgenic mouse muscle. J Muscle Res Cell Motil 30:199–207
Cornachione AS, Rassier DE (2012) A non-cross-bridge, static tension is present in permeabilized skeletal muscle fibers after active force inhibition or actin extraction. Am J Physiol Cell Physiol 302(3):C566–C574
Edman KA (2012) Residual force enhancement after stretch in striated muscle. A consequence of increased myofilament overlap? J Physiol 590(Pt 6):1339–1345
Ford LE, Huxley AF, Simmons RM (1981) The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol 311:219–249
Fryer MW, Gage PW, Neering IR, Dulhunty AF, Lamb GD (1988) Paralysis of skeletal muscle by butanedione monoxime, a chemical phosphatase. Pflugers Arch 411(1):76–79
Fujita H, Labeit D, Gerull B, Labeit S, Granzier HL (2004) Titin isoform-dependent effect of calcium on passive myocardial tension. Am J Physiol Heart Circ Physiol 287(6):H2528–H2534
Horowits R, Podolsky RJ (1987) The positional stability of thick filaments in activated skeletal muscle depends on sarcomere length: evidence for the role of titin filaments. J Cell Biol 105(5):2217–2223
Horowits R, Kempner ES, Bisher ME, Podolsky RJ (1986) A physiological role for titin and nebulin in skeletal muscle. Nature 323(6084):160–164
Joumaa V, Rassier DE, Leonard TR, Herzog W (2007) Passive force enhancement in single myofibrils. Pflugers Arch 455(2):367–371
Joumaa V, Rassier DE, Leonard TR, Herzog W (2008) The origin of passive force enhancement in skeletal muscle. Am J Physiol Cell Physiol 294(1):C74–C78
Kato K, Kimura S (1985) S100ao (alpha alpha) protein is mainly located in the heart and striated muscles. Biochim Biophys Acta 842(2–3):146–150
Kellermayer MS, Granzier HL (1996a) Calcium-dependent inhibition of in vitro thin-filament motility by native titin. FEBS Lett 380(3):281–286
Kellermayer MS, Granzier HL (1996b) Elastic properties of single titin molecules made visible through fluorescent F-actin binding. Biochem Biophys Res Commun 221(3):491–497
Kolmerer B, Olivieri N, Witt CC, Herrmann BG, Labeit S (1996) Genomic organization of M line titin and its tissue-specific expression in two distinct isoforms. J Mol Biol 256(3):556–563
Kulke M, Fujita-Becker S, Rostkova E, Neagoe C, Labeit D, Manstein DJ, Gautel M, Linke WA (2001) Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ Res 89(10):874–881
Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H (2003) Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci USA 100(23):13716–13721
Linke WA, Ivemeyer M, Labeit S, Hinssen H, Ruegg JC, Gautel M (1997) Actin-titin interaction in cardiac myofibrils: probing a physiological role. Biophys J 73(2):905–919
Linke WA, Kulke M, Li H, Fujita-Becker S, Neagoe C, Manstein DJ, Gautel M, Fernandez JM (2002) PEVK domain of titin: an entropic spring with actin-binding properties. J Struct Biol 137(1–2):194–205
Lu H, Isralewitz B, Krammer A, Vogel V, Schulten K (1998) Unfolding of titin immunoglobulin domains by steered molecular dynamics simulation. Biophys J 75(2):662–671
Ma K, Wang K (2003) Malleable conformation of the elastic PEVK segment of titin: non-co-operative interconversion of polyproline II helix, beta-turn and unordered structures. Biochem J 374(Pt 3):687–695
Minozzo FC, Baroni BM, Correa JA, Vaz MA, Rassier DE (2013) Force produced after stretch in sarcomeres and half-sarcomeres isolated from skeletal muscles. Sci Rep 3:2320
Nagy A, Grama L, Huber T, Bianco P, Trombitas K, Granzier HL, Kellermayer MS (2005) Hierarchical extensibility in the PEVK domain of skeletal-muscle titin. Biophys J 89(1):329–336
Neagoe C, Opitz CA, Makarenko I, Linke WA (2003) Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness. J Muscle Res Cell Motil 24(2–3):175–189
Nocella M, Colombini B, Bagni MA, Bruton J, Cecchi G (2012) Non-crossbridge calcium-dependent stiffness in slow and fast skeletal fibres from mouse muscle. J Muscle Res Cell Motil 32(6):403–409
Pavlov I, Novinger R, Rassier DE (2009) The mechanical behavior of individual sarcomeres of myofibrils isolated from rabbit psoas muscle. Am J Physiol Cell Physiol 297(5):C1211–C1219
Rassier DE (2012a) The mechanisms of the residual force enhancement after stretch of skeletal muscle: non-uniformity in half-sarcomeres and stiffness of titin. Proc Biol Sci 279(1739):2705–2713
Rassier DE (2012b) Residual force enhancement in skeletal muscles: one sarcomere after the other. J Muscle Res Cell Motil 33(3–4):155–165
Rassier DE, Herzog W (2004) Effects of shortening on stretch-induced force enhancement in single skeletal muscle fibers. J Biomech 37(9):1305–1312
Rassier DE, Pavlov I (2012) Force produced by isolated sarcomeres and half-sarcomeres after an imposed stretch. Am J Physiol Cell Physiol 302(1):C240–C248
Rassier DE, Herzog W, Pollack GH (2003) Dynamics of individual sarcomeres during and after stretch in activated single myofibrils. Proc Biol Sci 270(1525):1735–1740
Rassier DE, Lee EJ, Herzog W (2005) Modulation of passive force in single skeletal muscle fibres. Biol Lett 1(3):342–345
Roots H, Offer GW, Ranatunga KW (2007) Comparison of the tension responses to ramp shortening and lengthening in intact mammalian muscle fibres: crossbridge and non-crossbridge contributions. J Muscle Res Cell Motil 28(2–3):123–139
Schoenberg M, Brenner B, Chalovich JM, Greene LE, Eisenberg E (1984) Cross-bridge attachment in relaxed muscle. Adv Exp Med Biol 170:269–284
Stuyvers BD, Miura M, Jin JP, ter Keurs HE (1998) Ca(2 +)-dependence of diastolic properties of cardiac sarcomeres: involvement of titin. Prog Biophys Mol Biol 69(2–3):425–443
Takahashi K, Hattori A, Tatsumi R, Takai K (1992) Calcium-induced splitting of connectin filaments into beta-connectin and a 1,200-kDa subfragment. J Biochem 111(6):778–782
Tatsumi R, Hattori A, Takahashi K (1996) Splitting of connectin/titin filaments into beta-connectin/T2 and a 1,200-kDa subfragment by 0.1 mM calcium ions. Adv Biophys 33:65–77
Tatsumi R, Maeda K, Hattori A, Takahashi K (2001) Calcium binding to an elastic portion of connectin/titin filaments. J Muscle Res Cell Motil 22(2):149–162
Trombitas K, Greaser ML, Pollack GH (1997) Interaction between titin and thin filaments in intact cardiac muscle. J Muscle Res Cell Motil 18(3):345–351
Watanabe K, Muhle-Goll C, Kellermayer MS, Labeit S, Granzier H (2002) Different molecular mechanics displayed by titin’s constitutively and differentially expressed tandem Ig segments. J Struct Biol 137(1–2):248–258
Yamasaki R, Berri M, Wu Y, Trombitas K, McNabb M, Kellermayer MS, Witt C, Labeit D, Labeit S, Greaser M, Granzier H (2001) Titin-actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/S100A1. Biophys J 81(4):2297–2313
Acknowledgments
We would like to thank Prof. Giovanni Cecchi for his significant contributions to the concepts introduced in this paper. The ideas reviewed in this paper results from the work of many investigators; due to space limitations, several references dealing directly or indirectly with the subject were omitted. This research was supported by the Canadian Institutes of Health Research (CIHR), the Natural Science and Engineering Research Council of Canada (NSERC) and University of Florence, Ente Cassa di Risparmio di Firenze (2011.0302) and PRIN 2010-11: Pr. 2010R8JK2X, MIUR, Italy. Felipe S. Leite is a recipient of a CNPq scholarship, Brazil. Anabelle S. Cornachione is funded by FAPESP, Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rassier, D.E., Leite, F.S., Nocella, M. et al. Non-crossbridge forces in activated striated muscles: a titin dependent mechanism of regulation?. J Muscle Res Cell Motil 36, 37–45 (2015). https://doi.org/10.1007/s10974-014-9397-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-014-9397-6