Abstract
Few studies are concerned with the stabilization of polymers using natural polymeric polyphenols. There are no literature reports on the use of poly(flavonoids) produced by bio-chemical polymerization as stabilizers. The aim of the research was to analyse the stabilizing potential (anti-ageing UV) of poly(catechin) and poly(naringenin) in polymer compositions based on the thermoplastic elastomer of ethylene-norbornene copolymer (TOPAS Elastomer E-140). Poly(flavonoids) were obtained in a polymerization reaction with a cross-linking compound and then introduced into cyclic olefin copolymer TOPAS. For comparison, materials with monomeric catechin and naringenin were also prepared. The scope of research included the thermal analysis of the polymer compositions (Oxidation induction time OIT, Thermogravimetry TG), determination of carbonyl indices and ageing coefficients K (based on changes in mechanical properties) after UV ageing (400 h). In addition, the colour change after ageing of the samples was investigated. Samples containing polymeric forms of catechin and naringenin were more susceptible to degradation than samples with monomeric flavonoids. Inferior stabilizing properties of poly(flavonoids) were associated with steric hindrances and limited availability of hydroxyl groups to provide the antioxidant activity of the polymeric compounds. The work extends the literature data by providing an analysis of the stabilizing effect of synthetic poly(flavonoids) in polymer compositions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For many years, attempts have been made to use substances of plant origin as natural, environmentally and human-friendly stabilizers of polymers. Polyphenol compounds, including flavonoids, are popular due to their antioxidant properties [1]. The literature describes tests on the use of natural polyphenolic polymer compounds, such as tannins and lignins, to improve the degradation resistance of polymers [2, 3]. The monomer flavan-3-ol is a repeating unit that forms proanthocyanidins or more condensed tannins. A rich source of natural tannins is galls as well as grape skin and seeds [2, 3]. Tannin especially increased the thermo-oxidative stability of poly(ethylene)-based films under specific conditions and also improved the UV stability during accelerated ageing, while maintaining greater strength and elongation of the samples [4]. Stability provided by the tannins can be attributed to radical scavenging by the chain-breaking donor mechanism. It was found that the use of condensed tannins to stabilize polymers is difficult because tannins as a polar material form a heterogeneous phase in the polymer mass, and phase separation increases with the increase in the polymeric flavan-3-ol content [4]. The results of improving the miscibility of tannins with polymers by esterification with anhydrides have been described [5, 6]. The use of tannic esters of pine bark increased the resistance to photodegradation caused by ultraviolet radiation in polylactide (PLA) [5], and also reduced photo- and thermo-oxidative degradation in polypropylene (PP) [6]. The second important natural phenolic polymer is lignin. Chemically, it is a polymer whose monomers are organic compounds derived from phenolic alcohols, such as coniferyl alcohol, sinapine alcohol and coumaryl alcohol [7]. Due to the presence of numerous phenolic hydroxyl groups in the structure, lignin has the ability to chelate metal ions [8] and scavenge free radicals. A significant number of attempts have been made to use lignin as a stabilizer to protect polymers from oxidation. The antioxidant and stabilizing properties of lignin were tested for several polymers. The research mainly concerned polyethylene (PE) and polypropylene (PP) [9, 10], but also biodegradable polymers of natural origin, polylactide (PLA) [11] and poly(3-hydroxybutyrate) (PHB) [12], as well as biodegradable synthetic polycaprolactone (PCL) [13] and natural rubber [14]. Despite its beneficial properties, it has been shown that lignin does not sufficiently stabilize polymers when added to the polymer at lower concentrations. On the other hand, the addition of lignin in larger amounts results in the formation of a separate phase in the polymeric materials. The heterogeneity of polymer composites is the main obstacle limiting the application of lignin as a stabilizer. The use of low molecular weight lignin or its chemical modification by alkylation, acetylation or grafting can improve the compatibility between lignin and polymers, but such modifications usually reduce the number of phenolic hydroxyl groups responsible for antiradical activity [15].
The literature lacks information on the use of synthetically obtained polymer flavonoids as functional substances in polymer compositions. Poly(flavonoids) obtained synthetically seem to be interesting potential stabilizing agents considering their properties described in the literature [16,17,18,19]. One of the methods of obtaining polymeric flavonoids is polymerization with a cross-linking compound. Sahiner described a method of obtaining polymeric quercetin and rutin by polymerization with glycerol diglycide ether (GDE) The process used L-α lecithin as a surface-active agent, in a cyclohexane environment in the case of obtaining poly(quercetin) [16] or in a gasoline environment when obtaining poly(rutin) [17]. Polymeric forms of catechin [18] and naringenin were also obtained in the polymerization reaction with GDE [19]. The epoxy groups in GDE cross-linking agent react with the OH groups in the flavonoids to form polymeric structures [16, 17]. Poly(catechin) and poly(naringenin) were characterized by greater resistance to oxidation and better thermal stability than monomeric flavonoids [18, 19]. Poly(catechin) had better ability to reduce transition metal ions than ( +)-catechin [18], whereas polymeric naringenin also had better ability to scavenge ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) and DPPH (2,2-diphenyl-1-picrylhydrazyl) free radicals [19].
The aim of this study was to analyse the stabilizing effect (anti-ageing UV) of poly(catechin) and poly(naringenin) in polymer compositions based on the thermoplastic elastomer of ethylene-norbornene copolymer (TOPAS Elastomer E-140).Poly(catechin) and poly(naringenin) were prepared by reaction with the GDE cross-linking compound and tested as stabilizers in the light of the beneficial properties of the powders described in the literature, including thermal stability and the ability to reduce radicals and transition metal ions [18, 19]. The scope of research included the analysis of thermal stability of polymer compositions, analysis of carbonyl indices and mechanical properties of samples before and after UV ageing. In addition, the colour change of materials after a controlled ageing process was examined. Cyclic olefin copolymer TOPAS was selected for the study due to the lack of cross-linking reaction (and thus the lack of cross-linking substances that could react with poly(flavonoids) during cross-linking and affect the stabilization process of the polymer material). Furthermore, TOPAS is a transparent material, so the observation of the colour change of the samples was not problematic. Few literature data concern the stabilization of TOPAS with plant-based substances, including polyphenols. Cannabidiol (CBD) extract [20], thyme and cloves [21], as well as flavonoids hesperidin [22] and quercetin [23] were examined as a potential natural stabilizer for thermoplastic elastomer ethylene-norbornene copolymer. However, natural polyphenolic polymers and synthetically obtained poly(flavonoids) based on plant flavonoids have not yet been tested as stabilizing substances in TOPAS.
Experimental
Materials
Preparation of polymeric forms of flavonoids by reaction with a cross-linking compound
Catechin (( +)-catechin hydrate ≥ 98% HPLC, MW: 290,27 g mol−1, Sigma-Aldrich, China) and naringenin (natural, 98%, MW: 272.25 g mol−1, Sigma-Aldrich, Shanghai, China) were polymerized prior to being incorporated in the polymer. Polymeric forms of catechin and naringenin were synthesized in reaction with the cross-linking compound glycerol diglycide ether (GDE). The authors described the method of synthesis and the properties of poly(catechin) and poly(naringenin) in previous publications [18, 19].
Preparation of polymer samples
Thermoplastic elastomer ethylene-norbornene copolymer (TOPAS Elastomer E-140 from TOPAS Advanced Polymers, Germany) was used as the polymer matrix. The Melt Volume-flow Rate (MVR) of the polymer was 11.9 cm3 10 min−1, and the melting temperature Tm reported to be 84 °C. The polymer granules and poly(flavonoids) were mixed in a laboratory micromixer (Plasti-Corder Brabender Lab-Station with Julabo cooling system) at 110 °C and 50 rpm for 20 min. Samples of TOPAS Elastomer E-140 with catechin or naringen flavonoids were also prepared for comparison purposes. The prepared polymer mixtures were passed through a two-roll mill with rolls of 200 mm, at a roll temperature of 25 °C and a friction ratio of 1:1.1 for about 30 s. The last stage was pressing the sheets between the two halves of a steel mold, lined with Teflon sheets, in a hydraulic press under the following conditions: temperature 160 °C, pressure 7.5 MPa, time 10 min. Table 1 shows the composition of the prepared samples.
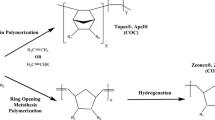
Figure 1 shows the preparation of poly(flavonoids) [18, 19] and samples based on TOPAS.
Methods
UV ageing
Samples of TOPAS® with flavonoids and poly(flavonoids) were placed in an ATLAS UV 2000 ageing chamber. The UV ageing process was carried out under the following conditions: ageing time 400 h, temperature 60 °C, UV radiation intensity 0.55 W m−2.
Thermogravimetric (TG) analysis
Thermal stability of flavonoids, poly(flavonoids) and polymeric composition was performed using a Mettler Toledo thermobalance (TA Instruments, Switzerland). Samples were heated from 25 to 1000 °C under air flow (50 mL min−1) at rate of 20 °C min−1.
Oxidation induction time OIT experiment
The determination of the resistance of the samples to oxidation was performed using a DSC1 differential scanning calorimeter (TA Instruments, Switzerland). The polymer compositions were heated under an inert gas atmosphere to a temperature of 240 °C. After the required measurement conditions were achieved and a stabilization period (5 min at 240 °C, argon), the inert gas was switched to air and the measurement of the oxidation induction time (OIT) was started. The OIT measurement of catechin, naringenin, poly(catechin) and poly(naringenin) powders was performed at 300 °C.
Carbonyl index (CI)
Based on the Fourier transform infrared (FTIR) spectrum, a carbonyl index (CI) which gives a measure of the amount of carbonyl groups formed during the ageing process of polymer compositions was calculated for samples after UV ageing according to Eq. (1):
where IC=O—intensity of peak that corresponds to the C = O groups (~ 1700 cm−1), and IC–H—intensity of peak that represents the –CH groups (~ 2800 cm−1).
FTIR spectra were recorded in the 4000–400 cm−1 range with a Thermo Scientific Nicolet 6700 FTIR spectrometer equipped with a diamond Smart Orbit ATR sampling accessory. Measurements were made for unaged and aged samples.
Determination of the ageing coefficient K
Based on changes in static mechanical properties of the samples after UV ageing, the ageing coefficients K were determined. The mechanical properties of tensile strength \(\left( {T_{{{\text{Fmax}}}} } \right)\) and elongation at break \(\left( {E_{{{\text{Fmax}}}} } \right)\) of the polymer compositions were tested with a Zwick-Roell 1435 device (Germany) based on ISO 37. Samples in the shape of a “dumbbell” with a thickness of about 1.5 mm and a centre portion width of 4 mm were prepared in accordance with ISO 37. The test parameters were as follows: tensile speed 500 mm min−1, initial force 0.1 N. Using Eq. (2), the ageing coefficients K were calculated:
where TFmax [MPa] is maximum tensile strength and EFmax [%] is elongation at ultimate strength.
Change of colour after UV aging
The sample colours were described by the CIE-Lab system (L—lightness, a—red–green, b—yellow–blue) using a Konica Minolta UV–VIS CM-36001 spectrophotometer (Japan). In the next step, the values of colour difference (ΔE) (3), whiteness index (Wi) (4), saturation (Cab) (5) and hue angle (hab) (6) were calculated according to the following equations [24]:
The Δa, Δb and ΔL denote the coordinate differences between aged and unaged samples.
Results and discussion
Adequate thermal stability of natural additives is very important in the processing of polymers. Plant-derived additives should not degrade at the elevated temperatures associated with extrusion, injection and other processing of polymeric materials. The first step was the thermal analysis of flavonoids and poly(flavonoids) and comparing their thermal stability in air. Figures 2 and 3, and Tables 2 and 3 show the curves of the TG and OIT of catechins, naringenin and their polymeric forms.
The thermal decomposition of catechin and poly(catechin) was multi-stage (Fig. 2). Table 2 shows the temperatures at which the mass loss of the samples amounted to 5% (T5), 50% (T50), 80% (T80) and 90% (T90). The beginning of decomposition and the half decomposition of poly(catechin) started at lower temperatures than for catenin (T5 poly(catechin) = 126 °C, T5 catechin = 214 °C). However, the final degradation of the polymeric catechin occurred at a higher temperature (T80 poly(catechin) = 998 °C, T80 catechin = 638 °C). Thermal decomposition of naringenin was two-stage, while poly(naringenin) was multi-stage. As in the case of catechin and poly(catechin), increased thermal stability of poly(naringenin) was observed in the final stage of degradation (T90 poly(naringenin) = 998 °C, T90 naringenin = 643 °C). Similar results were obtained during the thermal analysis of poly(catechin) [18] and poly(naringenin) [19] in an inert gas—the polymeric forms had better thermal stability, especially visible in the final stage of decomposition of the compounds.
Oxygen induction time (OIT) is the time after which an exothermic oxidation peak begins to appear on the DSC curve. The OIT measurements were made at the temperature of 300 °C (Fig. 3, Table 3). Catechin was characterized by the longest oxidation induction time (onset = 14.5 min, peak = 30.7 min, endset = 37.6 min). After polymerization, the poly(catechin) OIT times were significantly shortened (endset = 15.1 min), but the value of the oxidation enthalpy was about 7 times higher. The polymeric naringenin had a longer OIT time than the monomer (naringenin endset = 4 min; poly(naringenin) endset = 26.4 min) and the oxidation was accompanied by a greater enthalpy of oxidation (about 99 times). The enthalpy of oxidation is the heat released during oxidation. Generally, the enthalpy of oxidation is larger, the oxidation process is more likely to occur, and the OIT value should be smaller. Polymerization appears to have decreased the thermal stability of poly(flavonoids), which may have been caused by a reduction in the amount of OH groups. Thermal degradation of polymeric compounds probably occurred during the OIT measurement, which correlated with the mass loss results from thermogravimetry.
A different OIT curve of catechin profile may be related to the specificity of the catechin compound itself. According to the literature, polyphenols have good charring properties. In particular, tannins have excellent charring capacity and therefore can be used in flame- retardant applications [25, 26]. A different course of the catechin OIT curve may be related to formation of a char layer on the sample surface during combustion. As a result, a protective foamed coke layer may be formed, which prevents free heat exchange and oxygen transport to the deeper layers of the sample, thanks to which the course of catechin oxidation is prolonged and spread over time compared to other tested samples.
Thermal analyses TG and OIT of flavonoids and poly(flavonoids) powders suggested that the compounds showed sufficient resistance to thermo-oxidation to be introduced into TOPAS. Therefore, the next step was thermogravimetric and oxidation induction time determination of TOPAS compositions containing plant-derived additives. During the controlled ageing, apart from UV radiation, the samples were subjected to a temperature of 60 °C in an air atmosphere. Thus, thermo-oxidative ageing was part of the controlled UV ageing. Therefore, it was reasonable to test the resistance of the samples to thermal oxidation.
The TG analysis of polymeric composition showed no differences in the thermal decomposition of the TOPAS samples containing flavonoids and poly(flavonoids), as well as for the reference copolymer sample (Fig. 4 and Table 4). On the other hand, changes were observed in the oxidation induction time of TOPAS with the individual additives (Fig. 5 and Table 5). Among all the analysed samples, the shortest OIT time was shown by the standard TOPAS (1.9 min). Both the addition of flavonoids and poly(flavonoids) extended the OIT time found for the polymer compositions, i.e., the additives increased the resistance of all samples to oxidation. The addition of catechin significantly extended the OIT times of the samples—with the increase in polyphenol concentration, the onset of oxidation was prolonged. For samples containing poly(catechin), the OIT times were identical regardless of the additive concentration, but they were longer than the OIT time of the reference TOPAS. In the case of materials containing the second polyphenol, the oxidation induction time for the sample with poly(naringenin) was 2.2 min (onset) and was about 1 min longer than the OIT time of the sample with monomeric naringenin. In general, polymeric flavonoids, due to their higher molar mass, can thermally degrade more slowly than monomers.
All polymer compositions containing flavonoids and poly(flavonoids) were characterized by a longer oxidation induction time than the reference TOPAS, which meant that both additives increased the oxidation resistance of the materials.
It was interesting to compare the OIT times for TOPAS samples containing flavonoids and poly(flavonoids). The OIT parameter allows determination of the resistance to oxidation of polymeric materials containing various antioxidants. For compositions containing poly(catechin), OIT times were shorter than for samples with monomeric catechin. On the other hand, for materials with naringenin, a contrasting situation was found—samples with a polymeric compound had longer OIT times than samples with naringenin.
As a result of the polymerization of catechin and naringenin, the hydroxyl groups of flavonoids, which are responsible for the antioxidant activity, participated in the polymerization reactions so that, due to the lack of these active OH groups, the OIT times of both poly(flavonoid) powders and samples with polymeric flavonoids were shorter than those of flavonoids and TOPAS with monomeric forms of flavonoids. Koontz et al. [27] reported similar observations for the flavonoid quercetin and its complex compounds. In their paper, they analysed the effect and efficiency of quercetin, α-tocopherol and cyclodextrin complexes on polyethylene stability. The additives increased the oxidation induction times of the polymer, but the efficiency of the quercetin complexes was lower compared with the free flavonoid molecule. Therefore, it can be concluded that the hydroxyl groups and other structural elements responsible for the antioxidant activity of complex flavonoid compounds and polymeric flavonoids were blocked as a result of steric hindrance, and their activity was limited, which resulted in lower resistance to oxidation of polymeric materials containing these compounds (shorter OIT time).
Subsequent research was focused on analysis of changes in materials after UV ageing combined with thermo-oxidation. The next step was to analyse the carbonyl indices of the TOPAS composition (Fig. 6). Carbonyl indices were calculated from structural changes recorded in FTIR spectra (Supplementary material S.1.). The carbonyl index is considered a measure of the progress of oxidation and decomposition of polymeric materials because it corresponds to the carbonyl groups (acids, aldehydes and ketones) accompanying the degradation of the composition. It indicates the course of the degradation process, where the first stage is the oxidation of the polymer chain to carbonyl groups, which are crucial during oxidation (decompose into CO2 and H2O). The increase in the carbonyl index indicates more advanced degradation processes of polymer blends. This means that the TOPAS reference sample with the highest carbonyl index (CI TOPAS = 3.23 [–]) showed the lowest resistance to UV ageing at an elevated temperature of 60 °C. Comparing the carbonyl indexes of compositions containing catechin and poly(catechin), it was found that samples containing polymerized flavonoid had lower index values, which meant that the addition of poly(catechin) better improved the UV ageing resistance of the materials. The polymeric forms of catechin may be more resistant to UV irradiation than the monomer. In addition, catechin monomer can undergo chemical reactions, including polymerization, under the influence of UV. These phenomena may explain why samples containing cross-linked catechin showed lower carbonyl indices and thus were better protected against UV ageing. By analysing the carbonyl indexes of polymer compositions with naringenin and poly(naringenin), it was concluded that less destructive changes occurred in the flavonoid-containing sample (lower carbonyl index value, CI naringenin = 0 0.20 [–], CI poly(naringenin) = 0.61 [–]), which suggested that naringenin gave TOPAS better protection against degrading agents. All the additives used, i.e., monomeric and polymeric flavonoids, improved the resistance of TOPAS to UV ageing, but no clear trends in the action of additives were observed. Monomeric catechin and polymeric naringenin showed a better stabilizing effect. These ambiguous trends in the results may be related to the surface character of the measurement of the samples and the inhomogeneity of the materials.
As a basis for indicating changes in the mechanical properties (Table 6) of polymeric materials after UV ageing, ageing coefficients K (Fig. 7) were determined. For the reference sample of TOPAS, the ageing coefficient K reached a value close to zero (K = 0.06 [–]), which meant that it was susceptible to ageing caused by UV radiation and an elevated temperature of 60 °C for 400 h. The addition of flavonoids and poly(flavonoids) improved the resistance of TOPAS to UV ageing (the values of K coefficients were higher). Polymer compositions containing catechin (0.5 phr, 1 phr and 1.5 phr), naringenin and poly(naringenin) were characterized by ageing coefficient values close to one, so these materials were resistant to degradation. The K values of the samples of TOPAS containing catechin (0.5–1.5 phr) were 0.81–1.01 [–], while the samples with poly(catechin) remained at 0.26–0.38 [–]. The highest values of ageing coefficients were found for samples containing the highest concentration of catechin (K = 1.01[–]) and poly(catechin) (K = 0.38[–]). The values of the ageing coefficients for samples containing catechin and poly(catechin) differed significantly—the K values for materials with poly(catechin) were much lower. This meant that the catechin was better at protecting the TOPAS from ultraviolet ageing than its polymeric form. The better resistance to ageing of the samples with catechin could be due to the better availability of functional groups, including hydroxyl groups, responsible for the polyphenol’s antioxidant properties. In the polymeric form, active functional groups may be less accessible, spherically limited, which contributes to the reduction of their activity. In addition, the TOPAS samples with poly(flavonoids) were inhomogeneous, which could affect their degree of crystallinity, and thus the mechanical properties of the polymer compositions. A lower degree of crystallinity of the materials could have resulted in poorer mechanical properties of the samples. The use of condensed catechins, i.e., tannins, as substances to stabilize polymeric materials may be difficult due to the poor miscibility of the components—the resulting materials were inhomogeneous and contained visible inclusions of the additive.
The problem with obtaining homogeneous samples of polymers with the addition of phenolic polymers (tannin and lignin) has also been described in the literature. It has been observed that natural additives form a separate phase in polymer matrices, which limits their stabilizing effect [1]. Improving the miscibility of polymers with polymeric poly(flavonoids) can be achieved by modifying polyphenols, but this issue is poorly researched and requires further analysis.
The K coefficient of samples with naringenin and poly(naringenin) was comparable and amounted to K = 1.00 [–] and K = 0.98 [–], respectively. Monomeric and polymeric naringenin were characterized by similar TOPAS stabilizing properties, which could be related to the similar availability and reactivity of functional groups involved in antioxidant reactions protecting the polymer against degradation. Moreover, the reactivity and antioxidant activity of polymeric flavonoids may depend on their spatial structure, the availability of OH groups and their susceptibility to oxidation, as well as the solubility of the compound in the polymer. Additionally, the ageing factors K were determined based on the mechanical properties of the samples before and after ageing. The mechanical properties of samples can be influenced by many factors, including the crystallinity of the samples or their imperfect homogeneity, which will also affect the K-factor results.
The last stage of the research was to determine the colour change of the samples after UV ageing. Figure 8 summarizes the colour change parameters of ΔE colour change factor, saturation, whiteness index and hue angle. Figure 9 shows the visual colour changes of the polymeric materials.
The colour change coefficient ΔE is a numerical reflection of the colour change of the tested material after ageing compared to the unaged sample. The smallest values of this parameter were for the TOPAS sample and had values in the range 1 < ΔE < 2, which meant that statistically only an experienced observer could detect the difference between the colours. The remaining samples had colour change coefficient above 5, so these colours were perceived as completely different. The colour change factor (Fig. 8A) and the colour change parameters (Fig. 8B, C, D) correspond to the visual observation of the samples (Fig. 9). The colour of samples containing catechin changed from orange to light brown, and the colour of materials with poly(catechin) took on a shade of darker brown after UV ageing. Compositions with naringenin and poly(naringenin) became more milky in colour as a result of ageing with ultraviolet radiation. Chemical reactions of flavonoids under the influence of UV radiation were responsible for the colour change of the samples. According to the literature, catechin is sensitive to UV radiation, which causes changes in the structure of this polyphenol [28]. Shi et al. showed that catechin and epicatechin are also sensitive to ultraviolet B radiation, causing these two compounds to form yellow products [29]. Illumination of catechins and epicatechins with UV light opens the rings of these polyphenols and causes isomerization between the catechins [30, 31].
Summing up the research described above, it can be concluded that the phytochemicals (catechin and naringenin) and their polymeric forms acted as stabilizers, allowing control of the oxidation time of polymer compositions. Ultimately, the life time of the material can be controlled by adding the appropriate amount and form of substances of plant origin.
Comparing the resistance to thermo-oxidation (OIT, TG) of materials based on TOPAS with the addition of catechin or poly(catechin), it was found that the monomeric flavonoid significantly improved the resistance to these parameters compared to its polymerized form. This was best seen in the results of OIT times followed by oxidation of the samples, where catechin extended the time to start of oxidation by an average of about 11 min compared to poly(catechin). Along with the increase in the concentration of catechin and poly(catechin) in polymer compositions, their resistance to degradation also increased—in most studies the best results were obtained for materials containing 1.0 phr or 1.5 phr of this flavonoid or poly(flavonoid).
The analysis of materials with the addition of naringenin and poly(naringenin) showed that they stabilize TOPAS to a similar extent, as evidenced by similar oxidation times of the samples and similar values of ageing coefficients. In the case of the FTIR study, on the basis of which the carbonyl index was determined, it was found that the monomeric form gave the material a better resistance to degradation. However, it should be taken into account that the samples containing poly(naringenin) were inhomogeneous, which could have influenced the results obtained.
The analysis of mechanical properties, which was necessary to determine the ageing coefficient K, allowed the conclusion that the addition of flavonoids and their polymerized forms increased the resistance of TOPAS to 400 h of ultraviolet ageing.
In the case of samples with catechin and poly(catechin), the monomeric flavonoid had better stabilizing properties. Different results were obtained for samples containing naringenin and poly(naringenin)—both compounds had a similar stabilizing effect, only the analysis of CI indices indicated a stronger stabilizing effect of the monomer. The stronger stabilizing effect of monomeric forms of flavonoids may be related to the better availability of hydroxyl groups and other structural elements that take an active part in antioxidant reactions that determine the resistance of samples to oxidation. Poly(flavonoids) may have steric hindrances that hinder the occurrence of stabilizing chemical reactions.
During polymerization with GDE, the phenolic OH groups in catechin and naringenin react with the epoxy groups in the cross-linking compound. As a result of the reaction, polymeric molecules of flavonoids were formed. Catechin is the flavan-3-ol group of flavonoids, whereas the structure of naringenin is based on a carbon skeleton with a ketone group in position 4 in ring C. Catechin, in ring B, has two OH groups in the 4ʹ and 5ʹ positions, while naringenin has only one OH group in the 4ʹ position. Taking into account the differences in the structure of catechin and naringenin, cross-linking reactions with GDE may follow different paths. The catechin cross-linking reaction involving OH groups in the 5, 4ʹ and 5ʹ positions was proposed [18]. However, for naringenin, a reaction scheme involving OH groups in the 5, 7 and 4ʹ positions was suggested. Different proposals for reaction pathways of catechin and naringenin polymerization with GDE resulted from structural differences of both flavonoids and could be related to the electron density of these polyphenols [19]. Considering the polymerization reaction paths, it can be concluded that the hydroxyl groups, which to a large extent determine the antioxidant activity of flavonoids, were unavailable or sterically limited in poly(flavonoids). Thus, the greater ageing susceptibility of TOPAS with poly(flavonoids) was justified.
Hydroxyl groups largely determine the antioxidant activity of flavonoids. Poly(catechin) has two OH groups in the monomer unit, while poly(naringenin) has only one OH group. Poly(catechin) has more phenolic hydroxyl groups than poly(naringenin), but the TOPAS/poly(naringenin) composition with the same content (1 phr) of poly(flavonoid) showed better results. For example, OIT values TOPAS/Poly(catechin)1 (2.6 min) and TOPAS /Poly(naringenin)1 (3.2 min), carbonyl index TOPAS/Poly(catechin)1 (CI = approx. 1.5 [–]) and TOPAS/Poly(naringenin)1 (CI = 0.61 [–]), ageing factors K TOPAS/Poly(catechin)1 (K = about 0.3 [–]) and TOPAS/Poly(naringenin)1 (K = 0.98 [–]). These results did not correlate with the theory that the more hydroxyl groups, the better the results. In the case of the research described in this paper, it cannot be assumed that only hydroxyl groups from poly(flavonoids) determine the ageing resistance of samples. For flavonoids, their antioxidant activity depends on their structural structure, including the number and position of hydroxyl groups and other structural elements. SAR structure activity relationships for flavonoids have been well described in the literature [32, 33]. In contrast, such information is lacking for polymeric flavonoids. There is little literature data on SAR relationships in poly(flavonoids) and these relationships have not yet been precisely defined [32]. In this manuscript, it cannot be assumed that only the amount of hydroxyl groups will be responsible for the antioxidant activity of poly(flavonoids). For poly(flavonoids), the antioxidant activity, or lack thereof, may also be related to, for example, the spatial structure of the poly(flavonoids), to possible steric hindrance limiting the availability of hydroxyl groups, or to other factors related to the spatial structure of the compounds, as well as to the molecular weight of the polymeric compound. The investigation and determination of the structure of polymeric flavonoids and SAR relationships still needs to be developed in the literature. Unfortunately, these studies may be hampered by the limited solubility of poly(flavonoids). The research described in this work is basic research and one of the first to focus on the use of polymeric flavonoids in polymers and to determine their effect on the ageing process of polymeric materials. Moreover, not only OH hydroxyl groups may be responsible for antioxidant activity and limiting UV ageing in samples. The addition of poly(flavonoids) to polymeric materials could affect the interactions between polymer macromolecules and cause structural changes that determine the ageing processes. The samples were aged with UV radiation, and poly(flavonoids), due to the colour they gave to the materials, could absorb (dark samples with poly(catechin) or reflect UV radiation (light samples with poly(naringenin), and thus cause the phenomenon of polymer photostability.
The mechanism of photodegradation of ethylene-norbornene random copolymers with different norbornene content has been investigated in the scientific literature [34]. During photo-irradiation of copolymers, the hydrogen atom bonded to the tertiary and secondary carbon atoms is abstracted, resulting in the formation of formyl, formate, acyl and hydroxyl groups and a carbon–carbon double bond. The processes of photodegradation of ethylene-norbornene copolymers occur with the participation of free radicals. Stabilization of polymeric materials by natural antioxidants may consist of capturing free radicals involved in the degradation of the polymer matrix and slowing down their oxidation processes. The process of stabilizing polymeric materials by natural polyphenols is very complex and depends on many factors, including the polymer matrix and factors causing degradation. It has been shown that catechin may have an antioxidant or pro-oxidative effect [35]. During photo-oxidation, catechin can cause a pro-oxidative effect. Prooxidative activity was observed for very specific conditions—during photo-oxidation in a solid and non-polar environment (polyolefin matrix), while typical environments for natural stabilizers are very different (in vivo conditions). Natural phenolic stabilizers can show pro-oxidative activity during photo-oxidation, but still show antioxidant activity during thermo-oxidation. It was shown that polyolefins with phenolic stabilizers (commercial Irganox®1010, vitamin E or catechin) were characterized by good resistance to thermal oxidation [35]. During the controlled ageing of TOPAS samples with catechin, poly(catechin), naringenin and poly(naringenin), the materials were exposed to elevated temperature (60 °C) as well as to UV radiation. The process of thermo-oxidation of the samples could be the dominant process at a given time of ageing. Therefore, it was observed that the natural additives acted as antioxidants and reduced the ageing of the TOPAS.
The heterogeneous structure of polymeric materials and the presence of poly(flavonoids) agglomerates could also adversely affect and limit the stabilizing effect of poly(catechin) and poly(naringenin) in TOPAS. The disadvantages associated with the limited miscibility of natural polymeric polyphenolic compounds such as tannins and lignin have been described in the literature [6, 11, 14, 15, 31, 36]. The heterogeneity of polymer composites is the main obstacle limiting the application of natural polymeric polyphenols as stabilizers. Both lignin and condensed tannins are cheap and readily available polymer-stabilizing raw materials that will probably be used in the future, but further research is needed to improve their miscibility with polymers.
The compositions prepared based on TOPAS, flavonoids and poly(flavonoids) were characterized by an intense colour change. A clear change in the colour of materials may find potential application in intelligent materials. By designing an appropriate scale of colour change as a function of the time of exposure to external factors, it would be possible to assess the lifetime of the polymer composition.
The analyses performed in this work are the first studies on the stabilizing effect of synthetic poly(flavonoids) in polymeric materials and require continuation. However, they prove the possibility of using poly(catechin) and poly(naringenin) as stabilizing agents and indicators of ageing time. The study provides new data on the effect of synthetic poly(catechin) and poly(naringenin) on ageing and stabilization processes of thermoplastic elastomer ethylene-norbornene copolymer. Moreover, the research carried out in the manuscript was of an exploratory nature and was intended to test poly(flavonoids) in terms of their behaviour in polymers, dispersion, and influence on the properties of polymer compositions.
Conclusions
Polymeric flavonoids obtained in the reaction with a cross-linking compound showed stabilizing properties in the TOPAS copolymer (determination of carbonyl indexes, ageing factors) during controlled UV ageing. However, the stabilizing abilities of poly(catechins) and poly(naringenins) were inferior to reference flavonoids. Poly(flavonoids) after a cross-linking reaction (polymerization) had less OH groups in their structure responsible for antioxidant protection in polymeric materials. Moreover, due to the highly cross-linked structure, i.e., the presence of steric hindrance, the antioxidant effect of hydroxyl groups could be limited. However, compared with additive-free TOPAS, poly(flavonoids) showed ability to stabilize this material.
Another interesting property of flavonoids and poly(flavonoids) added to the cyclic olefin copolymer was an intense and clear change in the colour of the samples during UV ageing. Catechin and naringenin, as well as their synthetically obtained polymeric forms, can successfully act as indicators of the ageing time of polymeric materials, especially materials intended for the production of intelligent packaging or their components.
References
Kirschweng B, Tátraaljai D, Földes E, Pukánszky B. Natural antioxidants as stabilizers for polymers. Polym Degrad Stab. 2017;145:25–40.
Oroian M, Escriche I. Antioxidants: characterization, natural sources, extraction and analysis. Food Res Int. 2015;74:10–36.
Nanni A, Ricci A, Versari A, Messori M. Wine derived additives as poly(butylene succinate) (PBS) natural stabilizers for different degradative environments. Polym Degrad Stab. 2020;182: 109381.
Bridson JH, Kaur J, Zhang Z, Donaldson L, Fernyhough A. Polymeric flavonoids processed with co-polymers as UV and thermal stabilisers for polyethylene films. Polym Degrad Stab. 2015;122:18–24.
Grigsby W, Bridson J, Lomas C, Elliot JA. Esterification of condensed tannins and their impact on the properties of poly(lactic acid). Polymers. 2013;5:344–60.
Grigsby WJ, Bridson JH, Lomas C, Frey H. Evaluating modified tannin esters as functional additives in polypropylene and biodegradable aliphatic polyester. Macromol Mater Eng. 2014;299:1251–8.
Vibha K, Negi S. Enzymatic plasticising of lignin and styrene with adipic acid to synthesize a biopolymer with high antioxidant and thermostability. Polym Degrad Stab. 2020;174: 109081.
Guo X, Zhang S, Shan X. Adsorption of metal ions on lignin. J Hazard Mater. 2008;151:134–42.
Alexy P, Košı́ková B, Podstránska G. The effect of blending lignin with polyethylene and polypropylene on physical properties. Polymer. 2000;41:4901–8.
Slavikova E, Košíková B. Use of lignin products derived from wood pulping as environmentally desirable additives of polypropylene films. Wood Res. 2010;55:87–92.
Gordobil O, Delucis R, Egüés I, Labidi J. Kraft lignin as filler in PLA to improve ductility and thermal properties. Ind Crops Prod. 2015;72:46–53.
Bertini F, Canetti M, Cacciamani A, Elegir G, Orlandi M, Zoia L. Effect of ligno-derivatives on thermal properties and degradation behavior of poly(3-hydroxybutyrate)-based biocomposites. Polym Degrad Stab. 2012;97:1979–87.
Pucciariello R, D’Auria M, Villani V, Giammarino G, Gorrasi G, Shulga G. Lignin/poly(ε-Caprolactone) blends with tuneable mechanical properties prepared by high energy ball-milling. J Polym Environ. 2010;18:326–34.
Košíková B, Gregorová A, Osvald A, Krajčovičová J. Role of lignin filler in stabilization of natural rubber–based composites. J Appl Polym Sci. 2007;103:1226–31.
Duval A, Lawoko M. A review on lignin-based polymeric, micro- and nano-structured materials. React Funct Polym. 2014;85:78–96.
Sahiner N. One step poly(quercetin) particle preparation as biocolloid and its characterization. Colloids Surf A Physicochem Eng Asp. 2014;452:173–80.
Sahiner N. One step poly(rutin) particle preparation as biocolloid and its characterization. Mater Sci Eng, C. 2014;44:9–16.
Latos-Brozio M, Masek A, Piotrowska M. Thermally stable and antimicrobial active poly(Catechin) obtained by reaction with a cross-linking agent. Biomolecules. 2021;11:1–15.
Latos-Brozio M, Masek A, Piotrowska M. Novel polymeric biomaterial based on naringenin. Materials. 2021;14:2142.
Plota A, Masek A. Plant-origin stabilizer as an alternative of natural additive to polymers used in packaging materials. Int J Mol Sci. 2021;22:4012.
Masek A, Cichosz S, Piotrowska M. Comparison of aging resistance and antimicrobial properties of ethylene-norbornene copolymer and poly(lactic acid) impregnated with phytochemicals embodied in thyme (Thymus vulgaris) and clove (Syzygium aromaticum). Int J Mol Sci. 2021;22:13025.
Masek A, Plota A. Influence of a natural plant antioxidant on the aging process of ethylene-norbornene copolymer (topas). Int J Mol Sci. 2021;22:4018.
Masek A, Latos M, Piotrowska M, Zaborski M. The potential of quercetin as an effective natural antioxidant and indicator for packaging materials. Food Packag Shelf Life. 2018;16:51–8.
Cichosz S, Masek A, Wolski K. Innovative cellulose fibres reinforced ethylene-norbornene copolymer composites of an increased degradation potential. Polym Degrad Stab. 2019;159:174–83.
Drabble E, Nierenstein M. On the rôle of phenols, tannic acids, and oxybenzoic acids in cork formation. Biochem J. 1907;2:96-102.1.
Xia Z, Kiratitanavit W, Facendola P, Thota S, Yu S, Kumar J, et al. Fire resistant polyphenols based on chemical modification of bio-derived tannic acid. Polym Degrad Stab. 2018;153:227–43.
Koontz JL, Marcy JE, O’Keefe SF, Duncan SE, Long TE, Moffitt RD. Polymer processing and characterization of LLDPE films loaded with α-tocopherol, quercetin, and their cyclodextrin inclusion complexes. J Appl Polym Sci. 2010;117:2299–309.
Yuann JMP, Lee SY, Yang MJ, Huang ST, Cheng CW, Liang JY. A study of catechin photostability using photolytic processing. Processes. 2021;9:1–12.
Shi M, Nie Y, Zheng XQ, Lu JL, Liang YR, Ye JH. Ultraviolet B (UVB) photosensitivities of tea catechins and the relevant chemical conversions. Molecules. 2016;21:1345.
Wilhelm-Mouton A, Bonnet SL, Ding Y, Li XC, Ferreira D, van der Westhuizen JH. Photochemistry synthesis. Part 2: enantiomerically pure polyhydroxy-1,1,3-triarylpropan-2-ols. J Photochem Photobiol A Chem. 2012;227:18–24.
Forest K, Wan P, Preston CM. Catechin and hydroxybenzhydrols as models for the environmental photochemistry of tannins and lignins. Photochem Photobiol Sci. 2004;3:463–72.
Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure–activity relationships. J Nutr Biochem. 2002;13(10):572–84.
Amic D, Davidovic-Amic D, Beslo D, Rastija V, Lucic B, Trinajstic N. SAR and QSAR of the antioxidant activity of flavonoids. Curr Med Chem. 2007;14:827–45.
Nakade K, Nagai Y, Ohishi F. Photodegradation of some ethylene–norbornene random copolymers. Polym Degrad Stab. 2010;95:2654–8.
Gajdošová V, Šlouf M, Michálková D, Dybal J, Pilař J. Pro-oxidant activity of biocompatible catechin stabilizer during photooxidation of polyolefins. Polym Degrad Stab. 2021;193: 109735.
Grigsby WJ, Bridson JH, Schrade C. Modifying biodegradable plastics with additives based on condensed tannin esters. J Appl Polym Sci. 2015;132:41626.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
M.L.-B., A.M. contributed to conceptualization, resources, data curation, formal analysis, funding acquisition, investigation; M.L.-B., A.M., K.M. contributed to methodology, visualization and validation; M.L.-B. contributed to software and roles/writing—original draft; and A.M. supervised the study and contributed to writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Latos-Brozio, M., Milczarek, K. & Masek, A. Evaluation of the stabilizing effect and ageing time indicator properties of poly(flavonoids) in a cyclic olefin copolymer. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13431-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13431-x